Current Concepts in Diagnosis and Management of Patients Undergoing Total Hip Replacement with Concurrent Disorders of Spinopelvic Anatomy: A Narrative Review
Abstract
:1. Introduction
2. Nomenclature
3. Management Algorithms
4. Conclusions
5. Case Example
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrer, S.; Magnuson, J.A.; Toci, G.R.; Star, A.M.; Saxena, A. Bibliometric Analysis of Spinopelvic Alignment in Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2023, 7, e22.00182. [Google Scholar] [CrossRef]
- Innmann, M.M.; McGoldrick, N.P.; Ratra, A.; Merle, C.; Grammatopoulos, G. The Accuracy in Determining Pelvic Tilt from Anteroposterior Pelvic Radiographs in Patients Awaiting Hip Arthroplasty. J. Orthop. Res. 2022, 40, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Schwab, F.; Patel, A.; Demakakos, J.; Moal, B.; Farcy, J.P.; Lafage, V. Sacro-Femoral-Pubic Angle: A Coronal Parameterto Estimate Pelvic Tilt. Eur. Spine J. 2012, 21, 719–724. [Google Scholar] [CrossRef] [PubMed]
- McKnight, B.M.; Trasolini, N.A.; Dorr, L.D. Spinopelvic Motion and Impingement in Total Hip Arthroplasty. J. Arthroplast. 2019, 34, S53–S56. [Google Scholar] [CrossRef] [PubMed]
- Van Der Gronde, B.A.T.D.; Schlösser, T.P.C.; Van Erp, J.H.J.; Snijders, T.E.; Castelein, R.M.; Weinans, H.; De Gast, A. Current Evidence for Spinopelvic Characteristics Influencing Total Hip Arthroplasty Dislocation Risk. JBJS Rev. 2022, 10, e22. [Google Scholar] [CrossRef]
- Lazennec, J.-Y.; Brusson, A.; Rousseau, M.-A. Hip-Spine Relations and Sagittal Balance Clinical Consequences. Eur. Spine J. 2011, 20 (Suppl. S5), 686–698. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, J.-Y.; Charlot, N.; Gorin, M.; Roger, B.; Arafati, N.; Bissery, A.; Saillant, G. Hip-Spine Relationship: A Radio-Anatomical Study for Optimization in Acetabular Cup Positioning. Surg. Radiol. Anat. 2004, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Lum, Z.C.; Coury, J.G.; Cohen, J.L.; Dorr, L.D. The Current Knowledge on Spinopelvic Mobility. J. Arthroplast. 2018, 33, 291–296. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, Z.; Dorr, L.D. Quantification of Pelvic Tilt in Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 571–575. [Google Scholar] [CrossRef]
- Kanawade, V.; Dorr, L.D.; Wan, Z. Predictability of Acetabular Component Angular Change with Postural Shift from Standing to Sitting Position. J. Bone Jt. Surg. 2014, 96, 978–986. [Google Scholar] [CrossRef]
