Mild Behavioral Impairment in Parkinson’s Disease: An Updated Review on the Clinical, Genetic, Neuroanatomical, and Pathophysiological Aspects
Abstract
1. Introduction
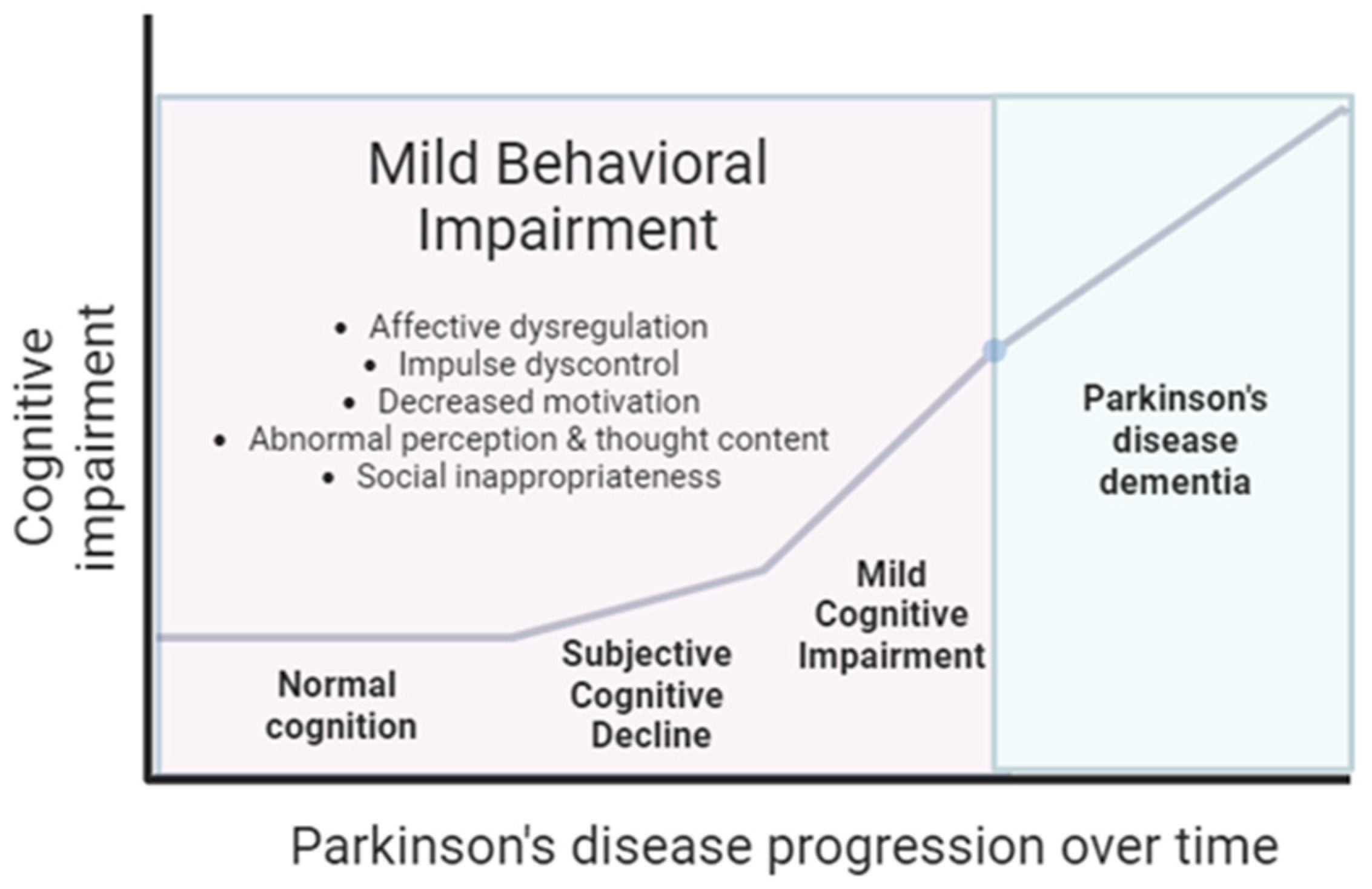
2. MBI Definition and Assessment
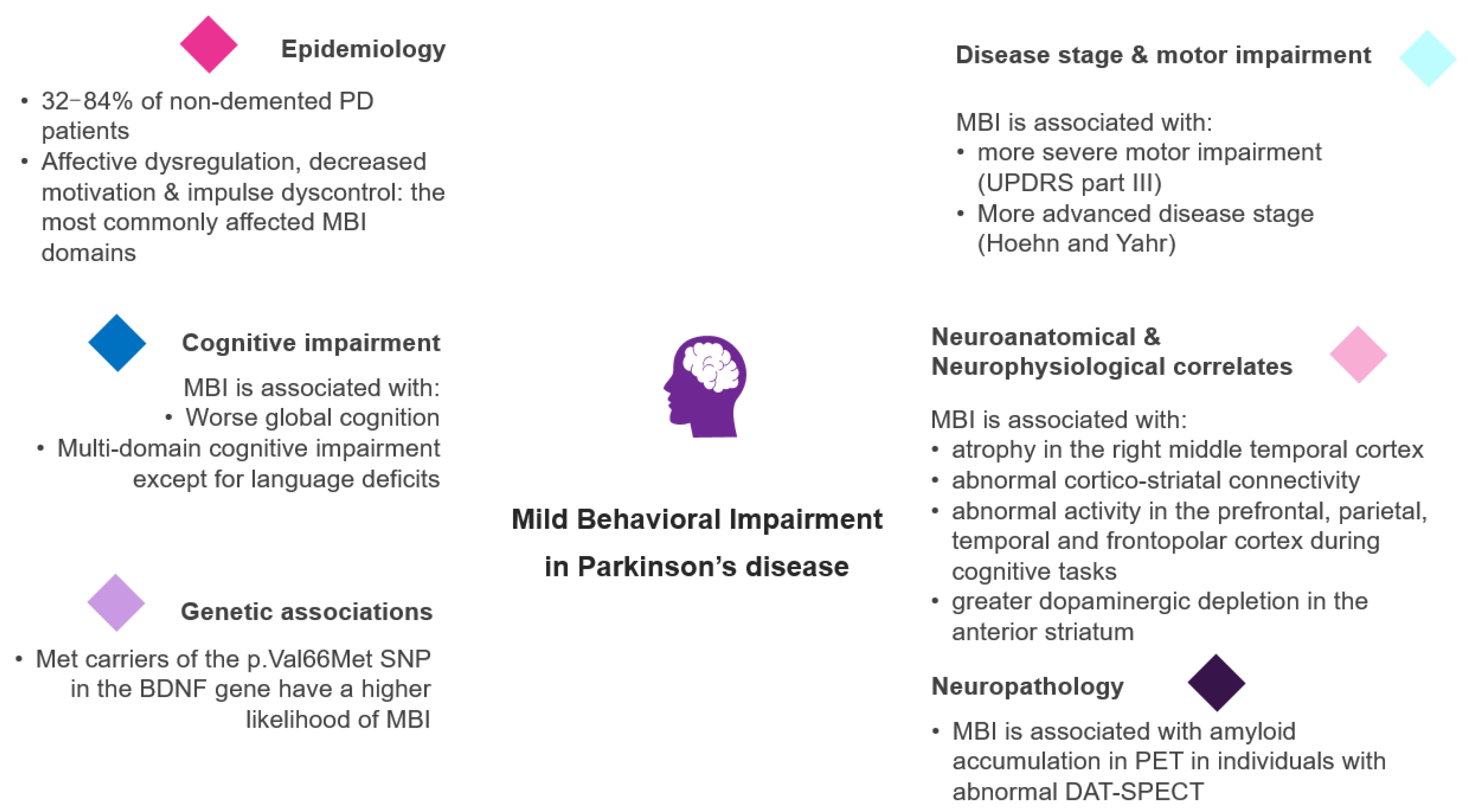
3. The Epidemiology of MBI in PD
4. The Relationship of MBI with Disease Stage and Motor Impairment in PD
5. The Link between MBI and Cognitive Impairment in PD
6. The Genetic Background of MBI in PD
7. Neuroanatomical Correlates of MBI in PD
8. The Relationship between MBI and Functional Connectivity in PD
9. MBI and Associated Neuronal Activity Patterns in PD
10. MBI and Nigrostriatal Dopaminergic Degeneration in PD
11. Neuropathological Correlates of MBI in PD
| Study | Study Type | Country or Region | Study Participants | PD & MCI Diagnosis | MBI Characterization | Main Results |
|---|---|---|---|---|---|---|
| [48] | Cross-sectional, hospital-based, data derived from PArkinson’s Disease COgnitive Impairment Study (PACOS) | Italy | Patients with PD without dementia, with or without MCI (n = 429) | PD: United Kingdom PD Society Brain Bank criteria MCI: MDS Task Force, Level II criteria | NPS assessed by NPI (algorithm proposed by Sheikh and colleagues, modified reference range for 6 months) Functionality assessed by BADL (no or minimal impairment was included) |
|
| [50] | Cross-sectional | Canada | Patients with PD without dementia, with or without MCI (n = 60), and age- and sex-matched healthy controls without MCI (n = 29) | PD: United Kingdom PD Society Brain Bank criteria MCI: MDS Task Force, Level II criteria | MBI-C (cut-off point: 7.5) |
|
| [49] | Cross-sectional | South Korea | Drug-naïve newly diagnosed PD patients without dementia (n = 275) | PD: United Kingdom PD Society Brain Bank criteria | NPSs assessed by the NPI with the modified reference range of 6 months MBI diagnosis was determined when there were deficits in at least two NPS items (either 2 impaired items in 1 domain, or 1 impaired item in 2 domains) |
|
| [55] | Cross-sectional | Canada | PD patients (n = 146) | PD: United Kingdom PD Society Brain Bank criteria | MBI-C (cut-off point: 8) |
|
| [56] | Cross-sectional | Canada | PD patients without dementia (n = 74) | PD: United Kingdom PD Society Brain Bank criteria MCI: MDS Task Force, Level II criteria | MBI-C (cut-off point: 7.5) |
|
| [54] | Cross-sectional | Canada | PD patients without dementia (n = 74), healthy controls without MCI (n = 28) | PD: United Kingdom PD Society Brain Bank criteria MCI: MDS Task Force, Level II criteria | MBI-C (cut-off point: 7.5) |
|
| [70] | Cross-sectional | Canada | PD patients without dementia (n = 59), healthy controls without MCI (n = 26) | PD: United Kingdom PD Society Brain Bank criteria MCI: MDS Task Force, Level II criteria | MBI-C (cut-off point: 7.5) |
|
| [121] | Cross-sectional | Japan | Individuals>=50 years of age without dementia (n = 103), divided into 5 groups (Group 1: amyloid-positive and abnormal DAT-SPECT, Group 2: amyloid-negative and abnormal DAT-SPECT, Group 3: amyloid-positive and normal DAT-SPECT, Group 4: mild cognitive impairment unlikely due to AD with normal DAT-SPECT, Group 5:cognitively normal with amyloid-negative and normal DAT-SPECT) | Prodromal PDD/ DLB defined by abnormal DAT-SPECT Preclinical/prodromal AD defined by positive amyloid PET | NPS assessed by NPI |
|
12. Future Perspectives
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Rijk, M.C.; Breteler, M.M.; Graveland, G.A.; Ott, A.; Grobbee, D.E.; van der Meche, F.G.; Hofman, A. Prevalence of Parkinson’s disease in the elderly: The Rotterdam Study. Neurology 1995, 45, 2143–2146. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.; Meara, J. Mild cognitive impairment in Parkinson’s disease and its progression onto dementia: A 16-year outcome evaluation of the Denbighshire cohort. Int. J. Geriatr. Psychiatry 2015, 30, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.K.; Jones, W.E.; Baker, K.A.; Boersma, I.M.; Kluger, B.M. Outcome measures for Parkinson’s disease dementia: A systematic review. Mov. Disord. Clin. Pract. 2016, 3, 9–18. [Google Scholar] [CrossRef]
- Degirmenci, Y.; Angelopoulou, E.; Georgakopoulou, V.E.; Bougea, A. Cognitive Impairment in Parkinson’s Disease: An Updated Overview Focusing on Emerging Pharmaceutical Treatment Approaches. Medicina 2023, 59, 1756. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Rocca, W.A.; Bower, J.H. Parkinson disease with and without Dementia: A prevalence study and future projections. Mov. Disord. 2018, 33, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Holden, S.K.; Litvan, I.; McKeith, I.; Stebbins, G.T.; Taylor, J.P. Evolution of diagnostic criteria and assessments for Parkinson’s disease mild cognitive impairment. Mov. Disord. 2018, 33, 503–510. [Google Scholar] [CrossRef]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J.M. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: A cohort study. Lancet Neurol. 2017, 16, 66–75. [Google Scholar] [CrossRef]
- Weintraub, D.; Dietz, N.; Duda, J.E.; Wolk, D.A.; Doshi, J.; Xie, S.X.; Davatzikos, C.; Clark, C.M.; Siderowf, A. Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain A J. Neurol. 2012, 135, 170–180. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Shang, H. Meta-analysis of risk factors for Parkinson’s disease dementia. Transl. Neurodegener. 2016, 5, 11. [Google Scholar] [CrossRef]
