The Association between the Extent of the Osteoarthritic Meniscus Degeneration and Cigarette Smoking—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
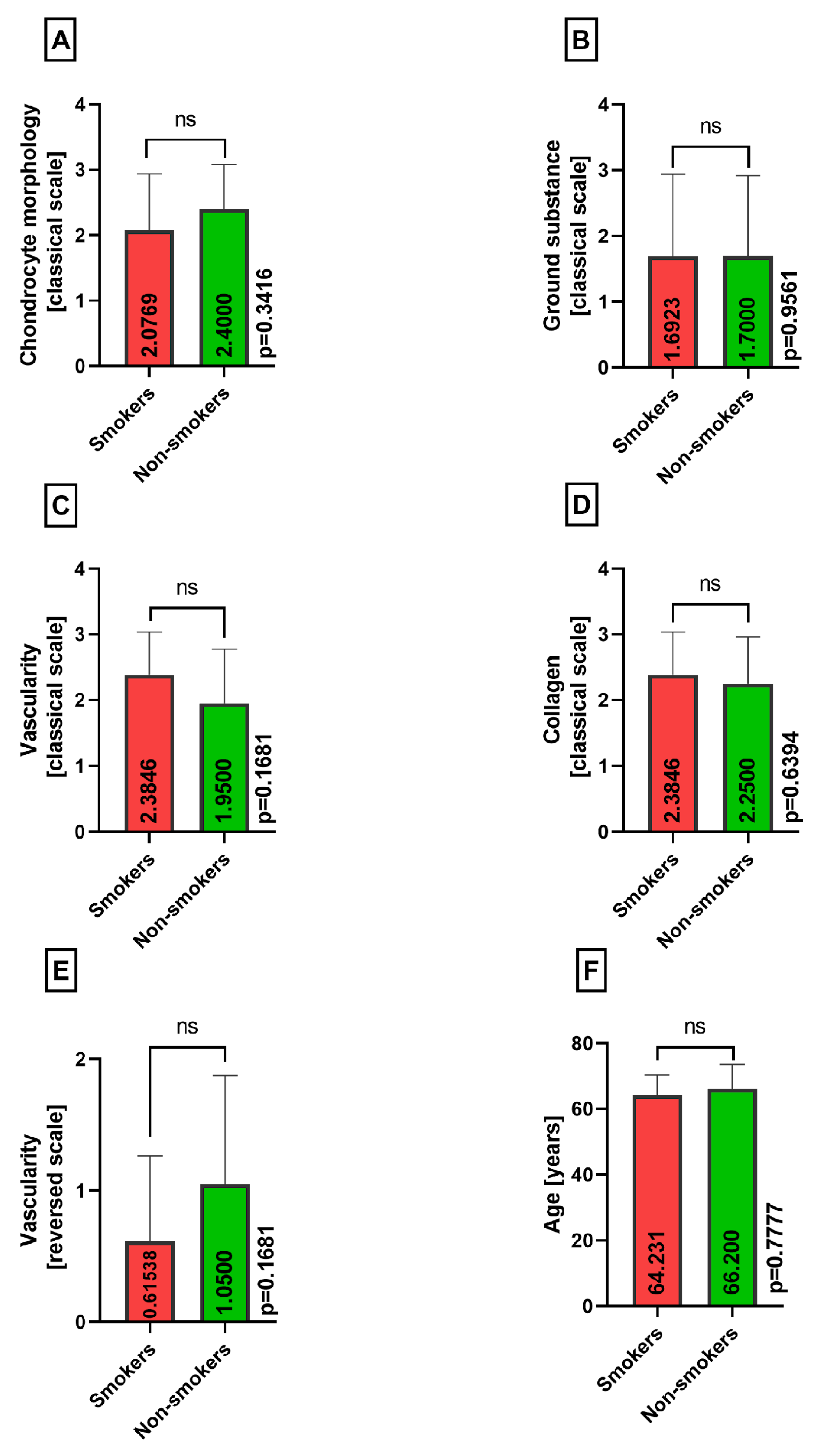
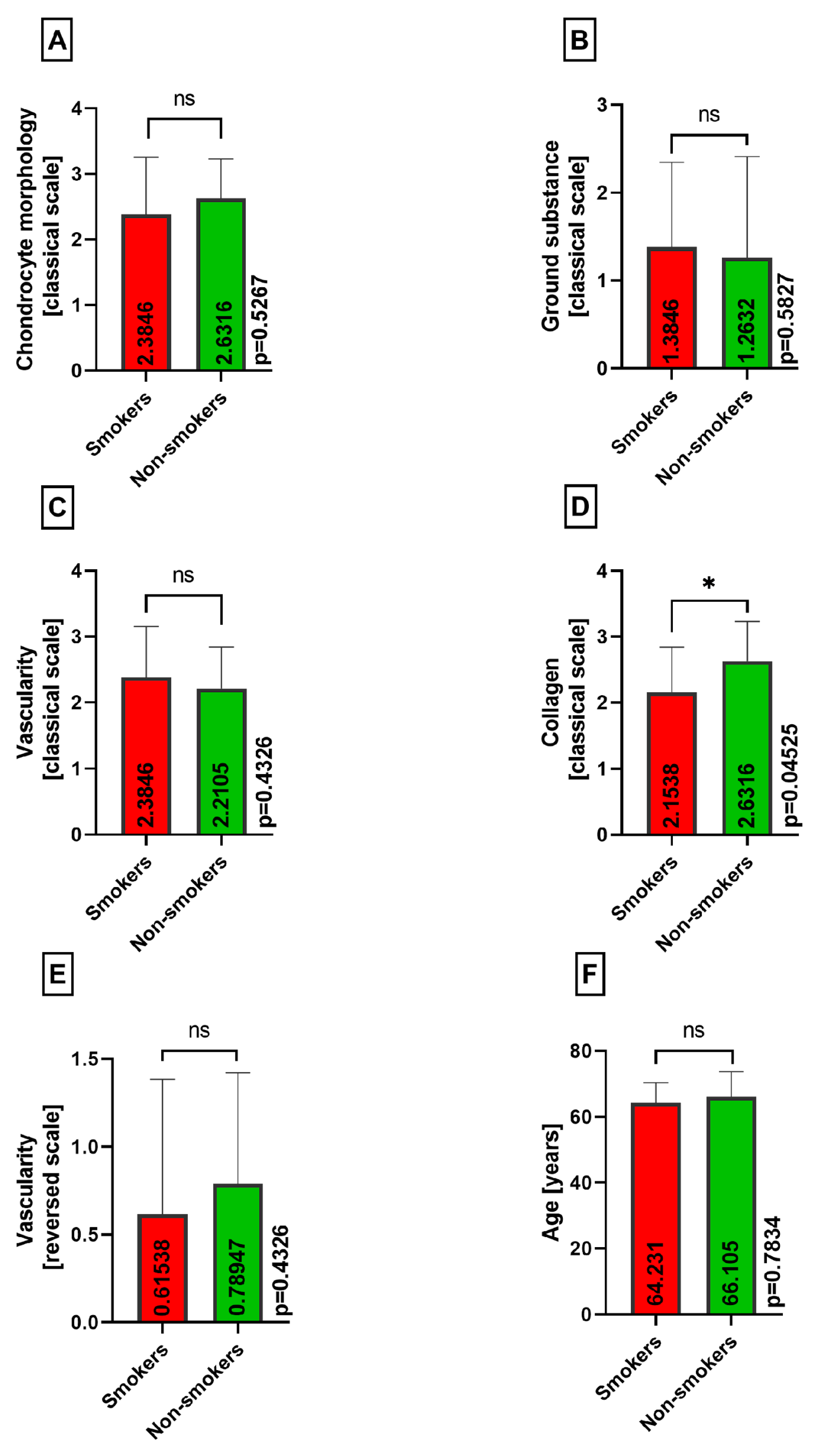
2.2. Histopathological Assessment
2.3. Statistical Analysis
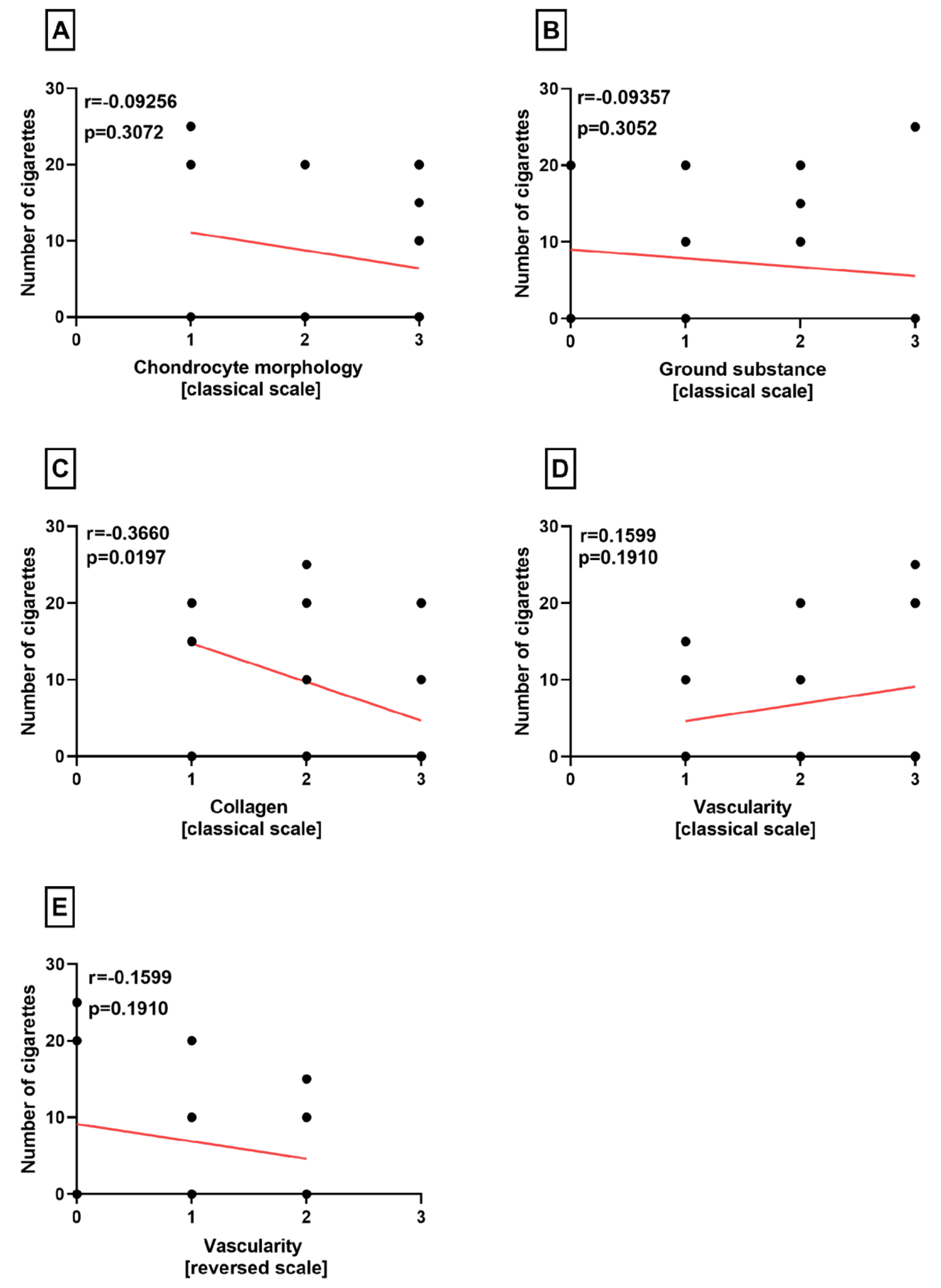
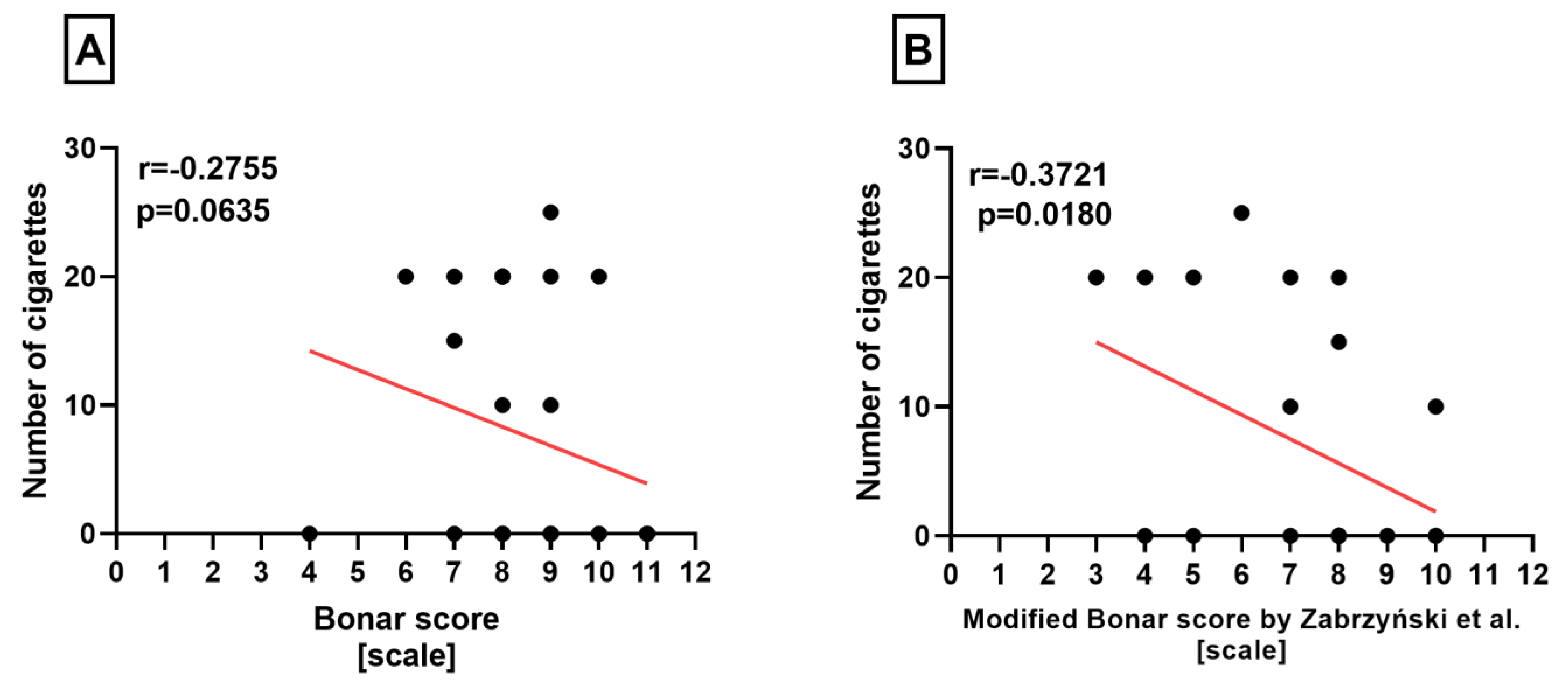
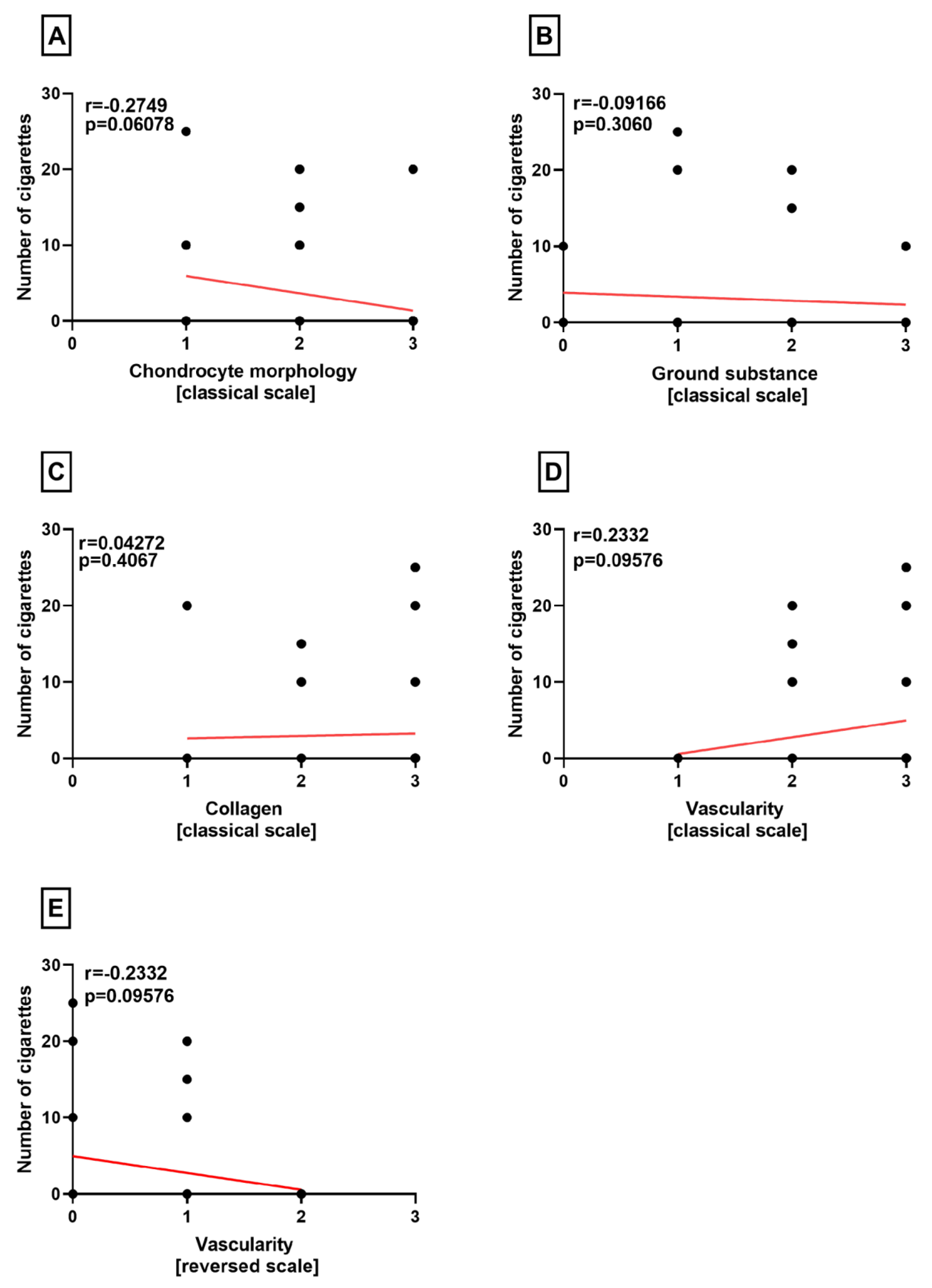
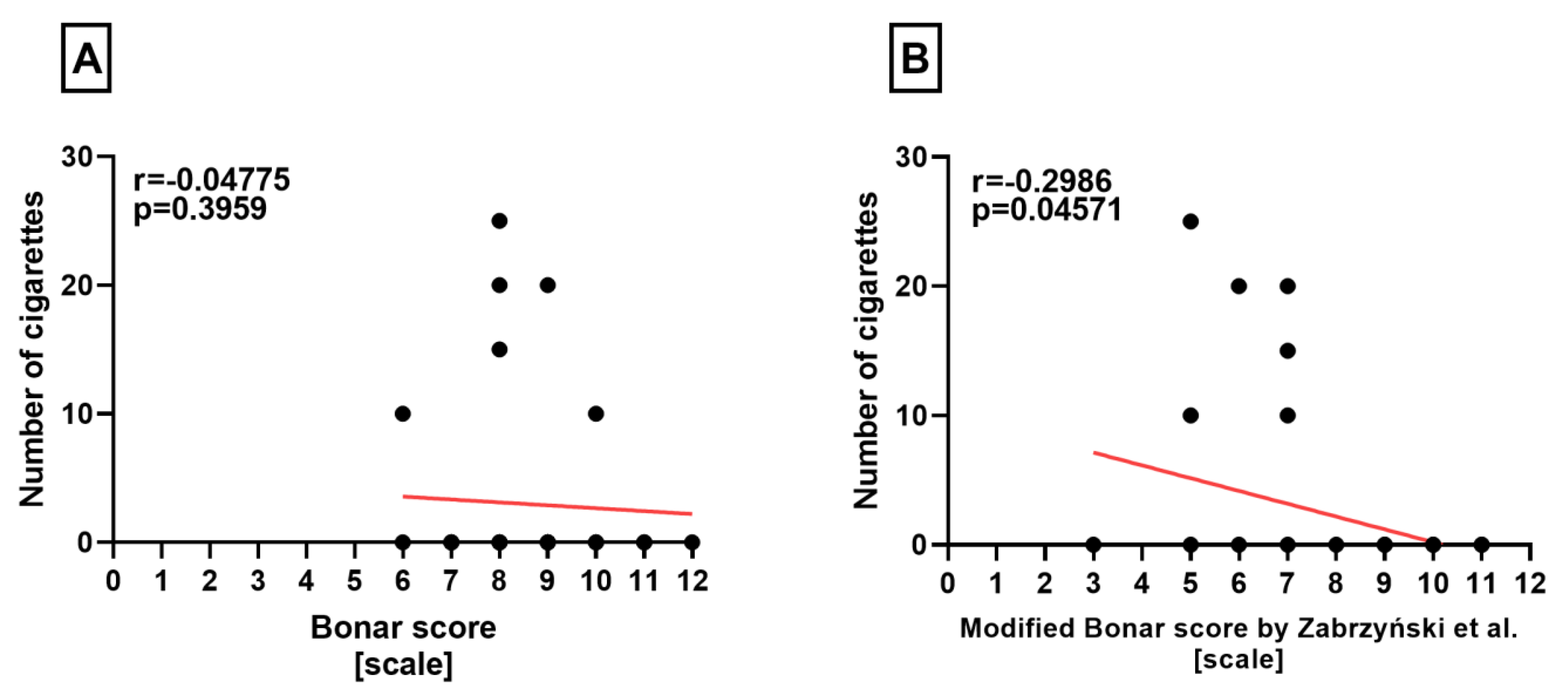
Correlation Analyses
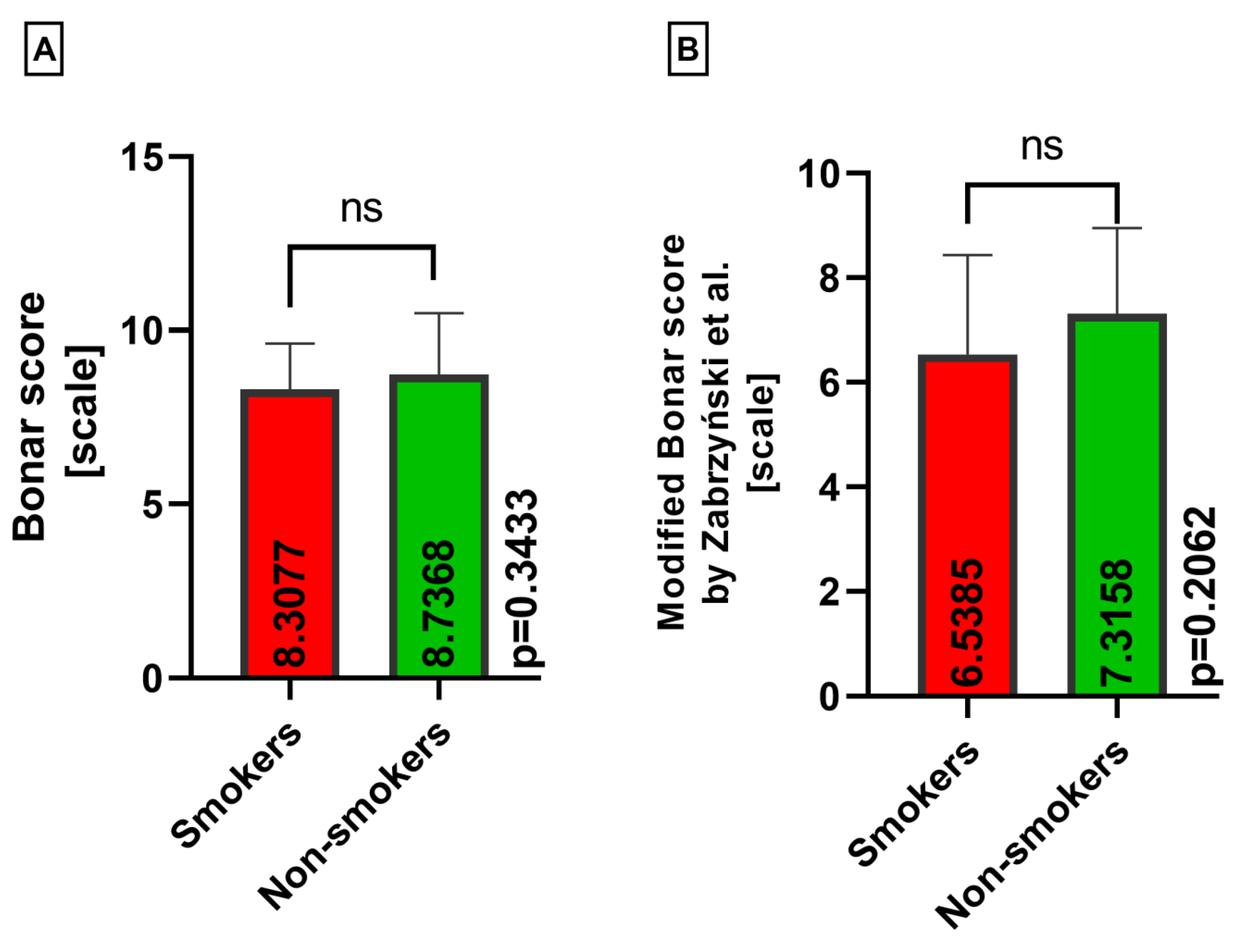
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasiński, M.; Zabrzyńska, M.; Adamczyk, M.; Sokołowski, M.; Głos, T.; Ziejka, M.; Augustynowicz, P.; Boguszewski, K.; Piotrowski, W.; Michał, B.; et al. A Current Insight into Human Knee Menisci. Transl. Res. Anat. 2023, 32, 100259. [Google Scholar] [CrossRef]
- Mordecai, S.C. Treatment of Meniscal Tears: An Evidence Based Approach. World J. Orthop. 2014, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Tarantino, D.; Aicale, R. Meniscal Tears. In Evidence-Based Orthopedics; Bhandari, M., Ed.; Wiley: New York, NY, USA, 2021; pp. 787–791. ISBN 978-1-119-41400-1. [Google Scholar]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Human Knee Menisci: Structure, Composition, and Function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Bryceland, J.K.; Powell, A.J.; Nunn, T. Knee Menisci: Structure, Function, and Management of Pathology. Cartilage 2017, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, I.D.; Moran, C.J.; Potter, H.G.; Warren, R.F.; Rodeo, S.A. Restoration of the Meniscus: Form and Function. Am. J. Sports Med. 2014, 42, 987–998. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The Knee Meniscus: Structure–Function, Pathophysiology, Current Repair Techniques, and Prospects for Regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef]
- Zabrzyńska, M.; Grzanka, D.; Zielińska, W.; Jaworski, Ł.; Pękala, P.; Gagat, M. The Bonar Score in the Histopathological Assessment of Tendinopathy and Its Clinical Relevance—A Systematic Review. Medicina 2021, 57, 367. [Google Scholar] [CrossRef]
