Direct Anterior Approach in Total Hip Arthroplasty for Severe Crowe IV Dysplasia: Retrospective Clinical and Radiological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
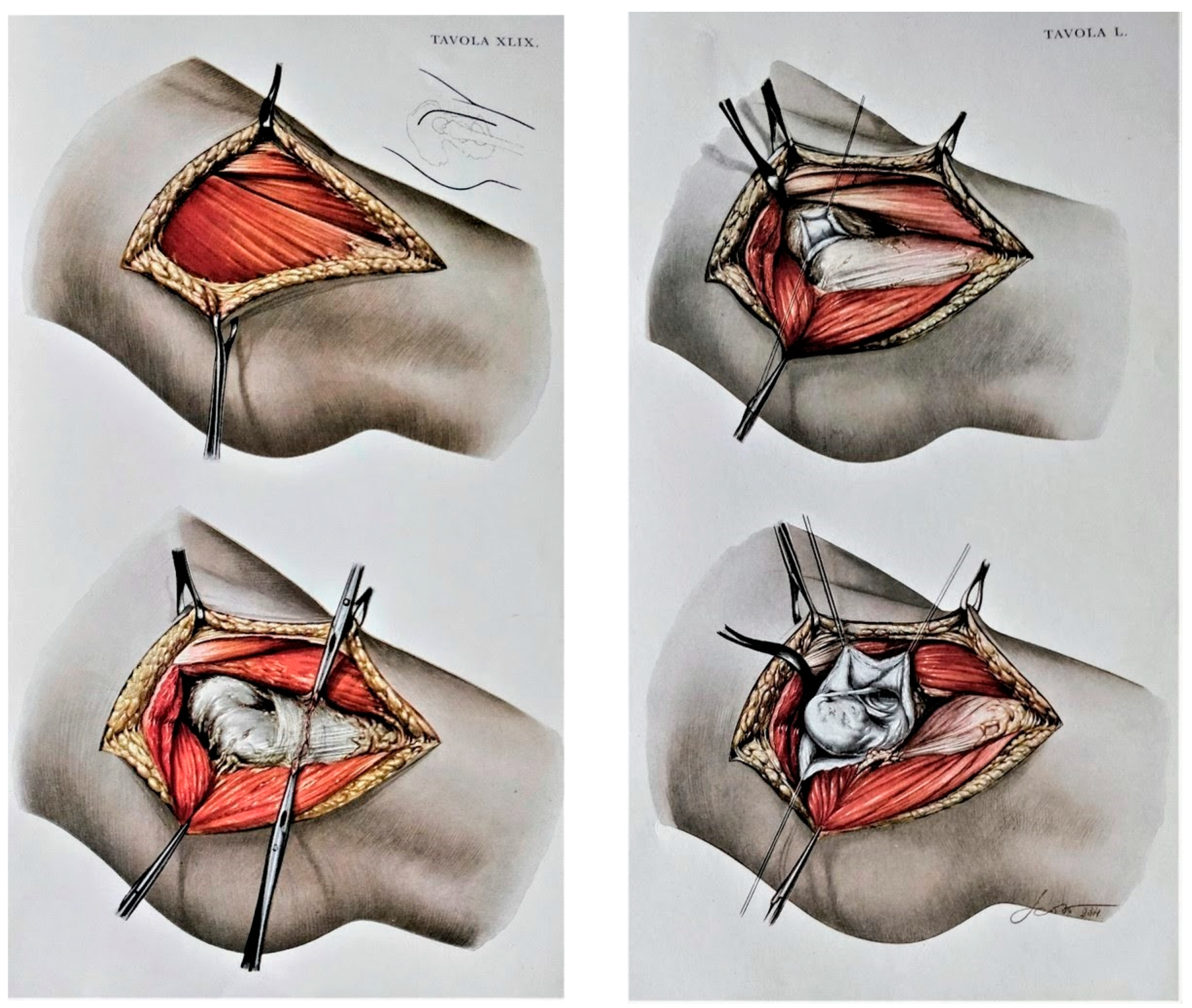
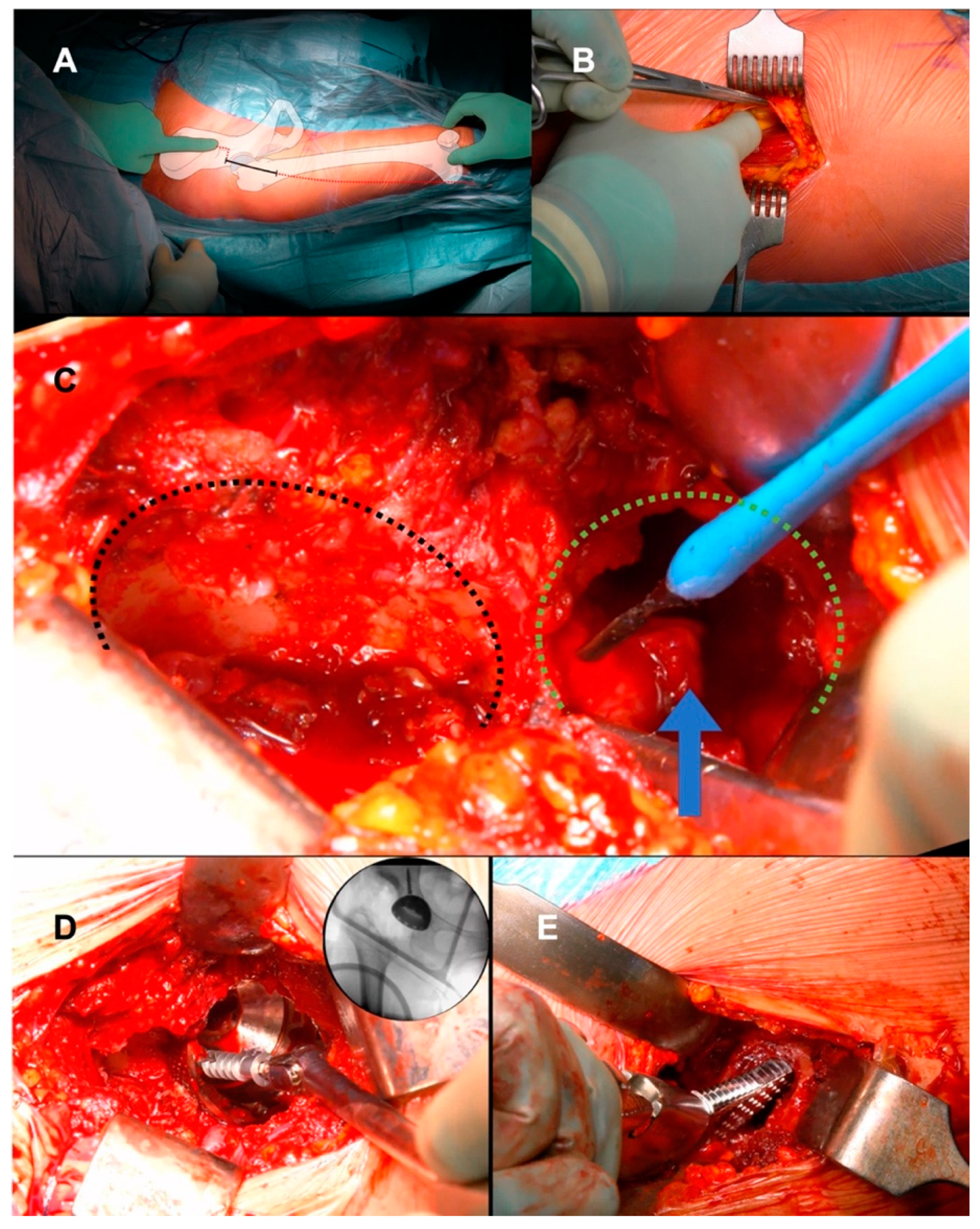
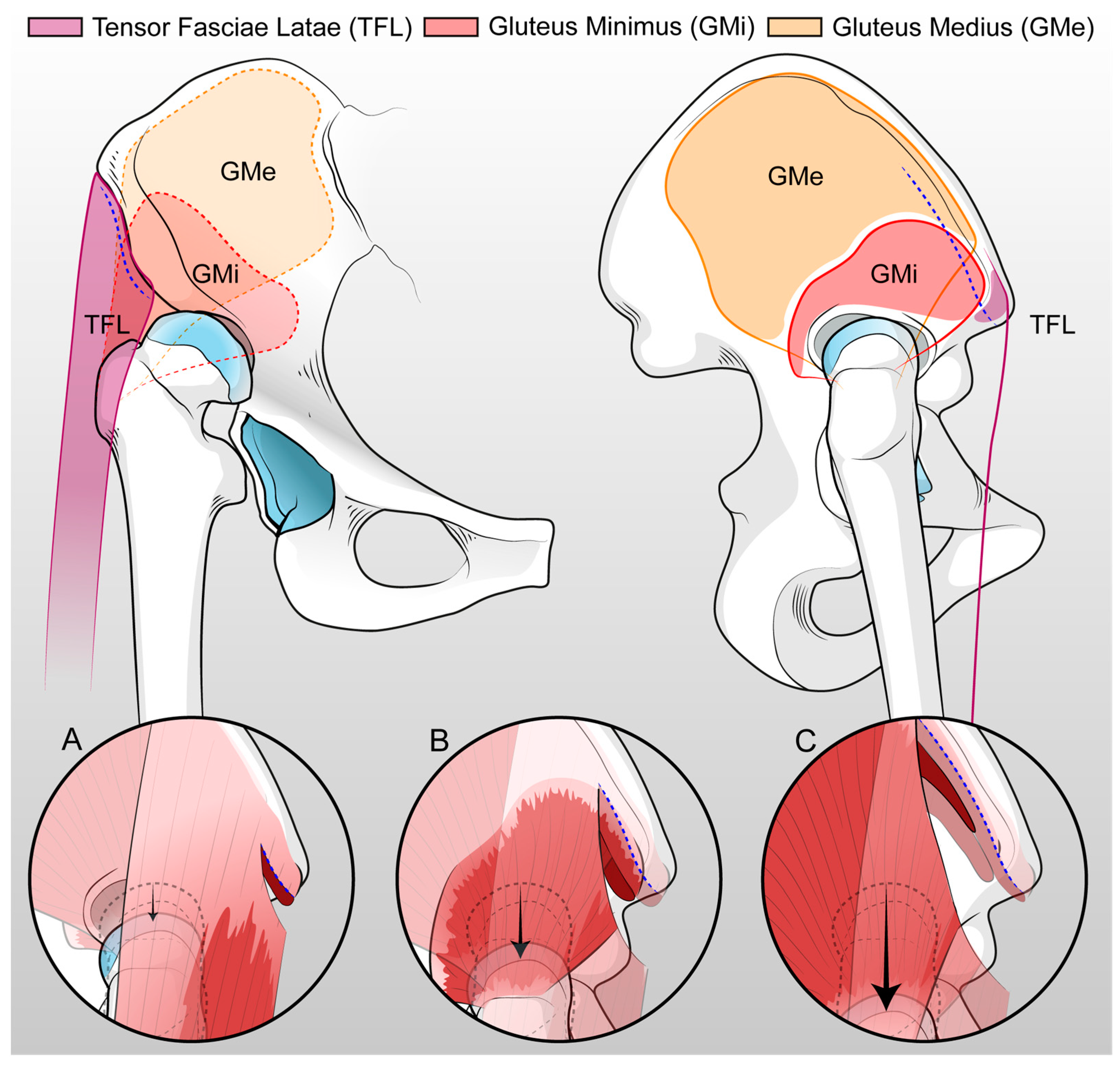
2.2. Surgical Technique
2.3. Post-Operative Management
2.4. Clinical Evaluation
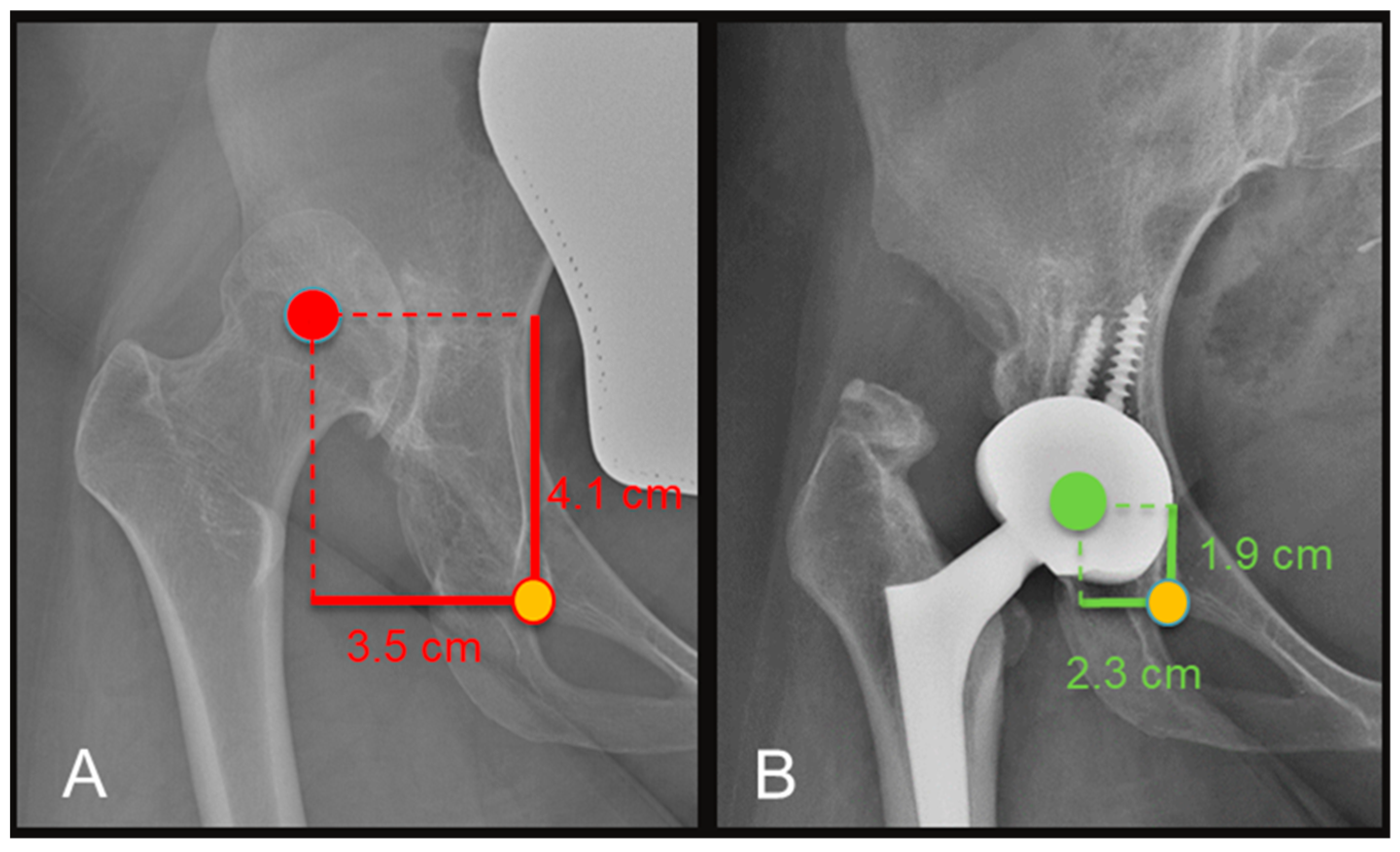
2.5. Radiographic Evaluation
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.2. Clinical Results
3.3. Radiographic Results
3.4. Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Husson, J.L.; Mallet, J.F.; Huten, D.; Odri, G.A.; Morin, C.; Parent, H.F. Applications in Hip Pathology. Orthop. Traumatol. Surg. Res. 2010, 96, S10–S16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greber, E.M.; Pelt, C.E.; Gililland, J.M.; Anderson, M.B.; Erickson, J.A.; Peters, C.L. Challenges in Total Hip Arthroplasty in the Setting of Developmental Dysplasia of the Hip. J. Arthroplast. 2017, 32, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Castagnini, F.; Giardina, F.; Tassinari, E.; Biondi, F.; Toni, A. Cementless Total Hip Arthroplasty in Crowe III and IV Dysplasia: High Hip Center and Modular Necks. J. Arthroplast. 2018, 33, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Wang, Q.; Peng, X.; Wang, Q.; Jiang, Y.; Chen, Y. Equalisation of Leg Lengths in Total Hip Arthroplasty for Patients with Crowe Type-IV Developmental Dysplasia of the Hip. Bone Jt. J. 2017, 99B, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, V.I.; Christodoulou, M.; Sasalos, G.; Babis, G.C. Reconstruction of the Acetabulum in Developmental Dysplasia of the Hip in Total Hip Replacement. Arch. Bone Jt. Surg. 2014, 2, 130–136. [Google Scholar] [PubMed]