- Anang, J.B.; Gagnon, J.F.; Bertrand, J.A.; Romenets, S.R.; Latreille, V.; Panisset, M.; Montplaisir, J.; Postuma, R.B. Predictors of dementia in Parkinson disease: A prospective cohort study. Neurology 2014, 83, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Marsh, L.; Schrag, A. Neuropsychiatric symptoms in Parkinson’s disease. Mov. Disord. 2009, 24, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Bronnick, K.; Ehrt, U.; De Deyn, P.P.; Tekin, S.; Emre, M.; Cummings, J.L. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: Frequency, profile and associated care giver stress. J. Neurol. Neurosurg. Psychiatry 2007, 78, 36–42. [Google Scholar] [CrossRef]
- Goel, A.; Narayan, S.K.; Sugumaran, R. Neuropsychiatric Features, Health-Related Quality of Life, and Caregiver Burden in Parkinson’s Disease. Ann. Indian Acad. Neurol. 2022, 25, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, K.; Sockeel, P.; Delliaux, M.; Destee, A.; Defebvre, L. Apathy may herald cognitive decline and dementia in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Treves, T.A.; Paleacu, D.; Shabtai, H.; Orlov, Y.; Kandinov, B.; Simon, E.S.; Korczyn, A.D. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J. Neural Transm. 2000, 107, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Jin, Z.; Wang, Y.; Fang, B. Longitudinal cognitive changes in patients with early Parkinson’s disease and neuropsychiatric symptoms. CNS Neurosci. Ther. 2023, 29, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Vriend, C.; Raijmakers, P.; Veltman, D.J.; van Dijk, K.D.; van der Werf, Y.D.; Foncke, E.M.; Smit, J.H.; Berendse, H.W.; van den Heuvel, O.A. Depressive symptoms in Parkinson’s disease are related to reduced [123I]FP-CIT binding in the caudate nucleus. J. Neurol. Neurosurg. Psychiatry 2014, 85, 159–164. [Google Scholar] [CrossRef]
- Picillo, M.; Santangelo, G.; Erro, R.; Cozzolino, A.; Amboni, M.; Vitale, C.; Barone, P.; Pellecchia, M.T. Association between dopaminergic dysfunction and anxiety in de novo Parkinson’s disease. Park. Relat. Disord. 2017, 37, 106–110. [Google Scholar] [CrossRef]
- Backman, E.A.; Luntamo, L.; Parkkola, R.; Koikkalainen, J.; Gardberg, M.; Kaasinen, V. Early cortical atrophy is related to depression in patients with neuropathologically confirmed Parkinson’s disease. J. Neurol. Sci. 2023, 455, 122804. [Google Scholar] [CrossRef]
- Yin, W.; Li, A.; Yang, B.; Gao, C.; Hu, Y.; Luo, Z.; Li, Y.; Zhu, Y.; Zhou, C.; Ren, H.; et al. Abnormal cortical atrophy and functional connectivity are associated with depression in Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 957997. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, F.; Martinez-Horta, S.; Marin-Lahoz, J.; Pagonabarraga, J.; Kulisevsky, J. Apathy Reflects Extra-Striatal Dopaminergic Degeneration in de novo Parkinson’s Disease. J. Park. Dis. 2022, 12, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.A.; Harrison, S.J.; Melzer, T.R.; Dalrymple-Alford, J.C.; Anderson, T.J.; MacAskill, M.R.; Le Heron, C.J. Altered nucleus accumbens functional connectivity precedes apathy in Parkinson’s disease. Brain A J. Neurol. 2023, 146, 2739–2752. [Google Scholar] [CrossRef] [PubMed]
- Pachi, I.; Koros, C.; Simitsi, A.M.; Papadimitriou, D.; Bougea, A.; Prentakis, A.; Papagiannakis, N.; Bozi, M.; Antonelou, R.; Angelopoulou, E.; et al. Apathy: An underestimated feature in GBA and LRRK2 non-manifesting mutation carriers. Park. Relat. Disord. 2021, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Comi, C.; Marino, F.; Magistrelli, L.; De Marchi, F.; Cantello, R.; Riboldazzi, G.; Bono, G.; Cosentino, M. Polymorphisms of dopamine receptor genes and risk of visual hallucinations in Parkinson’s patients. Eur. J. Clin. Pharmacol. 2016, 72, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Carriere, N.; Lopes, R.; Defebvre, L.; Delmaire, C.; Dujardin, K. Impaired corticostriatal connectivity in impulse control disorders in Parkinson disease. Neurology 2015, 84, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jeon, B.S.; Kim, H.J.; Park, S.S. Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson’s disease. Park. Relat. Disord. 2012, 18, 76–78. [Google Scholar] [CrossRef]
- Saczynski, J.S.; Beiser, A.; Seshadri, S.; Auerbach, S.; Wolf, P.A.; Au, R. Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology 2010, 75, 35–41. [Google Scholar] [CrossRef]
- Bock, M.A.; Bahorik, A.; Brenowitz, W.D.; Yaffe, K. Apathy and risk of probable incident dementia among community-dwelling older adults. Neurology 2020, 95, e3280–e3287. [Google Scholar] [CrossRef]
- Ismail, Z.; Aguera-Ortiz, L.; Brodaty, H.; Cieslak, A.; Cummings, J.; Fischer, C.E.; Gauthier, S.; Geda, Y.E.; Herrmann, N.; Kanji, J.; et al. The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J. Alzheimer’s Dis. 2017, 56, 929–938. [Google Scholar] [CrossRef]