- Abdel, M.P.; von Roth, P.; Jennings, M.T.; Hanssen, A.D.; Pagnano, M.W. What Safe Zone? The Vast Majority of Dislocated THAs Are Within the Lewinnek Safe Zone for Acetabular Component Position. Clin. Orthop. Relat. Res. 2016, 474, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, J.Y.; Thauront, F.; Robbins, C.B.; Pour, A.E. Acetabular and Femoral Anteversions in Standing Position Are Outside the Proposed Safe Zone After Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 3550–3556. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, D.C.; Schwarzkopf, R.; Buckland, A.J.; Paulino, C.B.; Vigdorchik, J.M. Prosthetic Dislocation and Revision After Primary Total Hip Arthroplasty in Lumbar Fusion Patients: A Propensity Score Matched-Pair Analysis. J. Arthroplast. 2017, 32, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Malkani, A.L.; Garber, A.T.; Ong, K.L.; Dimar, J.R.; Baykal, D.; Glassman, S.D.; Cochran, A.R.; Berry, D.J. Total Hip Arthroplasty in Patients With Previous Lumbar Fusion Surgery: Are There More Dislocations and Revisions? J. Arthroplast. 2018, 33, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- An, V.V.G.; Phan, K.; Sivakumar, B.S.; Mobbs, R.J.; Bruce, W.J. Prior Lumbar Spinal Fusion Is Associated With an Increased Risk of Dislocation and Revision in Total Hip Arthroplasty: A Meta-Analysis. J. Arthroplast. 2018, 33, 297–300. [Google Scholar] [CrossRef]
- Esposito, C.I.; Carroll, K.M.; Sculco, P.K.; Padgett, D.E.; Jerabek, S.A.; Mayman, D.J. Total Hip Arthroplasty Patients With Fixed Spinopelvic Alignment Are at Higher Risk of Hip Dislocation. J. Arthroplast. 2018, 33, 1449–1454. [Google Scholar] [CrossRef]
- Innmann, M.M.; Merle, C.; Phan, P.; Beaulé, P.E.; Grammatopoulos, G. How Can Patients With Mobile Hips and Stiff Lumbar Spines Be Identified Prior to Total Hip Arthroplasty? A Prospective, Diagnostic Cohort Study. J. Arthroplast. 2020, 35, S255–S261. [Google Scholar] [CrossRef]
- Innmann, M.M.; Merle, C.; Phan, P.; Beaulé, P.E.; Grammatopoulos, G. Response to Letter to the Editor on “How Can Patients With Mobile Hips and Stiff Lumbar Spines Be Identified Prior to Total Hip Arthroplasty?—A Prospective, Diagnostic Cohort Study”. J. Arthroplast. 2021, 36, e9–e10. [Google Scholar] [CrossRef]
- Heckmann, N.; McKnight, B.; Stefl, M.; Trasolini, N.A.; Ike, H.; Dorr, L.D. Late Dislocation Following Total Hip Arthroplasty: Spinopelvic Imbalance as a Causative Factor. J. Bone Jt. Surg. Am. 2018, 100, 1845–1853. [Google Scholar] [CrossRef]