- Bicanic, G.; Barbaric, K.; Bohacek, I.; Aljinovic, A.; Delimar, D. Current Concept in Dysplastic Hip Arthroplasty: Techniques for Acetabular and Femoral Reconstruction. World J. Orthop. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Pagnano, M.W.; Hanssen, A.D.; Lewallen, D.G.; Shaughnessy, W.J. The Effect of Superior Placement of the Acetabular Component on the Rate of Loosening after Total Hip Arthroplasty: Long-Term Results in Patients Who Have Crowe Type-II Congenital Dysplasia of the Hip. J. Bone Jt. Surg. 1996, 78, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-t.; Li, C.-f.; Han, Y.; Song, Y.; Li, S.-x.; Liu, J.-g. Total Hip Arthroplasty for Crowe Type IV Hip Dysplasia: Surgical Techniques and Postoperative Complications. Orthop. Surg. 2019, 11, 966–973. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.H.; Lou, L.M.; Cai, Z.D. The Techniques of Soft Tissue Release and True Socket Reconstruction in Total Hip Arthroplasty for Patients with Severe Developmental Dysplasia of the Hip. Int. Orthop. 2012, 36, 1795–1801. [Google Scholar] [CrossRef]
- Heuter, C. Grundriss Der Chirurgie. In Grundriss der Chirurgie; F.C.W. Vogel: Leipzig, Germany, 1883; pp. 129–200. [Google Scholar]
- Smith-Petersen, M.N.; Larson, C.B. Complications of Old Fractures of the Neck of the Femur; Results of Treatment of Vitallium-Mold Arthroplasty. J. Bone Jt. Surg. Am. 1947, 29, 41–48. [Google Scholar]
- Putti, V.; Faldini, G.; Pasquali, E. Anatomy of Congenital Dislocation of the Hip; Licinio Cappelli Editore: Bologna, Italy, 1935. [Google Scholar]
- Cadossi, M.; Sambri, A.; Tedesco, G.; Mazzotti, A.; Terrando, S.; Faldini, C. Anterior Approach in Total Hip Replacement. Orthopedics 2017, 40, e553–e556. [Google Scholar] [CrossRef] [PubMed]
- Lovell, T.P. Single-Incision Direct Anterior Approach for Total Hip Arthroplasty Using a Standard Operating Table. J. Arthroplast. 