- Fearon, A.; Dahlstrom, J.E.; Twin, J.; Cook, J.; Scott, A. The Bonar Score Revisited: Region of Evaluation Significantly Influences the Standardized Assessment of Tendon Degeneration. J. Sci. Med. Sport 2014, 17, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Lundgreen, K.; Lian, Ø.; Scott, A.; Engebretsen, L. Increased Levels of Apoptosis and P53 in Partial-Thickness Supraspinatus Tendon Tears. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.M.; Twin, J.; Dahlstrom, J.E.; Cook, J.L.; Cormick, W.; Smith, P.N.; Scott, A. Increased Substance P Expression in the Trochanteric Bursa of Patients with Greater Trochanteric Pain Syndrome. Rheumatol. Int. 2014, 34, 1441–1448. [Google Scholar] [CrossRef]
- Docking, S.I.; Cook, J.; Chen, S.; Scarvell, J.; Cormick, W.; Smith, P.; Fearon, A. Identification and Differentiation of Gluteus Medius Tendon Pathology Using Ultrasound and Magnetic Resonance Imaging. Musculoskelet. Sci. Pract. 2019, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Furumatsu, T.; Maehara, A.; Miyazawa, S.; Kamatsuki, Y.; Hino, T.; Ozaki, T. Histological Alterations to the Hamstring Tendon Caused by Cleaning during Autograft Preparation. Muscle Ligaments Tendons J. 2019, 09, 217. [Google Scholar] [CrossRef]
- Zabrzyński, J.; Paczesny, Ł.; Łapaj, Ł.; Grzanka, D.; Szukalski, J. Process of Neovascularisation Compared with Pain Intensity in Tendinopathy of the Long Head of the Biceps Brachii Tendon Associated with Concomitant Shoulder Disorders, after Arthroscopic Treatment. Microscopic Evaluation Supported by Immunohistochemical. Folia Morphol. 2018, 77, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Min, B.-H.; Choi, B.H.; Kim, Y.J.; Kim, M.; Suh-Kim, H.; Kim, J.H. The Degeneration of Meniscus Roots Is Accompanied by Fibrocartilage Formation, Which May Precede Meniscus Root Tears in Osteoarthritic Knees. Am. J. Sports Med. 2015, 43, 3034–3044. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Jarraya, M.; Roemer, F.W.; Englund, M.; Crema, M.D.; Gale, H.I.; Hayashi, D.; Katz, J.N.; Guermazi, A. Meniscus Morphology: Does Tear Type Matter? A Narrative Review with Focus on Relevance for Osteoarthritis Research. Semin. Arthritis Rheum. 