- Ike, H.; Dorr, L.D.; Trasolini, N.; Stefl, M.; McKnight, B.; Heckmann, N. Spine-Pelvis-Hip Relationship in the Functioning of a Total Hip Replacement. J. Bone Jt. Surg. Am. 2018, 100, 1606–1615. [Google Scholar] [CrossRef]
- Heckmann, N.D.; Lieberman, J.R. Spinopelvic Biomechanics and Total Hip Arthroplasty: A Primer for Clinical Practice. JAAOS-J. Am. Acad. Orthop. Surg. 2021, 29, e888–e903. [Google Scholar] [CrossRef] [PubMed]
- Debbi, E.M.; Quevedo González, F.J.; Jerabek, S.A.; Wright, T.M.; Vigdorchik, J.M. Three-Dimensional Functional Impingement in Total Hip Arthroplasty: A Biomechanical Analysis. J. Arthroplast. 2022, 37, S678–S684. [Google Scholar] [CrossRef]
- Miki, H.; Kyo, T.; Kuroda, Y.; Nakahara, I.; Sugano, N. Risk of Edge-Loading and Prosthesis Impingement Due to Posterior Pelvic Tilting after Total Hip Arthroplasty. Clin. Biomech. 2014, 29, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Li, J.; Wang, L.; Jin, Z.; Wilcox, R.; Fisher, J. Contact Mechanics of Modular Metal-on-Polyethylene Total Hip Replacement under Adverse Edge Loading Conditions. J. Biomech. 2014, 47, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.H.; Chiu, K.Y.; Tang, W.M. Review Article: Polyethylene Wear and Osteolysis in Total Hip Arthroplasty. J. Orthop. Surg. 2001, 9, 91–99. [Google Scholar] [CrossRef]
- Hua, X.; Li, J.; Jin, Z.; Fisher, J. The Contact Mechanics and Occurrence of Edge Loading in Modular Metal-on-Polyethylene Total Hip Replacement during Daily Activities. Med. Eng. Phys. 2016, 38, 518–525. [Google Scholar] [CrossRef]
- Jones, M.D.; Buckle, C.L. How Does Aseptic Loosening Occur and How Can We Prevent It? Orthop. Trauma 2020, 34, 146–152. [Google Scholar] [CrossRef]
- Hua, Z.; Yan, X.; Liu, D.; Jin, Z.; Wang, X.; Liu, L. Analysis of the Friction-Induced Squeaking of Ceramic-on-Ceramic Hip Prostheses Using a Pelvic Bone Finite Element Model. Tribol. Lett. 2016, 61, 26. [Google Scholar] [CrossRef]
- Zagra, L.; Benazzo, F.; Dallari, D.; Falez, F.; Solarino, G.; D’Apolito, R.; Castelli, C.C. Current Concepts in Hip–Spine Relationships: Making Them Practical for Total Hip Arthroplasty. EFORT Open Rev. 2022, 7, 59–69. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Martins, F.L.M.; Barbosa, M.C.S.A.; dos Santos, R.T. Manipulation and Selective Exercises Decrease Pelvic Anteversion and Low-Back Pain: A Pilot Study. J. Back Musculoskelet. Rehabil. 2013, 26, 33–36. [Google Scholar] [CrossRef]
- Mills, E.S.; Talehakimi, A.; Urness, M.; Wang, J.C.; Piple, A.S.; Chung, B.C.; Tezuka, T.; Heckmann, N.D. Anteroposterior Pelvic Radiograph Findings Correlate with Sagittal Spinopelvic Motion. Bone Jt. J. 2023, 105, 496–503. [Google Scholar] [CrossRef]