2008, 23, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.J.; Lombardi, A.V., Jr.; Morris, M.J.; Berend, K.R. A Mini-Anterior Approach to the Hip for Total Joint Replacement: Optimising Results: Improving Hip Joint Replacement Outcomes. Bone Jt. J. 2014, 96B, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Restrepo, C.; Maltenfort, M.G. Total Hip Arthroplasty Performed Through Direct Anterior Approach Provides Superior Early Outcome: Results of a Randomized, Prospective Study. Orthop. Clin. N. Am. 2016, 47, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Nogler, M.M.; Thaler, M.R. The Direct Anterior Approach for Hip Revision: Accessing the Entire Femoral Diaphysis Without Endangering the Nerve Supply. J. Arthroplast. 2017, 32, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Taunton, M.J.; Trousdale, R.T.; Sierra, R.J.; Kaufman, K.; Pagnano, M.W. John Charnley Award: Randomized Clinical Trial of Direct Anterior and Miniposterior Approach THA: Which Provides Better Functional Recovery? Clin. Orthop. Relat. Res. 2018, 476, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Oinuma, K.; Tamaki, T.; Miura, Y.; Kaneyama, R.; Shiratsuchi, H. Total Hip Arthroplasty with Subtrochanteric Shortening Osteotomy for Crowe Grade 4 Dysplasia Using the Direct Anterior Approach. J. Arthroplast. 2014, 29, 626–629. [Google Scholar] [CrossRef]
- Kawasaki, M.; Hasegawa, Y.; Okura, T.; Ochiai, S.; Fujibayashi, T. Muscle Damage After Total Hip Arthroplasty Through the Direct Anterior Approach for Developmental Dysplasia of the Hip. J. Arthroplast. 2017, 32, 2466–2473. [Google Scholar] [CrossRef]
- Viamont-Guerra, M.R.; Chen, A.F.; Stirling, P.; Nover, L.; Guimarães, R.P.; Laude, F. The Direct Anterior Approach for Total Hip Arthroplasty for Severe Dysplasia (Crowe III and IV) Provides Satisfactory Medium to Long-Term Outcomes. J. Arthroplast. 2020, 35, 1642–1650. [Google Scholar] [CrossRef]
- Viamont-Guerra, M.R.; Saffarini, M.; Laude, F. Surgical Technique and Case Series of Total Hip Arthroplasty with the Hueter Anterior Approach for Crowe Type-IV Dysplasia. J. Bone Jt. Surg. Am. 2020, 102, 99–106. [Google Scholar] [CrossRef]