2017, 46, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Zhang, Y.; Niu, J.; Lynch, J.A.; Crema, M.D.; Marra, M.D.; Nevitt, M.C.; Felson, D.T.; Hughes, L.B.; El-Khoury, G.Y.; et al. Tibiofemoral Joint Osteoarthritis: Risk Factors for MR-Depicted Fast Cartilage Loss over a 30-Month Period in the Multicenter Osteoarthritis Study. Radiology 2009, 252, 772–780. [Google Scholar] [CrossRef]
- Lamplot, J.D.; Tompkins, W.P.; Friedman, M.V.; Nguyen, J.T.; Rai, M.F.; Brophy, R.H. Radiographic and Clinical Evidence for Osteoarthritis at Medium-Term Follow-up after Arthroscopic Partial Medial Meniscectomy. Cartilage 2021, 13, 588S–594S. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Lohmander, L.S. The Meniscus in Knee Osteoarthritis. Rheum. Dis. Clin. N. Am. 2009, 35, 579–590. [Google Scholar] [CrossRef]
- Olivotto, E.; Trisolino, G.; Belluzzi, E.; Lazzaro, A.; Strazzari, A.; Pozzuoli, A.; Cigolotti, A.; Ruggieri, P.; Evangelista, A.; Ometto, F.; et al. Macroscopic Synovial Inflammation Correlates with Symptoms and Cartilage Lesions in Patients Undergoing Arthroscopic Partial Meniscectomy: A Clinical Study. J. Clin. Med. 2022, 11, 4330. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Paczesny, Ł.; Zabrzyńska, A.; Huri, G.; Graboń, K.; Pielak, T.; Kruczyński, J.; Łapaj, Ł. Smoking Has No Influence on Outcomes after Repair of the Medial Meniscus in the Hypo and Avascular Zones—A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 16127. [Google Scholar] [CrossRef] [PubMed]
- Doral, M.N.; Bilge, O.; Huri, G.; Turhan, E.; Verdonk, R. Modern Treatment of Meniscal Tears. EFORT Open Rev. 2018, 3, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, R.; Schmitt, L.; Flanigan, D.C.; Magnussen, R.A. Smoking Increases the Risk of Early Meniscus Repair Failure. Orthop. J. Sports Med. 2014, 2, 2325967114S0011. [Google Scholar] [CrossRef]
- AL-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Kelly, D.L.; Chengguo, X.; Yoon, S. The Effect of Tobacco Smoking on Musculoskeletal Health: A Systematic Review. J. Environ. Public Health 2018, 2018, 4184190. [Google Scholar] [CrossRef] [PubMed]