- Chai, Y.; Khadra, S.; Boudali, A.M.; Darwish, I.; Walter, W.L. Evaluating pelvic tilt using measurements from anteroposterior pelvic radiographs: A meta-analysis of sacro-femoral-pubic (sfp) method. Orthop. Proc. 2023, 105, 116. [Google Scholar] [CrossRef]
- Sharma, A.K.; Grammatopoulos, G.; Pierrepont, J.W.; Madurawe, C.S.; Innmann, M.M.; Vigdorchik, J.M.; Shimmin, A.J. Sacral Slope Change From Standing to Relaxed-Seated Grossly Overpredicts the Presence of a Stiff Spine. J. Arthroplast. 2023, 38, 713–718. [Google Scholar] [CrossRef]
- Pour, A.E.; Green, J.H.; Christensen, T.H.; Muthusamy, N.; Schwarzkopf, R. Arthroplasty Today Is It Necessary to Obtain Lateral Pelvic Radiographs in Flexed Seated Position for Preoperative Total Hip Arthroplasty Planning? Arthroplast. Today 2023, 21, 101133. [Google Scholar] [CrossRef]
- Behery, O.A.; Vasquez-Montes, D.; Cizmic, Z.; Vigdorchik, J.M.; Buckland, A.J. Can Flexed-Seated and Single-Leg Standing Radiographs Be Useful in Preoperative Evaluation of Lumbar Mobility in Total Hip Arthroplasty? J. Arthroplast. 2020, 35, 2124–2130. [Google Scholar] [CrossRef]
- Bodner, R.J.; Tezuka, T.; Heckmann, N.; Chung, B.; Jadidi, S. The Dorr Classification for Spinopelvic Functional Safe Component Positioning in Total Hip Replacement: A Primer for All. J. Orthop. Exp. Innov. 2022. [Google Scholar] [CrossRef]
- Grammatopoulos, G.; Falsetto, A.; Sanders, E.; Weishorn, J.; Gill, H.S.; Beaulé, P.E.; Innmann, M.M.; Merle, C. Integrating the Combined Sagittal Index Reduces the Risk of Dislocation Following Total Hip Replacement. J. Bone Jt. Surg.—Am. Vol. 2022, 104, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Dorr, L.D. CORR Insights(®): Does Degenerative Lumbar Spine Disease Influence Femoroacetabular Flexion in Patients Undergoing Total Hip Arthroplasty? Clin. Orthop. Relat. Res. 2016, 474, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Eftekhary, N.; Shimmin, A.; Lazennec, J.Y.; Buckland, A.; Schwarzkopf, R.; Dorr, L.D.; Mayman, D.; Padgett, D.; Vigdorchik, J. A Systematic Approach to the Hip-Spine Relationship and Its Applications to Total Hip Arthroplasty. Bone Jt. J. 2019, 101, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.P. How Much Hip Motion Is Used in Real-Life Activities? Assessment of Hip Flexion by a Wearable Sensor and Implications After Total Hip Arthroplasty. J. Arthroplast. 2022, 37, S871–S875. [Google Scholar] [CrossRef]