- Jawad, M.U.; Scully, S.P. In Brief: Crowe’s Classification: Arthroplasty in Developmental Dysplasia of the Hip. Clin. Orthop. Relat. Res. 2011, 469, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Kamara, E.; Cooper, H.J. Applied Anatomy of the Direct Anterior Approach for Femoral Mobilization. JBJS Essent. Surg. Tech. 2017, 7, e18. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, P.A. Traumatic Arthritis of the Hip after Dislocation and Acetabular Fractures: Treatment by Mold Arthroplasty: An End-Result Study Using a New Method of Result Evaluation. In Classic Papers in Orthopaedics; Springer: London, UK, 2014; pp. 13–17. ISBN 9781447154518. [Google Scholar]
- Abraham, W.; Dimon, J., 3rd. Leg Length Discrepancy in Total Hip Arthroplasty. Orthop. Clin. N. Am. 1992, 23, 201–209. [Google Scholar] [CrossRef]
- Sabharwal, S.; Kumar, A. Methods for Assessing Leg Length Discrepancy. Clin. Orthop. Relat. Res. 2008, 466, 2910–2922. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.S.; McAuley, J.P.; Young, A.M.; Engh, C.A. Radiographic Signs of Osseointegration in Porous-Coated Acetabular Components. Clin. Orthop. Relat. Res. 2006, 444, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-Coated Hip Replacement. The Factors Governing Bone Ingrowth, Stress Shielding, and Clinical Results. J. Bone Jt. Surg. Ser. B 1987, 69, 45–55. [Google Scholar] [CrossRef]
- Komiyama, K.; Fukushi, J.-i.; Motomura, G.; Hamai, S.; Ikemura, S.; Fujii, M.; Nakashima, Y. Does High Hip Centre Affect Dislocation after Total Hip Arthroplasty for Developmental Dysplasia of the Hip? Int. Orthop. 2019, 43, 2057–2063. [Google Scholar] [CrossRef]
- Della Valle, A.G.; Padgett, D.E.; Salvati, E.A. Preoperative Planning for Primary Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2005, 13, 455–462. [Google Scholar] [CrossRef]