- Banks, S.J.; Raman, R.; He, F.; Salmon, D.P.; Ferris, S.; Aisen, P.; Cummings, J. The Alzheimer’s disease cooperative study prevention instrument project: Longitudinal outcome of behavioral measures as predictors of cognitive decline. Dement. Geriatr. Cogn. Disord. Extra 2014, 4, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Mielke, M.M.; Appleby, B.S.; Oh, E.S.; Geda, Y.E.; Lyketsos, C.G. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am. J. Geriatr. Psychiatry 2013, 21, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Donovan, N.J.; Amariglio, R.E.; Zoller, A.S.; Rudel, R.K.; Gomez-Isla, T.; Blacker, D.; Hyman, B.T.; Locascio, J.J.; Johnson, K.A.; Sperling, R.A.; et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2014, 22, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Roberts, R.O.; Mielke, M.M.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Boeve, B.F.; Sochor, O.; Tangalos, E.G.; Petersen, R.C.; et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: A population-based study. Am. J. Psychiatry 2014, 171, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Coble, D.W.; Xiong, C.; Schindler, S.E.; Perrin, R.J.; Gordon, B.A.; Benzinger, T.L.S.; Grant, E.; Fagan, A.M.; Harari, O.; et al. Preclinical Alzheimer’s disease biomarkers accurately predict cognitive and neuropathological outcomes. Brain A J. Neurol. 2022, 145, 4506–4518. [Google Scholar] [CrossRef]
- Ismail, Z.; Smith, E.E.; Geda, Y.; Sultzer, D.; Brodaty, H.; Smith, G.; Aguera-Ortiz, L.; Sweet, R.; Miller, D.; Lyketsos, C.G.; et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 195–202. [Google Scholar] [CrossRef]
- Mallo, S.C.; Ismail, Z.; Pereiro, A.X.; Facal, D.; Lojo-Seoane, C.; Campos-Magdaleno, M.; Juncos-Rabadan, O. Assessing Mild Behavioral Impairment with the Mild Behavioral Impairment-Checklist in People with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2018, 66, 83–95. [Google Scholar] [CrossRef]
- Chen, T.H.; Yeh, Y.C.; Huang, M.F.; Chen, H.M.; Lee, J.I.; Chen, C.S. Validation and Comparison of the Informant-Rated and Self-Rated Versions of the Mild Behavioral Impairment Checklist. J. Alzheimer’s Dis. 2022, 90, 1203–1213. [Google Scholar] [CrossRef]
- Sheikh, F.; Ismail, Z.; Mortby, M.E.; Barber, P.; Cieslak, A.; Fischer, K.; Granger, R.; Hogan, D.B.; Mackie, A.; Maxwell, C.J.; et al. Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int. Psychogeriatr. 2018, 30, 233–244. [Google Scholar] [CrossRef]
- Hu, S.; Patten, S.; Charlton, A.; Fischer, K.; Fick, G.; Smith, E.E.; Ismail, Z. Validating the Mild Behavioral Impairment Checklist in a Cognitive Clinic: Comparisons With the Neuropsychiatric Inventory Questionnaire. J. Geriatr. Psychiatry Neurol. 2023, 36, 107–120. [Google Scholar] [CrossRef]
- Cui, Y.; Dai, S.; Miao, Z.; Zhong, Y.; Liu, Y.; Liu, L.; Jing, D.; Bai, Y.; Kong, Y.; Sun, W.; et al. Reliability and Validity of the Chinese Version of the Mild Behavioral Impairment Checklist for Screening for Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 70, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, T.; Xiong, L.; Wang, X.; Ismail, Z.; Fukuda, M.; Sun, Z.; Wang, J.; Gauthier, S.; Yu, X.; et al. Reliability and Validity of the Chinese Version of Mild Behavioral Impairment Checklist in Mild Cognitive Impairment and Mild Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 81, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Xu, J.; Liao, Z.; Zhang, Y.; Wang, Y.; Sun, W.; Yu, E. A review of current evidence for mild behavioral impairment as an early potential novel marker of Alzheimer’s disease. Front. Psychiatry 2023, 14, 1099333. [Google Scholar] [CrossRef] [PubMed]
- Mortby, M.E.; Ismail, Z.; Anstey, K.J. Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int. Psychogeriatr. 2018, 30, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.N.; Cano, J.; Zhao, X.; Ismail, Z.; Chen, C.L.; Xu, X. Prevalence, Clinical Correlates, Cognitive Trajectories, and Dementia Risk Associated With Mild Behavioral Impairment in Asians. J. Clin. Psychiatry 2022, 83, 40123. [Google Scholar] [CrossRef]
- Mallo, S.C.; Ismail, Z.; Pereiro, A.X.; Facal, D.; Lojo-Seoane, C.; Campos-Magdaleno, M.; Juncos-Rabadan, O. Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int. Psychogeriatr. 2019, 31, 231–239. [Google Scholar] [CrossRef]
- Pan, Y.; Shea, Y.F.; Ismail, Z.; Mak, H.K.; Chiu, P.K.; Chu, L.W.; Song, Y.Q. Prevalence of mild behavioural impairment domains: A meta-analysis. Psychogeriatr. 2022, 22, 84–98. [Google Scholar] [CrossRef]
- Baschi, R.; Restivo, V.; Nicoletti, A.; Cicero, C.E.; Luca, A.; Recca, D.; Zappia, M.; Monastero, R. Mild Behavioral Impairment in Parkinson’s Disease: Data from the Parkinson’s Disease Cognitive Impairment Study (PACOS). J. Alzheimer’s Dis. JAD 2019, 68, 1603–1610. [Google Scholar] [CrossRef]
- Yoo, H.S.; Lee, S.; Chung, S.J.; Ye, B.S.; Sohn, Y.H.; Yun, M.; Lee, P.H. Clinical and Striatal Dopamine Transporter Predictors of Mild Behavioral Impairment in Drug-Naive Parkinson Disease. Clin. Nucl. Med. 2020, 45, e463–e468. [Google Scholar] [CrossRef]
- Yoon, E.J.; Ismail, Z.; Hanganu, A.; Kibreab, M.; Hammer, T.; Cheetham, J.; Kathol, I.; Sarna, J.R.; Martino, D.; Furtado, S.; et al. Mild behavioral impairment is linked to worse cognition and brain atrophy in Parkinson disease. Neurology 2019, 93, e766–e777. [Google Scholar] [CrossRef]