- Davies-Tuck, M.L.; Wluka, A.E.; Forbes, A.; Wang, Y.; English, D.R.; Giles, G.G.; Cicuttini, F. Smoking Is Associated with Increased Cartilage Loss and Persistence of Bone Marrow Lesions over 2 Years in Community-Based Individuals. Rheumatology 2009, 48, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Uzun, E.; Misir, A.; Kizkapan, T.B.; Ozcamdalli, M.; Akkurt, S.; Guney, A. Factors Affecting the Outcomes of Arthroscopically Repaired Traumatic Vertical Longitudinal Medial Meniscal Tears. Orthop. J. Sports Med. 2017, 5, 232596711771244. [Google Scholar] [CrossRef]
- Uzun, E.; Misir, A.; Kizkapan, T.B.; Ozcamdalli, M.; Akkurt, S.; Guney, A. Evaluation of Midterm Clinical and Radiographic Outcomes of Arthroscopically Repaired Vertical Longitudinal and Bucket-Handle Lateral Meniscal Tears. Orthop. J. Sports Med. 2019, 7, 232596711984320. [Google Scholar] [CrossRef]
- Snoeker, B.A.M.; Bakker, E.W.P.; Kegel, C.A.T.; Lucas, C. Risk Factors for Meniscal Tears: A Systematic Review Including Meta-Analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 352–367. [Google Scholar] [CrossRef]
- Laurendon, L.; Neri, T.; Farizon, F.; Philippot, R. Prognostic Factors for All-inside Meniscal Repair. A 87-Case Series. Orthop. Traumatol. Surg. Res. 2017, 103, 1017–1020. [Google Scholar] [CrossRef]
- Moses, M.J.; Wang, D.E.; Weinberg, M.; Strauss, E.J. Clinical Outcomes Following Surgically Repaired Bucket-Handle Meniscus Tears. Physician Sportsmed. 2017, 45, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, M.B.; Pihl, K.; Nissen, N.; Sørensen, R.R.; Jørgensen, U.; Englund, M.; Thorlund, J.B. The Association between Smoking and Knee Osteoarthritis in a Cohort of Danish Patients Undergoing Knee Arthroscopy. BMC Musculoskelet. Disord. 2019, 20, 141. [Google Scholar] [CrossRef]
- Kanneganti, P.; Harris, J.D.; Brophy, R.H.; Carey, J.L.; Lattermann, C.; Flanigan, D.C. The Effect of Smoking on Ligament and Cartilage Surgery in the Knee: A Systematic Review. Am. J. Sports Med. 2012, 40, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Haugen, I.K.; Magnusson, K.; Turkiewicz, A.; Englund, M. The Prevalence, Incidence, and Progression of Hand Osteoarthritis in Relation to Body Mass Index, Smoking, and Alcohol Consumption. J. Rheumatol. 