- Glorion, C. Surgical Reduction of Congenital Hip Dislocation. Orthop. Traumatol. Surg. Res. 2018, 104, S147–S157. [Google Scholar] [CrossRef]
- Rüdiger, H.A.; Parvex, V.; Terrier, A. Impact of the Femoral Head Position on Moment Arms in Total Hip Arthroplasty: A Parametric Finite Element Study. J. Arthroplast. 2016, 31, 715–720. [Google Scholar] [CrossRef]
- Hueter, C. Die Methodik Der Resectio Coxae Durch Vorderen Schrägschnitt. (Methode von Schede Und C. Hueter). Verletzungen Und Krankheiten Des Hüftgelenks. Resectio Coxae. In Grundriss der Chirurgie; F.C.W. Vogel: Leipzig, Germany, 1880; pp. 935–937. [Google Scholar]
- Rachbauer, F.; Kain, M.S.H.; Leunig, M. The History of the Anterior Approach to the Hip. Orthop. Clin. N. Am. 2009, 40, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Faldini, C.; Miscione, M.T.; Chehrassan, M.; Acri, F.; Pungetti, C.; D’Amato, M.; Luciani, D.; Giannini, S. Congenital Hip Dysplasia Treated by Total Hip Arthroplasty Using Cementless Tapered Stem in Patients Younger than 50 Years Old: Results after 12-Years Follow-Up. J. Orthop. Traumatol. 2011, 12, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Corten, K.; Holzapfel, B.M. Direct Anterior Approach for Total Hip Arthroplasty Using the “Bikini Incision”. Oper. Orthop. Traumatol. 2021, 33, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Faldini, C.; Brunello, M.; Pilla, F.; Geraci, G.; Stefanini, N.; Tassinari, L.; Di Martino, A. Femoral Head Autograft to Manage Acetabular Bone Loss Defects in THA for Crowe III Hips by DAA: Retrospective Study and Surgical Technique. J. Clin. Med. 2023, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Sofu, H.; Kockara, N.; Gursu, S.; Issin, A.; Oner, A.; Sahin, V. Transverse Subtrochanteric Shortening Osteotomy During Cementless Total Hip Arthroplasty in Crowe Type-III or IV Developmental Dysplasia. J. Arthroplast. 