- Phan, D.; Bederman, S.S. The Influence of Sagittal Spinal Deformity on Anteversion of the Acetabular Component in Total Hip Arthroplasty. Bone Jt. J. 2015, 97, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Hardijzer, A.; Lazennec, J.-Y.; Beaulé, P.; Muirhead-Allwood, S.; Cobb, J. Spine-Hip Relations Add Understandings to the Pathophysiology of Femoro-Acetabular Impingement: A Systematic Review. Orthop. Traumatol. Surg. Res. 2017, 103, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Lazennec, J.-Y.; Van Der Straeten, C.; Auvinet, E.; Cobb, J.; Muirhead-Allwood, S. The Influence of Spine-Hip Relations on Total Hip Replacement: A Systematic Review. Orthop. Traumatol. Surg. Res. 2017, 103, 559–568. [Google Scholar] [CrossRef]
- Luthringer, T.A.; Vigdorchik, J.M. 2018 AAHKS Annual Meeting Symposium A Preoperative Workup of a “ Hip-Spine” Total Hip Arthroplasty Patient: A Simpli Fi Ed Approach to a Complex Problem. J Arthroplast. 2019, 34, S57–S70. [Google Scholar] [CrossRef]
- Vigdorchik, J.M.; Sharma, A.K.; Buckland, A.J.; Elbuluk, A.M.; Eftekhary, N.; Mayman, D.J.; Carroll, K.M.; Jerabek, S.A. 2021 Otto Aufranc Award: A Simple Hip-Spine Classification for Total Hip Arthroplasty: Validation and a Large Multicentre Series. Bone Jt. J. 2021, 103, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Heckmann, N.D.; Bodner, R.J.; Dorr, L.D. Functional Safe Zone Is Superior to the Lewinnek Safe Zone for Total Hip Arthroplasty: Why the Lewinnek Safe Zone Is Not Always Predictive of Stability. J. Arthroplast. 2019, 34, 3–8. [Google Scholar] [CrossRef]
- Heckmann, N.; Tezuka, T.; Bodner, R.J.; Dorr, L.D. Functional Anatomy of the Hip Joint. J. Arthroplast. 2023, 36, 374–378. [Google Scholar] [CrossRef]
- Vigdorchik, J.M.; Sharma, A.K.; Elbuluk, A.M.; Carroll, K.M.; Mayman, D.J.; Lieberman, J.R. High Offset Stems Are Protective of Dislocation in High-Risk Total Hip Arthroplasty. J. Arthroplast. 2021, 36, 210–216. [Google Scholar] [CrossRef]
- Dhawan, R.; Baré, J.V.; Shimmin, A. Modular Dual-Mobility Articulations in Patients with Adverse Spinopelvic Mobility. Bone Jt. J. 2022, 104, 820–825. [Google Scholar] [CrossRef]
- Haffer, H.; Adl Amini, D.; Perka, C.; Pumberger, M. The Impact of Spinopelvic Mobility on Arthroplasty: Implications for Hip and Spine Surgeons. J. Clin. Med. 2020, 9, 2569. [Google Scholar] [CrossRef]
- Grammatopoulos, G.; Alvand, A.; Monk, A.P.; Mellon, S.; Pandit, H.; Rees, J.; Gill, H.S.; Murray, D.W. Surgeons’ Accuracy in Achieving Their Desired Acetabular Component Orientation. J. Bone Jt. Surg.—Am. Vol. 2016, 98, e72. [Google Scholar] [CrossRef] [PubMed]