- Leroi, I.; Pantula, H.; McDonald, K.; Harbishettar, V. Neuropsychiatric symptoms in Parkinson’s disease with mild cognitive impairment and dementia. Park. Dis. 2012, 2012, 308097. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.K.; Goyal, V.; Kumar, N.; Shukla, G.; Srivastava, A.K.; Singh, S.; Behari, M. Neuropsychiatric co-morbidities in non-demented Parkinson’s disease. Ann. Indian Acad. Neurol. 2015, 18, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Di Fiore, P.; Ventimiglia, G.D.; Camarda, R.; Camarda, C. The neuropsychiatric profile of Parkinson’s disease subjects with and without mild cognitive impairment. J. Neural Transm. 2013, 120, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Yoon, E.J.; Kibreab, M.; Kathol, I.; Cheetham, J.; Hammer, T.; Sarna, J.; Ismail, Z.; Monchi, O. Mild behavioral impairment in Parkinson’s disease is associated with altered corticostriatal connectivity. NeuroImage Clin. 2020, 26, 102252. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Ruskey, J.A.; Martens, K.; Kibreab, M.; Javer, Z.; Kathol, I.; Hammer, T.; Cheetham, J.; Leveille, E.; Martino, D.; et al. Association Between BDNF Val66Met Polymorphism and Mild Behavioral Impairment in Patients With Parkinson’s Disease. Front. Neurol. 2020, 11, 587992. [Google Scholar] [CrossRef]
- Lang, S.; Ismail, Z.; Kibreab, M.; Kathol, I.; Sarna, J.; Monchi, O. Common and unique connectivity at the interface of motor, neuropsychiatric, and cognitive symptoms in Parkinson’s disease: A commonality analysis. Hum. Brain Mapp. 2020, 41, 3749–3764. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Stefanova, E.; Ziropadja, L.; Stojkovic, T.; Kostic, V.S. Neuropsychiatric symptoms in Serbian patients with Parkinson’s disease. J. Neurol. Sci. 2016, 367, 342–346. [Google Scholar] [CrossRef]
- Dlay, J.K.; Duncan, G.W.; Khoo, T.K.; Williams-Gray, C.H.; Breen, D.P.; Barker, R.A.; Burn, D.J.; Lawson, R.A.; Yarnall, A.J. Progression of Neuropsychiatric Symptoms over Time in an Incident Parkinson’s Disease Cohort (ICICLE-PD). Brain Sci. 2020, 10, 78. [Google Scholar] [CrossRef]
- Oguru, M.; Tachibana, H.; Toda, K.; Okuda, B.; Oka, N. Apathy and depression in Parkinson disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 35–41. [Google Scholar] [CrossRef]
- Weintraub, D.; Mamikonyan, E. The Neuropsychiatry of Parkinson Disease: A Perfect Storm. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2019, 27, 998–1018. [Google Scholar] [CrossRef]
- Owen, A.M.; James, M.; Leigh, P.N.; Summers, B.A.; Marsden, C.D.; Quinn, N.P.; Lange, K.W.; Robbins, T.W. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain A J. Neurol. 1992, 115, 1727–1751. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Zimmermann, R.; Gschwandtner, U.; Hatz, F.; Bousleiman, H.; Schwarz, N.; Fuhr, P. Apathy in Parkinson’s disease is related to executive function, gender and age but not to depression. Front. Aging Neurosci. 2014, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Silberman, C.D.; Laks, J.; Capitao, C.F.; Rodrigues, C.S.; Moreira, I.; Vasconcellos, L.F.; Engelhardt, E. Frontal functions in depressed and nondepressed Parkinson’s disease patients: Impact of severity stages. Psychiatry Res. 2007, 149, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.S.; Drago, V.; Yung, R.C.; Skidmore, F.M.; Skoblar, B.; Shenal, B.V.; Rhodes, R.D.; Heilman, K.M. Anxiety affects working memory only in left hemibody onset Parkinson disease patients. Cogn. Behav. Neurol. 2010, 23, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Wada-Isoe, K.; Kitayama, M.; Nakashima, K. Executive dysfunction in non-demented Parkinson’s disease patients with hallucinations. Acta Neurol. Scand. 2008, 117, 255–259. [Google Scholar] [CrossRef]
- Vitale, C.; Santangelo, G.; Trojano, L.; Verde, F.; Rocco, M.; Grossi, D.; Barone, P. Comparative neuropsychological profile of pathological gambling, hypersexuality, and compulsive eating in Parkinson’s disease. Mov. Disord. 2011, 26, 830–836. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Pagonabarraga, J.; Pascual-Sedano, B.; Garcia-Sanchez, C.; Gironell, A.; Trapecio Group, S. Prevalence and correlates of neuropsychiatric symptoms in Parkinson’s disease without dementia. Mov. Disord. 2008, 23, 1889–1896. [Google Scholar] [CrossRef]
- Dissanayaka, N.N.W.; Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Barker, R.A.; Burn, D.J.; on behalf of the ICICLE-PD study group. Anxiety is associated with cognitive impairment in newly-diagnosed Parkinson’s disease. Park. Relat. Disord. 2017, 36, 63–68. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.H.; Kim, Y.; Park, S.W.; Byeon, G.H.; Jang, J.W.; Parkinsons’s Progression Marker Initiative. Depressive symptoms are associated with worse cognitive prognosis in patients with newly diagnosed idiopathic Parkinson disease. Psychogeriatrics 2020, 20, 880–890. [Google Scholar] [CrossRef]
- Jin Yoon, E.; Ismail, Z.; Kathol, I.; Kibreab, M.; Hammer, T.; Lang, S.; Ramezani, M.; Auclair-Ouellet, N.; Sarna, J.R.; Martino, D.; et al. Patterns of brain activity during a set-shifting task linked to mild behavioral impairment in Parkinson’s disease. NeuroImage Clin. 2021, 30, 102590. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive impairment in Parkinson’s disease: The dual syndrome hypothesis. Neuro-Degener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef]