2015, 30, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Yang, D.; Xu, B.; Mamtimin, A.; Guo, W.; Cao, L. Midterm Outcome of Cementless Total Hip Arthroplasty in Crowe IV-Hartofilakidis Type III Developmental Dysplasia of the Hip. J. Arthroplast. 2016, 31, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Ibrahim, E.G.; Ayman, B. Total Hip Arthroplasty with Subtrochanteric Osteotomy in Neglected Dysplastic Hip. Int. Orthop. 2015, 39, 27–33. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Sun, J.; Lin, X.; Tang, T. Treatment of Crowe Type-IV Hip Dysplasia Using Cementless Total Hip Arthroplasty and Double Chevron Subtrochanteric Shortening Osteotomy: A 5- to 10-Year Follow-Up Study. J. Arthroplast. 2017, 32, 475–479. [Google Scholar] [CrossRef]
- Gofton, J.P. Studies in Osteoarthritis of the Hip. IV. Biomechanics and Clinical Considerations. Can. Med. Assoc. J. 1971, 104, 1007–1011. [Google Scholar]
- Watts, C.D.; Abdel, M.P.; Hanssen, A.D.; Pagnano, M.W. Anatomic Hip Center Decreases Aseptic Loosening Rates after Total Hip Arthroplasty with Cement in Patients with Crowe Type-II Dysplasia: A Concise Follow-up Report at a Mean of Thirty-Six Years. J. Bone Jt. Surg. Am. Vol. 2016, 98, 910–915. [Google Scholar] [CrossRef]
- Linde, F.; Jensen, J. Socket Loosening in Arthroplasty for Congenital Dislocation of the Hip. Acta Orthop. Scand. 1988, 59, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Stans, A.A.; Pagnano, M.W.; Shaughnessy, W.J.; Hanssen, A.D. Results of Total Hip Arthroplasty for Crowe Type III Developmental Hip Dysplasia. Clin. Orthop. Relat. Res. 1998, 348, 149–157. [Google Scholar] [CrossRef]
- Nawabi, D.H.; Meftah, M.; Nam, D.; Ranawat, A.S.; Ranawat, C.S. Durable Fixation Achieved with Medialized, High Hip Center Cementless THAs for Crowe II and III Dysplasia. Clin. Orthop. Relat. Res. 2014, 472, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Traina, F.; De Fine, M.; Biondi, F.; Tassinari, E.; Galvani, A.; Toni, A. The Influence of the Centre of Rotation on Implant Survival Using a Modular Stem Hip Prosthesis. Int. Orthop. 