- Babisch, J.W.; Layher, F.; Amiot, L.-P. The Rationale for Tilt-Adjusted Acetabular Cup Navigation. J. Bone Jt. Surg. Am. 2008, 90, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Molho, D.A.; Kuether, J.P.; Rubin, L.E. Anatomic Navigation Using the Transverse Acetabular Ligament for Acetabular Component Positioning in Total Hip Arthroplasty Through the Direct Anterior Approach. J. Am. Acad. Orthop. Surg. 2022, 30, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Cambias, J.; Cleary, K.; Daimler, E.; Drake, J.; Dupont, P.E.; Hata, N.; Kazanzides, P.; Martel, S.; Patel, R.V.; et al. Medical Robotics—Regulatory, Ethical, and Legal Considerations for Increasing Levels of Autonomy. Sci. Robot. 2017, 2, eaam8638. [Google Scholar] [CrossRef]
- Fleiderman Valenzuela, J.G.; Cirillo Totera, J.I.; Turkieltaub, D.H.; Echaurren, C.V.; Álvarez Lemos, F.L.; Arriagada Ramos, F.I. Spino-Pelvic Radiological Parameters: Comparison of Measurements Obtained by Radiologists Using the Traditional Method versus Spine Surgeons Using a Semi-Automated Software (Surgimap). Acta Radiol. Open 2023, 12, 20584601231177404. [Google Scholar] [CrossRef]
- Helmya, N.A.; El-Sayyadb, M.M.; Kattabeib, O.M. Intra-Rater and Inter-Rater Reliability of Surgimap Spine Software for Measuring Spinal Postural Angles from Digital Photographs. Bull. Fac. Phys. Ther. 2015, 20, 193–199. [Google Scholar] [CrossRef]
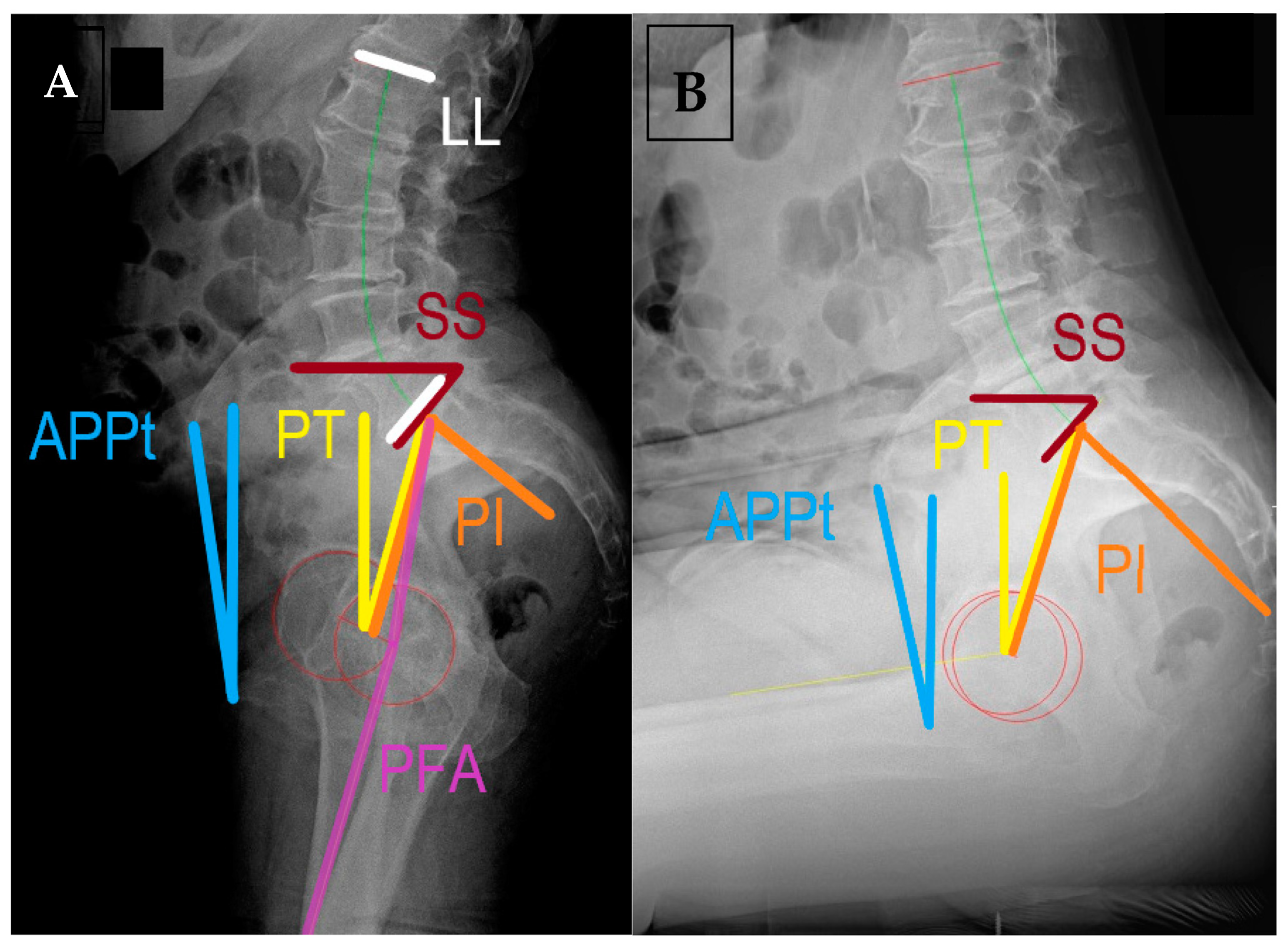
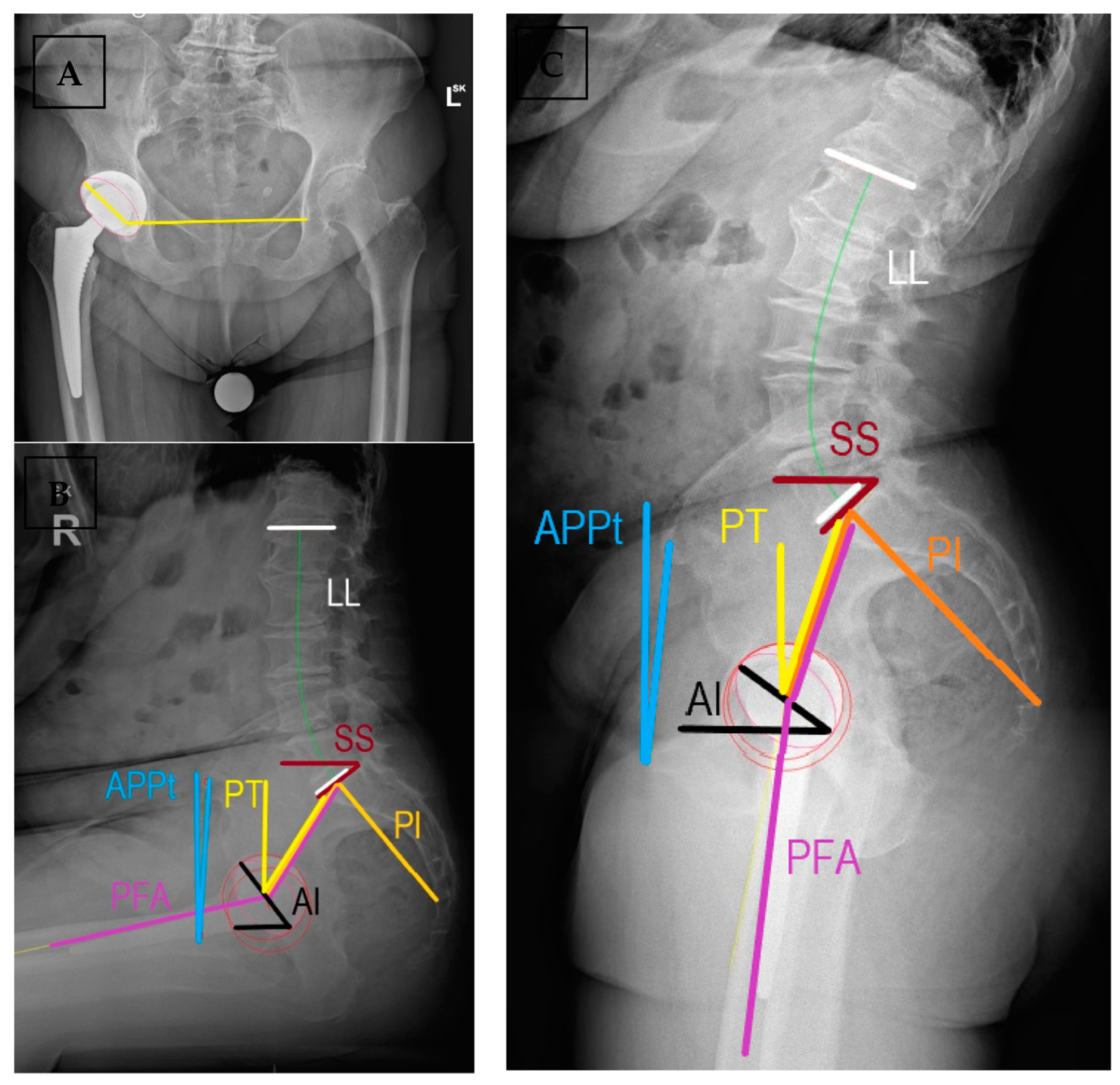
| Term | Definition | Relevance | Normal Values |
|---|---|---|---|
| Pelvic incidence (PI) | Angle between the perpendicular to the midpoint of sacral plate (S1) and the line connecting it to the center of the bicoxofemoral axis | Represents the relative anatomic position of the hip joint to the sacrum. | 40°–65° |
| Lumbar lordosis angle (LL) | Angle between the superior plate of L1 to the sacral end plate (S1) | Compensatory to pelvic morphology and position. | Within 10° of PI |
| Sacral slope (SS) | Angle of the sacral end plate (S1) and the horizontal line | Preferred parameter to assess spinopelvic motion, related to PI and PT | SSstanding > 30° OR 0.75 × PI SSsitting 5°–30° |
| Anterior pelvic plane tilt (APPt) | Functional pelvic plane as a triangle formed by ASIS and pubic symphysis relative to the vertical line | Used for pelvic tilt in arthroplasty literature, describes the rotation of pelvis in the sagittal plane | 0 or slightly anteverted in standing retroverted in sitting |