- Rozzini, L.; Vicini Chilovi, B.; Conti, M.; Delrio, I.; Borroni, B.; Trabucchi, M.; Padovani, A. Neuropsychiatric symptoms in amnestic and nonamnestic mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2008, 25, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Togari, A.; Kondo, T.; Mizuno, Y.; Komure, O.; Kuno, S.; Ichinose, H.; Nagatsu, T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999, 270, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Guo, Y.; Dong, G.; Zou, W.; Chen, Z. Association between BDNF G196A (Val66Met) polymorphism and cognitive impairment in patients with Parkinson’s disease: A meta-analysis. Braz. J. Med. Biol. Res. 2019, 52, e8443. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J. Critical Issues in BDNF Val66Met Genetic Studies of Neuropsychiatric Disorders. Front. Mol. Neurosci. 2018, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Duman, R.; Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef]
- Cagni, F.C.; Campelo, C.; Coimbra, D.G.; Barbosa, M.R.; Junior, L.G.O.; Neto, A.B.S.; Ribeiro, A.M.; Junior, C.O.G.; Gomes de Andrade, T.; Silva, R.H. Association of BDNF Val66MET Polymorphism With Parkinson’s Disease and Depression and Anxiety Symptoms. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 142–147. [Google Scholar] [CrossRef]
- Schrag, A.; Schott, J.M. Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol. 2006, 5, 355–363. [Google Scholar] [CrossRef]
- Belarbi, S.; Hecham, N.; Lesage, S.; Kediha, M.I.; Smail, N.; Benhassine, T.; Ysmail-Dahlouk, F.; Lohman, E.; Benhabyles, B.; Hamadouche, T.; et al. LRRK2 G2019S mutation in Parkinson’s disease: A neuropsychological and neuropsychiatric study in a large Algerian cohort. Park. Relat. Disord. 2010, 16, 676–679. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Bozi, M.; Simitsi, A.M.; Koros, C.; Antonelou, R.; Papagiannakis, N.; Maniati, M.; Poula, D.; Stamelou, M.; Vassilatis, D.K.; et al. Clinical differences between early-onset and mid-and-late-onset Parkinson’s disease: Data analysis of the Hellenic Biobank of Parkinson’s disease. J. Neurol. Sci. 2022, 442, 120405. [Google Scholar] [CrossRef]
- Hanganu, A.; Bruneau, M.A.; Degroot, C.; Bedetti, C.; Mejia-Constain, B.; Lafontaine, A.L.; Chouinard, S.; Monchi, O. Depressive symptoms in Parkinson’s disease correlate with cortical atrophy over time. Brain Cogn. 2017, 111, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.S.; Jeon, S.; Yoon, S.; Kang, S.W.; Baik, K.; Lee, Y.; Chung, S.J.; Oh, J.S.; Moon, H.; Kim, J.S.; et al. Effects of dopaminergic depletion and brain atrophy on neuropsychiatric symptoms in de novo Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Udaka, F.; Kameyama, M.; Sawamoto, N.; Hashikawa, K.; Fukuyama, H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology 2005, 65, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.; Venneri, A. Cognitive and neuroanatomical correlates of neuropsychiatric symptoms in Parkinson’s disease: A systematic review. J. Neurol. Sci. 2015, 356, 32–44. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.; Shine, J.M.; Lewis, S.J.; Hornberger, M. Neuropsychiatric symptoms in Parkinson’s disease: Fronto-striatal atrophy contributions. Park. Relat. Disord. 2014, 20, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, A.; Bedetti, C.; Jubault, T.; Gagnon, J.F.; Mejia-Constain, B.; Degroot, C.; Lafontaine, A.L.; Chouinard, S.; Monchi, O. Mild cognitive impairment in patients with Parkinson’s disease is associated with increased cortical degeneration. Mov. Disord. 2013, 28, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Melzer, T.R.; Watts, R.; MacAskill, M.R.; Pitcher, T.L.; Livingston, L.; Keenan, R.J.; Dalrymple-Alford, J.C.; Anderson, T.J. Grey matter atrophy in cognitively impaired Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 188–194. [Google Scholar] [CrossRef]
- Mak, E.; Zhou, J.; Tan, L.C.; Au, W.L.; Sitoh, Y.Y.; Kandiah, N. Cognitive deficits in mild Parkinson’s disease are associated with distinct areas of grey matter atrophy. J. Neurol. Neurosurg. Psychiatry 2014, 85, 576–580. [Google Scholar] [CrossRef]
- Pereira, J.B.; Svenningsson, P.; Weintraub, D.; Bronnick, K.; Lebedev, A.; Westman, E.; Aarsland, D. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 2014, 82, 2017–2025. [Google Scholar] [CrossRef]
- Hanganu, A.; Bedetti, C.; Degroot, C.; Mejia-Constain, B.; Lafontaine, A.L.; Soland, V.; Chouinard, S.; Bruneau, M.A.; Mellah, S.; Belleville, S.; et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain A J. Neurol. 2014, 137, 1120–1129. [Google Scholar] [CrossRef]
- Mak, E.; Su, L.; Williams, G.B.; Firbank, M.J.; Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Owen, A.M.; Khoo, T.K.; Brooks, D.J.; et al. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain A J. Neurol. 2015, 138, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Pereira, J.B.; Rios, J.; Ibarretxe-Bilbao, N.; Junque, C.; Bargallo, N.; Camara, A.; Buongiorno, M.; Fernandez, M.; Pont-Sunyer, C.; et al. Combined dementia-risk biomarkers in Parkinson’s disease: A prospective longitudinal study. Park. Relat. Disord. 2013, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; Mihaescu, A.; Strafella, A.P. Imaging behavioural complications of Parkinson’s disease. Brain Imaging Behav. 2019, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Castrioto, A.; Thobois, S.; Carnicella, S.; Maillet, A.; Krack, P. Emotional manifestations of PD: Neurobiological basis. Mov. Disord. 2016, 31, 1103–1113. [Google Scholar] [CrossRef]
- van Eimeren, T.; Monchi, O.; Ballanger, B.; Strafella, A.P. Dysfunction of the default mode network in Parkinson disease: A functional magnetic resonance imaging study. Arch. Neurol. 2009, 66, 877–883. [Google Scholar] [CrossRef]