2017, 44, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Zabrzyński, J.; Szukalski, J.; Paczesny, Ł.; Szwedowski, D.; Grzanka, D. Cigarette Smoking Intensifies Tendinopathy of the LHBT. A Microscopic Study after Arthroscopic Treatment. Pol. J. Pathol. 2019, 70, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Cheng, S.; Shen, Y.; Cheng, X.; An Rompis, F.; Wang, W.; Lin, Z.; Chen, Q.; Zhang, W.; Kou, D.; et al. Nicotine Promotes Proliferation and Collagen Synthesis of Chondrocytes Isolated from Normal Human and Osteoarthritis Patients. Mol. Cell. Biochem. 2012, 359, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Bitterman, H. Nicotine and Angiogenesis: A New Paradigm for Tobacco-related Diseases. Ann. Med. 2004, 36, 33–40. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Salehi, N.; Janjani, P.; Tadbiri, H.; Rozbahani, M.; Jalilian, M. Effect of Cigarette Smoking on Coronary Arteries and Pattern and Severity of Coronary Artery Disease: A Review. J. Int. Med. Res. 2021, 49, 030006052110598. [Google Scholar] [CrossRef]
- Darcey, E.; Boyle, T. Tobacco Smoking and Survival after a Prostate Cancer Diagnosis: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2018, 70, 30–40. [Google Scholar] [CrossRef]
- Neczypor, E.W.; Mears, M.J.; Ghosh, A.; Sassano, M.F.; Gumina, R.J.; Wold, L.E.; Tarran, R. E-Cigarettes and Cardiopulmonary Health: Review for Clinicians. Circulation 2022, 145, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Banks, E.; Yazidjoglou, A.; Brown, S.; Nguyen, M.; Martin, M.; Beckwith, K.; Daluwatta, A.; Campbell, S.; Joshy, G. Electronic Cigarettes and Health Outcomes: Umbrella and Systematic Review of the Global Evidence. Med. J. Aust. 2023, 218, 267–275. [Google Scholar] [CrossRef]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.-N.H.; Jaspers, I.; Chen, L.C. E-Cigarette Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of Tendinopathies: Inflammation or Degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef]
- Abate, M.; Vanni, D.; Pantalone, A.; Salini, V. Cigarette Smoking and Musculoskeletal Disorders. Muscles Ligaments Tendons J. 2013, 3, 63–69. [Google Scholar] [CrossRef]