2009, 33, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Traina, F.; De Fine, M.; Tassinari, E.; Sudanese, A.; Calderoni, P.P.; Toni, A. Modular Neck Prostheses in DDH Patients: 11-Year Results. J. Orthop. Sci. 2011, 16, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, L.L.; Wang, H.Y.; Pei, F.X.; Zhou, Z.K. Long-Term Results of Cementless Total Hip Arthroplasty With Subtrochanteric Shortening Osteotomy in Crowe Type IV Developmental Dysplasia. J. Arthroplast. 2017, 32, 1211–1219. [Google Scholar] [CrossRef]
- Krych, A.J.; Howard, J.L.; Trousdale, R.T.; Cabanela, M.E.; Berry, D.J. Total Hip Arthroplasty with Shortening Subtrochanteric Osteotomy in Crowe Type-IV Developmental Dysplasia. J. Bone Jt. Surg. 2009, 91, 2213–2221. [Google Scholar] [CrossRef]
- Zhu, B.; Su, C.; He, Y.; Chai, X.; Li, Z.; Hou, Z.; Lou, T.; Yan, X. Combined Anteversion Technique in Total Hip Arthroplasty for Crowe IV Developmental Dysplasia of the Hip. HIP Int. 2017, 27, 589–594. [Google Scholar] [CrossRef]
- Shi, X.-t.; Li, C.-f.; Cheng, C.-m.; Feng, C.-y.; Li, S.-x.; Liu, J.-g. Preoperative Planning for Total Hip Arthroplasty for Neglected Developmental Dysplasia of the Hip. Orthop. Surg. 2019, 11, 348–355. [Google Scholar] [CrossRef]
- Yang, S.; Cui, Q. Total Hip Arthroplasty in Developmental Dysplasia of the Hip: Review of Anatomy, Techniques and Outcomes. World J. Orthop. 2012, 3, 42–48. [Google Scholar] [CrossRef]
- Edwards, B.N.; Tullos, H.S.; Noble, P.C. Contributory Factors and Etiology of Sciatic Nerve Palsy in Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 1987, 218, 136–141. [Google Scholar] [CrossRef]
- Kawai, T.; Tanaka, C.; Kanoe, H. Total Hip Arthroplasty for Crowe IV Hip without Subtrochanteric Shortening Osteotomy—A Long Term Follow up Study. BMC Musculoskelet. Disord. 2014, 15, 72. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pre-Operative Mean (Range) | Post-Operative Mean (Range) | p-Value |