- Hua, J.P.Y.; Karcher, N.R.; Merrill, A.M.; O’Brien, K.J.; Straub, K.T.; Trull, T.J.; Kerns, J.G. Psychosis risk is associated with decreased resting-state functional connectivity between the striatum and the default mode network. Cogn. Affect. Behav. Neurosci. 2019, 19, 998–1011. [Google Scholar] [CrossRef]
- Vatansever, D.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Cognitive Flexibility: A Default Network and Basal Ganglia Connectivity Perspective. Brain Connect. 2016, 6, 201–207. [Google Scholar] [CrossRef]
- Putcha, D.; Ross, R.S.; Cronin-Golomb, A.; Janes, A.C.; Stern, C.E. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. NeuroImage Clin. 2015, 7, 449–455. [Google Scholar] [CrossRef]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- Pasquini, J.; Durcan, R.; Wiblin, L.; Gersel Stokholm, M.; Rochester, L.; Brooks, D.J.; Burn, D.; Pavese, N. Clinical implications of early caudate dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1098–1104. [Google Scholar] [CrossRef]
- Shenhav, A.; Cohen, J.D.; Botvinick, M.M. Dorsal anterior cingulate cortex and the value of control. Nat. Neurosci. 2016, 19, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.A.; Mian, M.K.; Patel, S.R.; Asaad, W.F.; Williams, Z.M.; Dougherty, D.D.; Bush, G.; Eskandar, E.N. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 2012, 488, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Le Heron, C.; Apps, M.A.J.; Husain, M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia 2018, 118, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Singh-Curry, V.; Husain, M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 2009, 47, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.A.; Berg, E.A. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 1948, 38, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Monchi, O.; Petrides, M.; Doyon, J.; Postuma, R.B.; Worsley, K.; Dagher, A. Neural bases of set-shifting deficits in Parkinson’s disease. J. Neurosci. 2004, 24, 702–710. [Google Scholar] [CrossRef]
- Nagano-Saito, A.; Habak, C.; Mejia-Constain, B.; Degroot, C.; Monetta, L.; Jubault, T.; Bedetti, C.; Lafontaine, A.L.; Chouinard, S.; Soland, V.; et al. Effect of mild cognitive impairment on the patterns of neural activity in early Parkinson’s disease. Neurobiol. Aging 2014, 35, 223–231. [Google Scholar] [CrossRef]
- Schultz, D.H.; Ito, T.; Solomyak, L.I.; Chen, R.H.; Mill, R.D.; Anticevic, A.; Cole, M.W. Global connectivity of the fronto-parietal cognitive control network is related to depression symptoms in the general population. Netw. Neurosci. 2019, 3, 107–123. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Liu, J.; Li, D.; Feng, Z.; Chen, C.; Xue, G. Dissociated roles of the parietal and frontal cortices in the scope and control of attention during visual working memory. NeuroImage 2017, 149, 210–219. [Google Scholar] [CrossRef]
- Nagano-Saito, A.; Leyton, M.; Monchi, O.; Goldberg, Y.K.; He, Y.; Dagher, A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J. Neurosci. Off. 2008, 28, 3697–3706. [Google Scholar] [CrossRef]
- Graham, S.; Phua, E.; Soon, C.S.; Oh, T.; Au, C.; Shuter, B.; Wang, S.C.; Yeh, I.B. Role of medial cortical, hippocampal and striatal interactions during cognitive set-shifting. NeuroImage 2009, 45, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Collette, F.; Van der Linden, M.; Laureys, S.; Delfiore, G.; Degueldre, C.; Luxen, A.; Salmon, E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum. Brain Mapp. 2005, 25, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Blangero, J.; Sanghera, M.K.; Pessoa, L.; Fox, P.M.; Uecker, A.; Friehs, G.; Young, K.A.; et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage 2012, 60, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Packard, M.G.; Knowlton, B.J. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Vitale, C.; Picillo, M.; Cuoco, S.; Moccia, M.; Pezzella, D.; Erro, R.; Longo, K.; Vicidomini, C.; Pellecchia, M.T.; et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Park. Relat. Disord. 2015, 21, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Newberg, A.B.; Cary, M.S.; Siderowf, A.D.; Moberg, P.J.; Kleiner-Fisman, G.; Duda, J.E.; Stern, M.B.; Mozley, D.; Katz, I.R. Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J. Nucl. Med. 2005, 46, 227–232. [Google Scholar]
- Chung, S.J.; Yoo, H.S.; Oh, J.S.; Kim, J.S.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Park. Relat. Disord. 2018, 51, 43–48. [Google Scholar] [CrossRef]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Park. Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef]
- Wile, D.J.; Agarwal, P.A.; Schulzer, M.; Mak, E.; Dinelle, K.; Shahinfard, E.; Vafai, N.; Hasegawa, K.; Zhang, J.; McKenzie, J.; et al. Serotonin and dopamine transporter PET changes in the premotor phase of LRRK2 parkinsonism: Cross-sectional studies. Lancet. Neurol. 2017, 16, 351–359. [Google Scholar] [CrossRef]
- Matsuoka, T.; Narumoto, J.; Morii-Kitani, F.; Niwa, F.; Mizuno, T.; Abe, M.; Takano, H.; Wakasugi, N.; Shima, A.; Sawamoto, N.; et al. Contribution of amyloid and putative Lewy body pathologies in neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry 2023, 38, e5993. [Google Scholar] [CrossRef] [PubMed]