- Rose, B.J.; Weyand, J.A.; Liu, B.; Smith, J.F.; Perez, B.R.; Clark, J.C.; Goodman, M.; Budge, K.M.H.; Eggett, D.L.; Arroyo, J.A.; et al. Exposure to Second-Hand Cigarette Smoke Exacerbates the Progression of Osteoarthritis in a Surgical Induced Murine Model. Histol. Histopathol. 2021, 36, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Domzalski, M.; Muszynski, K.; Mostowy, M.; Wojtowicz, J.; Garlinska, A. Smoking Is Associated with Prolonged Time of the Return to Daily and Sport Activities and Decreased Knee Function after Meniscus Repair with Outside-in Technique: Retrospective Cohort Study. J. Orthop. Surg. 2021, 29, 230949902110122. [Google Scholar] [CrossRef]
- Haklar, U.; Donmez, F.; Basaran, S.H.; Canbora, M.K. Results of Arthroscopic Repair of Partial- or Full-Thickness Longitudinal Medial Meniscal Tears by Single or Double Vertical Sutures Using the Inside-Out Technique. Am. J. Sports Med. 2013, 41, 596–602. [Google Scholar] [CrossRef]
- Franovic, S.; Kuhlmann, N.A.; Pietroski, A.; Schlosser, C.T.; Page, B.; Okoroha, K.R.; Moutzouros, V.; Makhni, E.C. Preoperative Patient-Centric Predictors of Postoperative Outcomes in Patients Undergoing Arthroscopic Meniscectomy. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 37, 964–971. [Google Scholar] [CrossRef]
- Astur, D.C.; Sbampato, I.N.; Arliani, G.G.; Franciozi, C.E.d.S.; Debieux, P.; Cohen, M. Association of Tobacco Dependence, Alcoholism and Anabolic Steroids with Meniscoligamentous Injuries. Acta Ortop. Bras. 2018, 26, 236–239. [Google Scholar] [CrossRef]
- Gittings, D.; Riggin, C.; Boorman-Padgett, J.; Weiss, S.; Fryhofer, G.; Farber, D.; Steinberg, D.; Soslowsky, L.J. Chronic Nicotine Exposure Alters Uninjured Tendon Vascularity and Viscoelasticity. Foot Ankle Orthop. 2019, 4, 247301141984252. [Google Scholar] [CrossRef]
- Ağladıoğlu, K.; Akkaya, N.; Güngör, H.R.; Akkaya, S.; Ök, N.; Özçakar, L. Effects of Cigarette Smoking on Elastographic Strain Ratio Measurements of Patellar and Achilles Tendons. J. Ultrasound Med. 2016, 35, 2431–2438. [Google Scholar] [CrossRef]