|---|---|---|---|
| Harris Hip Score | 44.6 (38–56) | 89.4 (82–96) | <0.001 |
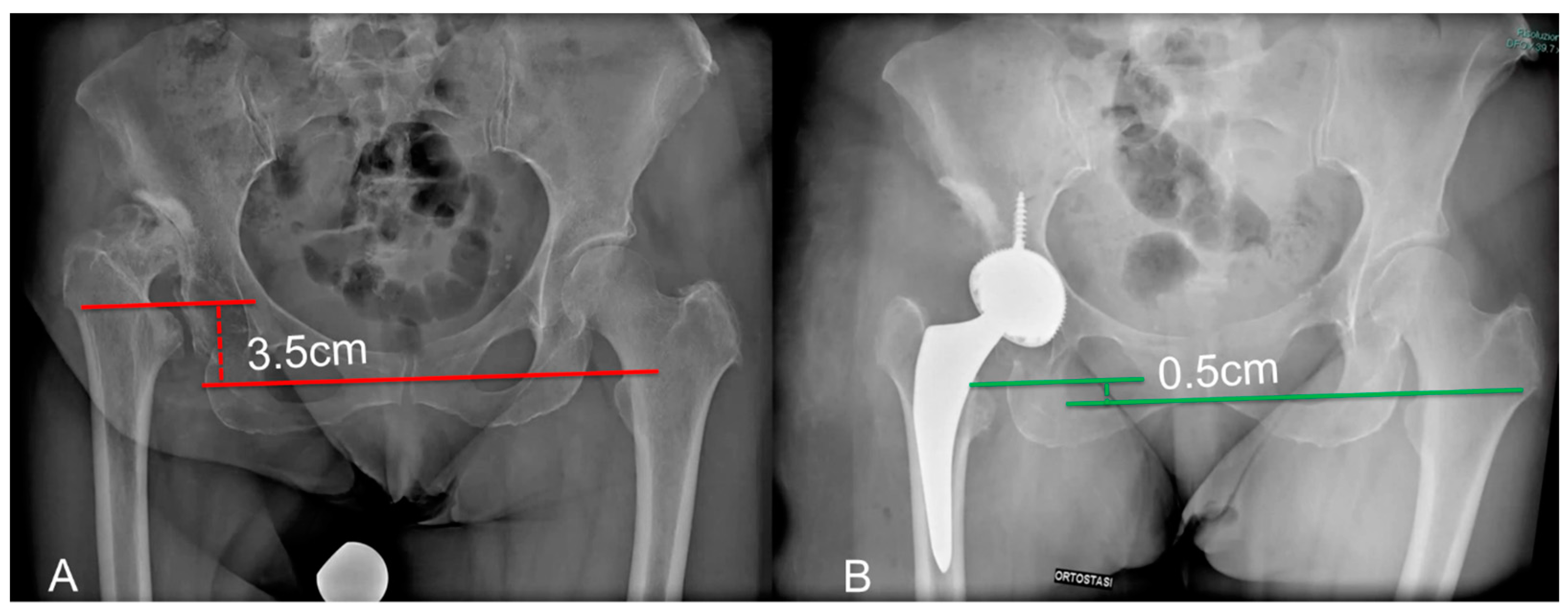
| Apparent LLD (cm) | 3.5 (2.5–4.3) | 1.2 (0.5–2.4) | <0.001 |
| COR (vertical distance, cm) | 4.6 (2.8–6.6) | 1.9 (1.1–2.4) | <0.002 |
| COR (horizontal distance, cm) | 4.4 (4–4.8) | 2.4 (1.3–3.4) | <0.0003 |
| True LLD (cm) | 3.4 (1.6–4) | 0.8 (0.3–1.1) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faldini, C.; Tassinari, L.; Pederiva, D.; Rossomando, V.; Brunello, M.; Pilla, F.; Geraci, G.; Traina, F.; Di Martino, A. Direct Anterior Approach in Total Hip Arthroplasty for Severe Crowe IV Dysplasia: Retrospective Clinical and Radiological Study. Medicina 2024, 60, 114. https://doi.org/10.3390/medicina60010114
Faldini C, Tassinari L, Pederiva D, Rossomando V, Brunello M, Pilla F, Geraci G, Traina F, Di Martino A. Direct Anterior Approach in Total Hip Arthroplasty for Severe Crowe IV Dysplasia: Retrospective Clinical and Radiological Study. Medicina. 2024; 60(1):114. https://doi.org/10.3390/medicina60010114
Chicago/Turabian StyleFaldini, Cesare, Leonardo Tassinari, Davide Pederiva, Valentino Rossomando, Matteo Brunello, Federico Pilla, Giuseppe Geraci, Francesco Traina, and Alberto Di Martino. 2024. "Direct Anterior Approach in Total Hip Arthroplasty for Severe Crowe IV Dysplasia: Retrospective Clinical and Radiological Study" Medicina 60, no. 1: 114. https://doi.org/10.3390/medicina60010114
APA StyleFaldini, C., Tassinari, L., Pederiva, D., Rossomando, V., Brunello, M., Pilla, F., Geraci, G., Traina, F., & Di Martino, A. (2024). Direct Anterior Approach in Total Hip Arthroplasty for Severe Crowe IV Dysplasia: Retrospective Clinical and Radiological Study. Medicina, 60(1), 114. https://doi.org/10.3390/medicina60010114