- Ffytche, D.H.; Pereira, J.B.; Ballard, C.; Chaudhuri, K.R.; Weintraub, D.; Aarsland, D. Risk factors for early psychosis in PD: Insights from the Parkinson’s Progression Markers Initiative. J. Neurol. Neurosurg. Psychiatry 2017, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Bougea, A.; Paudel, Y.N.; Georgakopoulou, V.E.; Papageorgiou, S.G.; Piperi, C. Genetic Insights into the Molecular Pathophysiology of Depression in Parkinson’s Disease. Medicina 2023, 59, 1138. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Bougea, A.; Papageorgiou, S.G.; Villa, C. Psychosis in Parkinson’s Disease: A Lesson from Genetics. Genes 2022, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Ushakova, A.; Doitsidou, M.; Tzoulis, C.; Tysnes, O.B.; Dalen, I.; Pedersen, K.F.; Alves, G.; Maple-Grodem, J. The impact of common genetic variants in cognitive decline in the first seven years of Parkinson’s disease: A longitudinal observational study. Neurosci. Lett. 2021, 764, 136243. [Google Scholar] [CrossRef] [PubMed]
- Mitaki, S.; Isomura, M.; Maniwa, K.; Yamasaki, M.; Nagai, A.; Nabika, T.; Yamaguchi, S. Apathy is associated with a single-nucleotide polymorphism in a dopamine-related gene. Neurosci. Lett. 2013, 549, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Huertas, I.; Jesus, S.; Garcia-Gomez, F.J.; Lojo, J.A.; Bernal-Bernal, I.; Bonilla-Toribio, M.; Martin-Rodriguez, J.F.; Garcia-Solis, D.; Gomez-Garre, P.; Mir, P. Genetic factors influencing frontostriatal dysfunction and the development of dementia in Parkinson’s disease. PLoS ONE 2017, 12, e0175560. [Google Scholar] [CrossRef]
- de la Fuente-Fernandez, R.; Nunez, M.A.; Lopez, E. The apolipoprotein E epsilon 4 allele increases the risk of drug-induced hallucinations in Parkinson’s disease. Clin. Neuropharmacol. 1999, 22, 226–230. [Google Scholar]
- Umeh, C.C.; Mahajan, A.; Mihailovic, A.; Pontone, G.M. APOE4 Allele, Sex, and Dementia Risk in Parkinson’s Disease: Lessons From a Longitudinal Cohort. J. Geriatr. Psychiatry Neurol. 2022, 35, 810–815. [Google Scholar] [CrossRef]
- Getz, S.J.; Levin, B. Cognitive and Neuropsychiatric Features of Early Parkinson’s Disease. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2017, 32, 769–785. [Google Scholar] [CrossRef]
- Creese, B.; Arathimos, R.; Brooker, H.; Aarsland, D.; Corbett, A.; Lewis, C.; Ballard, C.; Ismail, Z. Genetic risk for Alzheimer’s disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimer’s Dement. 2021, 13, e12164. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Imarisio, A.; Conforti, F.; Scalvini, A.; Masciocchi, S.; Nocivelli, S.; Turrone, R.; Gipponi, S.; Cottini, E.; Borroni, B.; et al. Plasma NfL, clinical subtypes and motor progression in Parkinson’s disease. Park. Relat. Disord. 2021, 87, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Ma, L.Z.; Liu, J.Y.; Ou, Y.N.; Zhao, B.; Ma, Y.H.; Tan, L. Cerebrospinal fluid neurofilament dynamic profiles predict cognitive progression in individuals with de novo Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 1061096. [Google Scholar] [CrossRef] [PubMed]
- Naude, J.P.; Gill, S.; Hu, S.; McGirr, A.; Forkert, N.D.; Monchi, O.; Stys, P.K.; Smith, E.E.; Ismail, Z.; Alzheimer’s Disease Neuroimaging, I. Plasma Neurofilament Light: A Marker of Neurodegeneration in Mild Behavioral Impairment. J. Alzheimer’s Dis. 2020, 76, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Charisis, S.; Ntanasi, E.; Stamelou, M.; Xiromerisiou, G.; Maraki, M.; Veskoukis, A.S.; Yannakoulia, M.; Kosmidis, M.H.; Anastasiou, C.A.; Giagkou, N.; et al. Plasma Glutathione and Prodromal Parkinson’s Disease Probability. Mov. Disord. 2022, 37, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Poladian, N.; Navasardyan, I.; Narinyan, W.; Orujyan, D.; Venketaraman, V. Potential Role of Glutathione Antioxidant Pathways in the Pathophysiology and Adjunct Treatment of Psychiatric Disorders. Clin. Pract. 2023, 13, 768–779. [Google Scholar] [CrossRef]
- Ntanasi, E.; Maraki, M.; Yannakoulia, M.; Stamelou, M.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Gargalionis, A.; et al. Frailty and Prodromal Parkinson’s Disease: Results From the HELIAD Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 622–629. [Google Scholar] [CrossRef]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef]
- Miao, R.; Chen, H.Y.; Robert, P.; Smith, E.E.; Ismail, Z.; Group, M.S. White matter hyperintensities and mild behavioral impairment: Findings from the MEMENTO cohort study. Cereb. Circ. Cogn. Behav. 2021, 2, 100028. [Google Scholar] [CrossRef]
- Linortner, P.; McDaniel, C.; Shahid, M.; Levine, T.F.; Tian, L.; Cholerton, B.; Poston, K.L. White Matter Hyperintensities Related to Parkinson’s Disease Executive Function. Mov. Disord. Clin. Pract. 2020, 7, 629–638. [Google Scholar] [CrossRef]
- Richey, L.N.; Daneshvari, N.O.; Young, L.; Bray, M.J.C.; Gottesman, R.F.; Mosley, T.; Walker, K.A.; Peters, M.E.; Schneider, A.L.C. Associations of Prior Head Injury With Mild Behavioral Impairment Domains. J. Head Trauma Rehabil. 2023. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Timblin, H.; Baxter, F. Cumulative Effect of Head Injuries on Nonmotor Outcomes in Parkinson’s Disease. J. Neuropsychiatry Clin. Neurosci. 2023, 35, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Delic, V.; Beck, K.D.; Pang, K.C.H.; Citron, B.A. Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.X.; Chen, H.Y.; Camicioli, R.; Montero-Odasso, M.; Smith, E.E.; Ismail, Z. Dual-task gait and mild behavioral impairment: The interface between non-cognitive dementia markers. Exp. Gerontol. 2022, 162, 111743. [Google Scholar] [CrossRef] [PubMed]