- Baumgarten, K.M.; Gerlach, D.; Galatz, L.M.; Teefey, S.A.; Middleton, W.D.; Ditsios, K.; Yamaguchi, K. Cigarette Smoking Increases the Risk for Rotator Cuff Tears. Clin. Orthop. Relat. Res. 2010, 468, 1534–1541. [Google Scholar] [CrossRef]
- Novikov, D.A.; Swensen, S.J.; Buza, J.A.; Gidumal, R.H.; Strauss, E.J. The Effect of Smoking on ACL Reconstruction: A Systematic Review. Physician Sportsmed. 2016, 44, 335–341. [Google Scholar] [CrossRef]
- Jensen, A.R.; Taylor, A.J.; Sanchez-Sotelo, J. Factors Influencing the Reparability and Healing Rates of Rotator Cuff Tears. Curr. Rev. Musculoskelet. Med. 2020, 13, 572–583. [Google Scholar] [CrossRef]
- Caughey, W.J.; Maher, A.; Leigh, W.B.; Brick, M.J.; Young, S.W.; Walker, C.G.; Caughey, M.A. Impact of Smoking on Pain and Function in Rotator Cuff Repair: A Prospective 5-year Cohort Follow-up of 1383 Patients. ANZ J. Surg. 2021, 91, 2153–2158. [Google Scholar] [CrossRef]
- Livesey, M.G.; Bains, S.S.; Weir, T.B.; Kolakowski, L.; Rocca, M.S.; Remily, E.A.; Gilotra, M.N.; Hasan, S.A. Does Timing Matter? The Effect of Preoperative Smoking Cessation on the Risk of Infection or Revision Following Rotator Cuff Repair. J. Shoulder Elb. Surg. 2023, 32, 1937–1944. [Google Scholar] [CrossRef]
- Carbone, S.; Gumina, S.; Arceri, V.; Campagna, V.; Fagnani, C.; Postacchini, F. The Impact of Preoperative Smoking Habit on Rotator Cuff Tear: Cigarette Smoking Influences Rotator Cuff Tear Sizes. J. Shoulder Elb. Surg. 2012, 21, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. New Insights into Tobacco-Induced Vascular Disease: Clinical Ramifications. Methodist. DeBakey Cardiovasc. J. 2015, 11, 156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Cooke, J.P. Nicotine and Pathological Angiogenesis. Life Sci. 2012, 91, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Khodabandeh, Z.; Valilo, M.; Velaei, K.; Pirpour Tazehkand, A. The Potential Role of Nicotine in Breast Cancer Initiation, Development, Angiogenesis, Invasion, Metastasis, and Resistance to Therapy. Breast Cancer 2022, 29, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Zabrzynski, J.; Gagat, M.; Paczesny, L.; Grzanka, D.; Huri, G. Correlation between Smoking and Neovascularization in Biceps Tendinopathy—A Functional Preoperative and Immunohistochemical Study. Ther. Adv. Chronic Dis. 2020, 11, 2040622320956418. [Google Scholar] [CrossRef] [PubMed]