| Pelvic tilt (PT) | Angle formed by the line from bicoxofemoral axis to the midpoint of S1 and a vertical line | It describes the position of the femoral heads to the base of the spine, related to SS and PI | PTstanding < 22° ΔPT ≈ 20° PI = SS + PT |
| Pelvic femoral angle (PFA) | Angle between the line connecting midpoint of S1 endplate with the center of the measured femoral head and femoral mechanical axis | Assesses flexion deformity and femoral motion. Does not change post-THR (≈3°) | ΔPFA 55°–75° PFAstanding 180–190 PFAsitting 120–130 Proportionality with PI |
| Acetabular anteinclination (AI) | Angle between the long axis of the cup and the horizontal on lateral radiographs | Sagittal plane orientation of the acetabular cup, represents anteversion | AIstanding 25°–45° AIsitting 45°–65° Surgeon dependent |
| PI-LL mismatch | Difference between PI and LL angle | Compensatory ability of lumbosacral spine to changes in pelvic tilt; sagittal balance | <10 in standing lateral radiographs |
| Combined sagittal index (CSI) | CSI = PFA + AI | Validated predictor for acute and late dislocations in postoperative assessment. Possible to plan AI based on PFA | CSIstanding 205°–245° If low PI, sagittal imbalance, stiffness: range 215°–235° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrus, R.; Douša, P.; Almási, J.; Šteňo, B. Current Concepts in Diagnosis and Management of Patients Undergoing Total Hip Replacement with Concurrent Disorders of Spinopelvic Anatomy: A Narrative Review. Medicina 2023, 59, 1591. https://doi.org/10.3390/medicina59091591
Ambrus R, Douša P, Almási J, Šteňo B. Current Concepts in Diagnosis and Management of Patients Undergoing Total Hip Replacement with Concurrent Disorders of Spinopelvic Anatomy: A Narrative Review. Medicina. 2023; 59(9):1591. https://doi.org/10.3390/medicina59091591
Chicago/Turabian StyleAmbrus, Richard, Pavel Douša, Jozef Almási, and Boris Šteňo. 2023. "Current Concepts in Diagnosis and Management of Patients Undergoing Total Hip Replacement with Concurrent Disorders of Spinopelvic Anatomy: A Narrative Review" Medicina 59, no. 9: 1591. https://doi.org/10.3390/medicina59091591
APA StyleAmbrus, R., Douša, P., Almási, J., & Šteňo, B. (2023). Current Concepts in Diagnosis and Management of Patients Undergoing Total Hip Replacement with Concurrent Disorders of Spinopelvic Anatomy: A Narrative Review. Medicina, 59(9), 1591. https://doi.org/10.3390/medicina59091591