- Subotic, A.; Gee, M.; Nelles, K.; Ba, F.; Dadar, M.; Duchesne, S.; Sharma, B.; Masellis, M.; Black, S.E.; Almeida, Q.J.; et al. Gray matter loss relates to dual task gait in Lewy body disorders and aging. J. Neurol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Buonomano, D.V.; Laje, R. Population clocks: Motor timing with neural dynamics. Trends Cogn. Sci. 2010, 14, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, Z.R.; Wang, X.P. The Fast Spiking Subpopulation of Striatal Neurons Coding for Temporal Cognition of Movements. Front. Cell. Neurosci. 2017, 11, 406. [Google Scholar] [CrossRef]
- Bougea, A.; Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Xiromerisiou, G.; Kosmidis, M.H.; Ntanasi, E.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; et al. Higher probability of prodromal Parkinson disease is related to lower cognitive performance. Neurology 2019, 92, e2261–e2272. [Google Scholar] [CrossRef]
- Liepelt-Scarfone, I.; Ophey, A.; Kalbe, E. Cognition in prodromal Parkinson’s disease. Prog. Brain Res. 2022, 269, 93–111. [Google Scholar] [CrossRef]
- Pachi, I.; Maraki, M.I.; Giagkou, N.; Kosmidis, M.H.; Yannakoulia, M.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Ntanasi, E.; Xiromerisiou, G.; et al. Late life psychotic features in prodromal Parkinson’s disease. Park. Relat. Disord. 2021, 86, 67–73. [Google Scholar] [CrossRef]
- Darweesh, S.K.; Verlinden, V.J.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain A J. Neurol. 2017, 140, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, R.D.; Troster, A.I.; Rubino, J.T.; Allen, L.A.; Gara, M.A.; Mark, M.H.; Menza, M. Neuropsychological outcomes after psychosocial intervention for depression in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 57–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jing, X.Z.; Yang, H.J.; Taximaimaiti, R.; Wang, X.P. Advances in the Therapeutic Use of Non-Ergot Dopamine Agonists in the Treatment of Motor and Non-Motor Symptoms of Parkinson’s Disease. Curr. Neuropharmacol. 2023, 21, 1224–1240. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Stanitsa, E.; Karpodini, C.C.; Bougea, A.; Kontaxopoulou, D.; Fragkiadaki, S.; Koros, C.; Georgakopoulou, V.E.; Fotakopoulos, G.; Koutedakis, Y.; et al. Pharmacological and Non-Pharmacological Treatments for Depression in Parkinson’s Disease: An Updated Review. Medicina 2023, 59, 1454. [Google Scholar] [CrossRef] [PubMed]
- Maraki, M.I.; Yannakoulia, M.; Xiromerisiou, G.; Stefanis, L.; Charisis, S.; Giagkou, N.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; et al. Mediterranean diet is associated with a lower probability of prodromal Parkinson’s disease and risk for Parkinson’s disease/dementia with Lewy bodies: A longitudinal study. Eur. J. Neurol. 2023, 30, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Balomenos, V.; Bounou, L.; Charisis, S.; Stamelou, M.; Ntanasi, E.; Georgiadi, K.; Mourtzinos, I.; Tzima, K.; Anastasiou, C.A.; Xiromerisiou, G.; et al. Dietary Inflammatory Index score and prodromal Parkinson’s disease incidence: The HELIAD study. J. Nutr. Biochem. 2022, 105, 108994. [Google Scholar] [CrossRef]
- Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutr. Neurosci. 2021, 24, 248–259. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Yao, Z.; Zhang, T.; Li, Z. Dietary inflammatory potential and the incidence of depression and anxiety: A meta-analysis. J. Health Popul. Nutr. 2022, 41, 24. [Google Scholar] [CrossRef]
- Orfei, M.D.; Assogna, F.; Pellicano, C.; Pontieri, F.E.; Caltagirone, C.; Pierantozzi, M.; Stefani, A.; Spalletta, G. Anosognosia for cognitive and behavioral symptoms in Parkinson’s disease with mild dementia and mild cognitive impairment: Frequency and neuropsychological/neuropsychiatric correlates. Park. Relat. Disord. 2018, 54, 62–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulou, E.; Bougea, A.; Hatzimanolis, A.; Stefanis, L.; Scarmeas, N.; Papageorgiou, S. Mild Behavioral Impairment in Parkinson’s Disease: An Updated Review on the Clinical, Genetic, Neuroanatomical, and Pathophysiological Aspects. Medicina 2024, 60, 115. https://doi.org/10.3390/medicina60010115
Angelopoulou E, Bougea A, Hatzimanolis A, Stefanis L, Scarmeas N, Papageorgiou S. Mild Behavioral Impairment in Parkinson’s Disease: An Updated Review on the Clinical, Genetic, Neuroanatomical, and Pathophysiological Aspects. Medicina. 2024; 60(1):115. https://doi.org/10.3390/medicina60010115
Chicago/Turabian StyleAngelopoulou, Efthalia, Anastasia Bougea, Alexandros Hatzimanolis, Leonidas Stefanis, Nikolaos Scarmeas, and Sokratis Papageorgiou. 2024. "Mild Behavioral Impairment in Parkinson’s Disease: An Updated Review on the Clinical, Genetic, Neuroanatomical, and Pathophysiological Aspects" Medicina 60, no. 1: 115. https://doi.org/10.3390/medicina60010115
APA StyleAngelopoulou, E., Bougea, A., Hatzimanolis, A., Stefanis, L., Scarmeas, N., & Papageorgiou, S. (2024). Mild Behavioral Impairment in Parkinson’s Disease: An Updated Review on the Clinical, Genetic, Neuroanatomical, and Pathophysiological Aspects. Medicina, 60(1), 115. https://doi.org/10.3390/medicina60010115