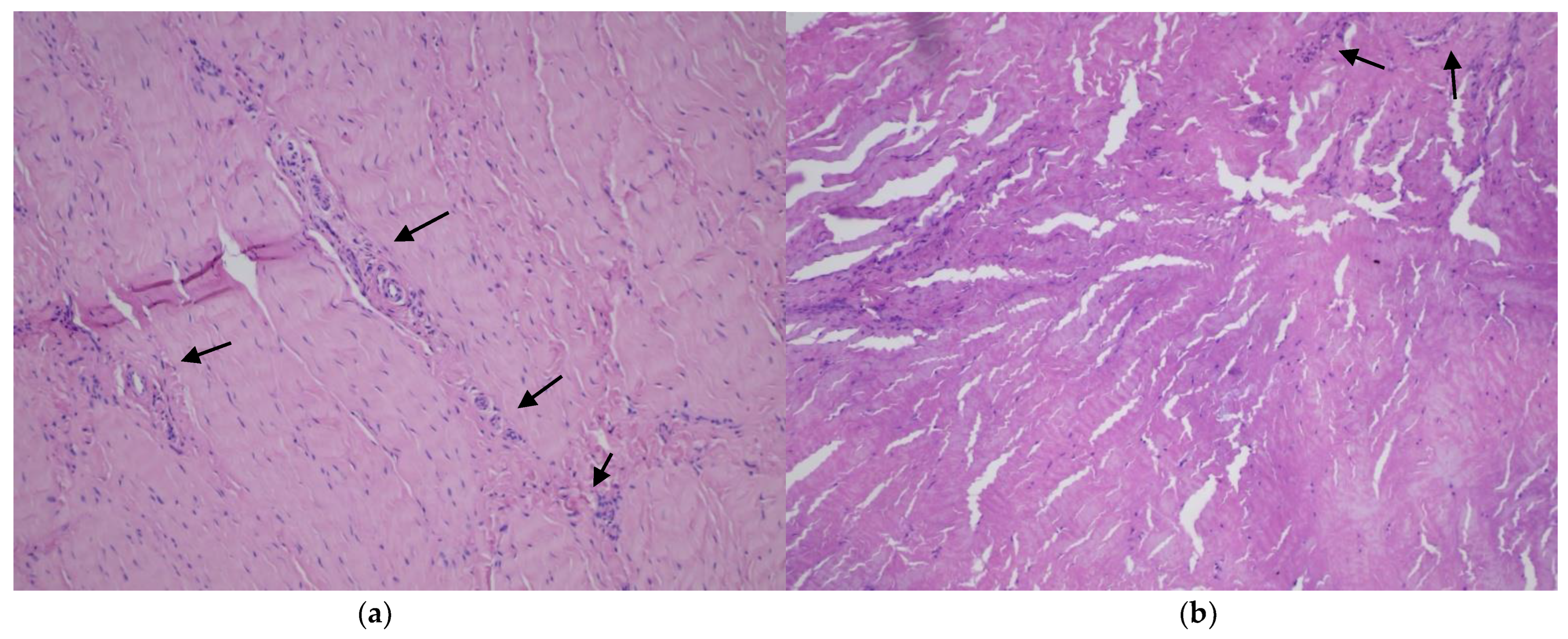


| Characteristics | Total | Smokers | Nonsmokers |
|---|---|---|---|
| No. of patients | 34 | 13 | 21 |
| Female | 21 | 7 | 14 |
| Male | 13 | 6 | 7 |
| Age | 65.385 (54–81) | 64.231 (54–72) | 66.154 (55–81) |
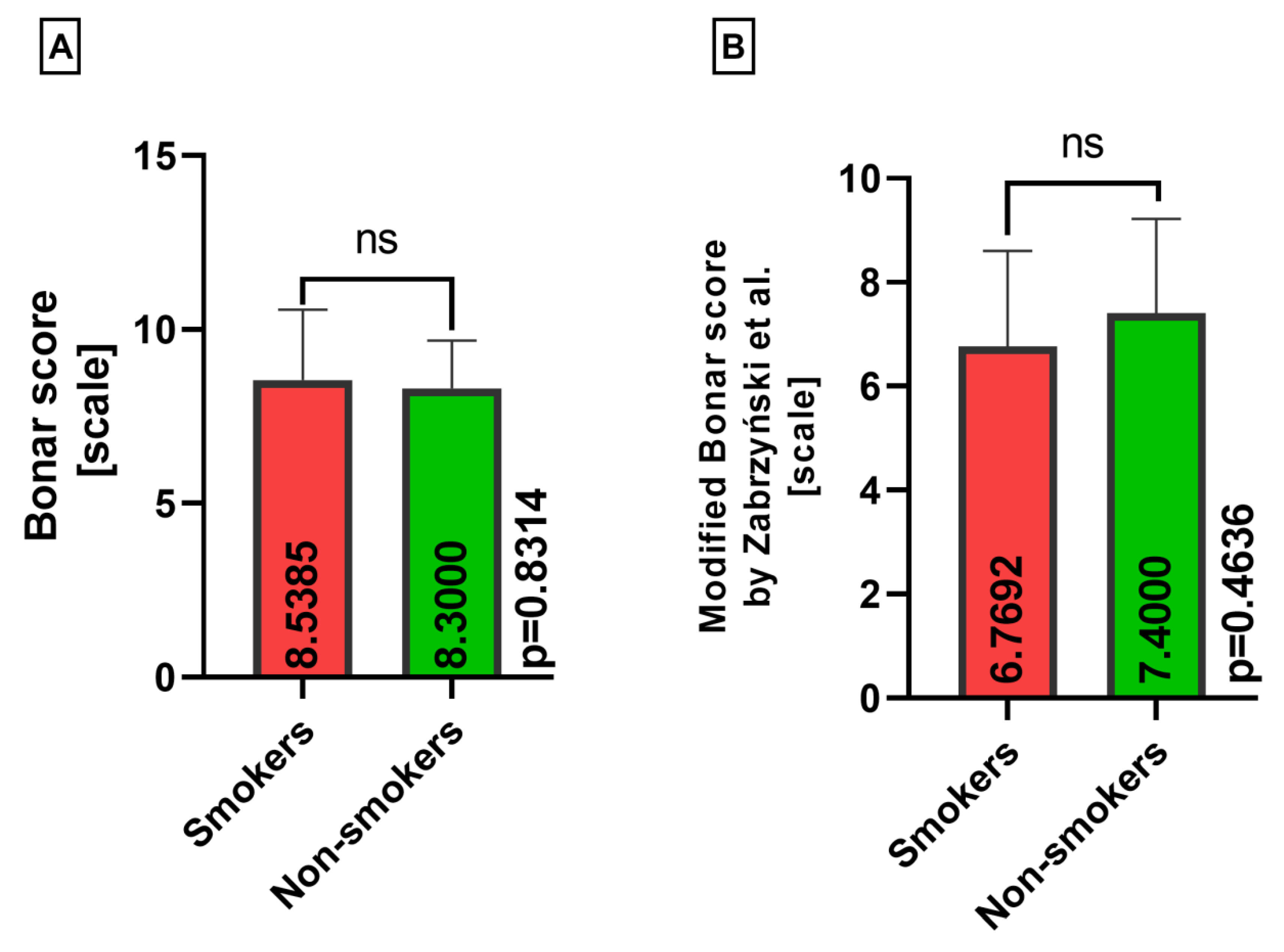
| Classical Bonar score | 8.4462 (4–12) | 8.4231 (6–12) | 8.5128 (4–11) |
| Modified Bonar score | 7.0462 (3–11) | 6.6538 (3–10) | 7.3590 (4–11) |
| Characteristic | Total | Meniscus | |
|---|---|---|---|
| Medial | Lateral | ||
| No. of samples | 65 | 32 | 33 |
| No. of cigarettes | 5.2308 (0–25) | 7.5000 (0–25) | 3.0303 (0–25) |
| Classical Bonar score | 8.4462 (4–12) | 8.5000(4–11) | 8.3939(6–12) |
| Modified Bonar score | 7.0462 (3–11) | 6.9375 (3–10) | 7.1515 (3–11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabrzyńska, M.; Pasiński, M.; Gagat, M.; Kułakowski, M.; Woźniak, Ł.; Elster, K.; Antosik, P.; Zabrzyński, J. The Association between the Extent of the Osteoarthritic Meniscus Degeneration and Cigarette Smoking—A Pilot Study. Medicina 2024, 60, 323. https://doi.org/10.3390/medicina60020323
Zabrzyńska M, Pasiński M, Gagat M, Kułakowski M, Woźniak Ł, Elster K, Antosik P, Zabrzyński J. The Association between the Extent of the Osteoarthritic Meniscus Degeneration and Cigarette Smoking—A Pilot Study. Medicina. 2024; 60(2):323. https://doi.org/10.3390/medicina60020323
Chicago/Turabian StyleZabrzyńska, Maria, Maciej Pasiński, Maciej Gagat, Michał Kułakowski, Łukasz Woźniak, Karol Elster, Paulina Antosik, and Jan Zabrzyński. 2024. "The Association between the Extent of the Osteoarthritic Meniscus Degeneration and Cigarette Smoking—A Pilot Study" Medicina 60, no. 2: 323. https://doi.org/10.3390/medicina60020323
APA StyleZabrzyńska, M., Pasiński, M., Gagat, M., Kułakowski, M., Woźniak, Ł., Elster, K., Antosik, P., & Zabrzyński, J. (2024). The Association between the Extent of the Osteoarthritic Meniscus Degeneration and Cigarette Smoking—A Pilot Study. Medicina, 60(2), 323. https://doi.org/10.3390/medicina60020323






