Assessment of the Phytochemical Profile, Antioxidant Capacity, and Hepatoprotective Effect of Andrographis paniculata against CCl4-Induced Liver Dysfunction in Wistar Albino Rats
Abstract
1. Introduction
2. Methods and Material
2.1. Plant Material
2.2. Animal Handling
2.3. Plant Extraction Method
2.4. Phytochemical Analysis
2.4.1. Qualitative Analysis of Phytoconstituents
2.4.2. Estimation of Flavonoids
2.4.3. Estimation of Tannin
2.4.4. Estimation of Terpenoids
2.4.5. Estimation of Steroids
2.4.6. Estimation of Alkaloids
2.4.7. Estimation of Quinone
2.4.8. Quantitative Estimation of Phenolic and Flavonoid Content
2.4.9. Antioxidant Activity of Different Extracts
- Xc = Absorbance of control;
- Xs = Absorbance of the sample.
2.5. Hepatoprotective Role of Ethanol Extracts of Leaves
2.6. GC-MS Analysis
2.7. Statistical Analysis
3. Results
3.1. Qualitative Assessment of Phytochemicals
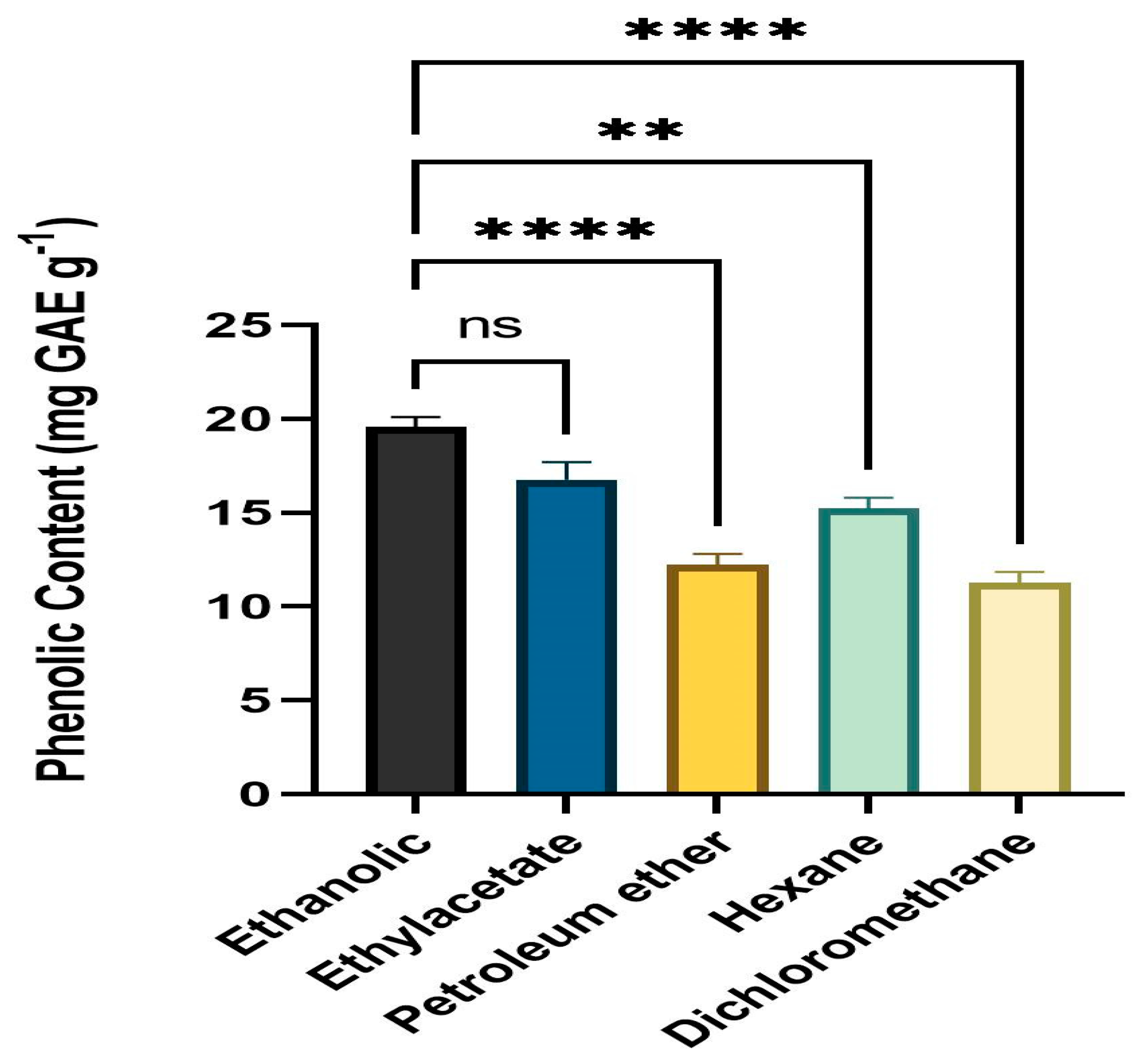
3.2. Quantitative Assessment of Phenolic Content
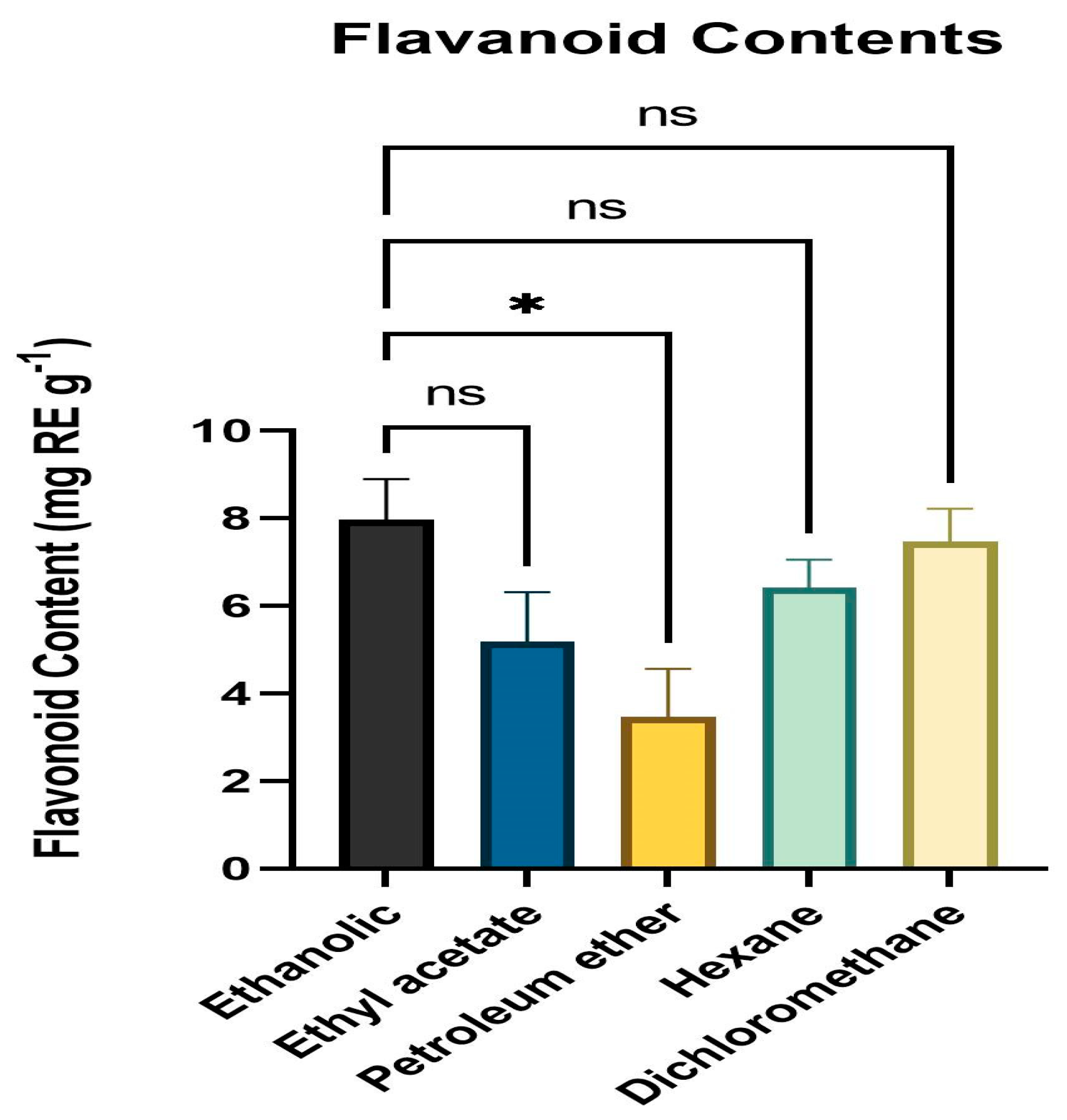
3.3. Quantitative Assessment of Flavonoid Content
3.4. Antioxidant Potential
3.5. Hepatoprotective Role of Ethanolic Extracts
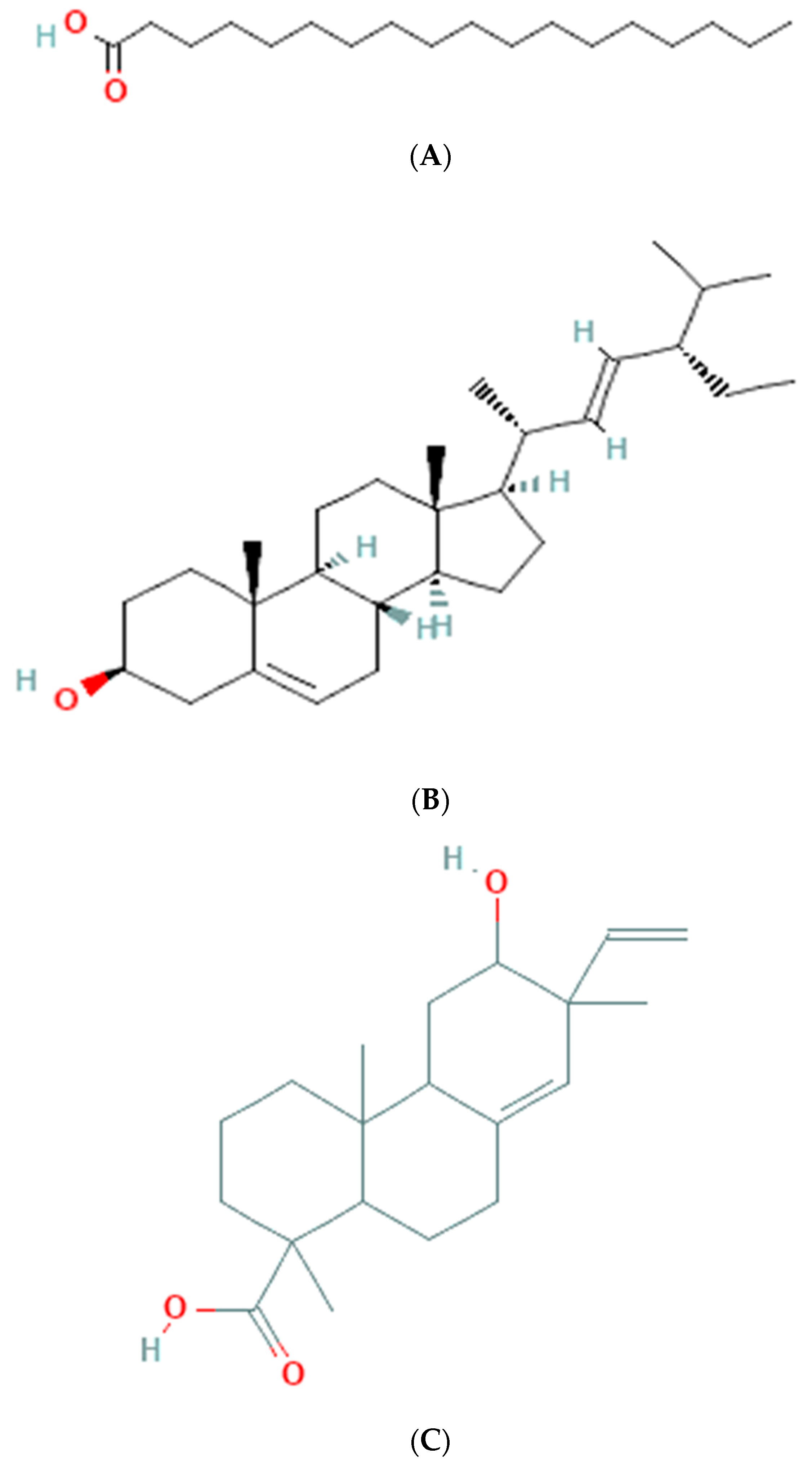
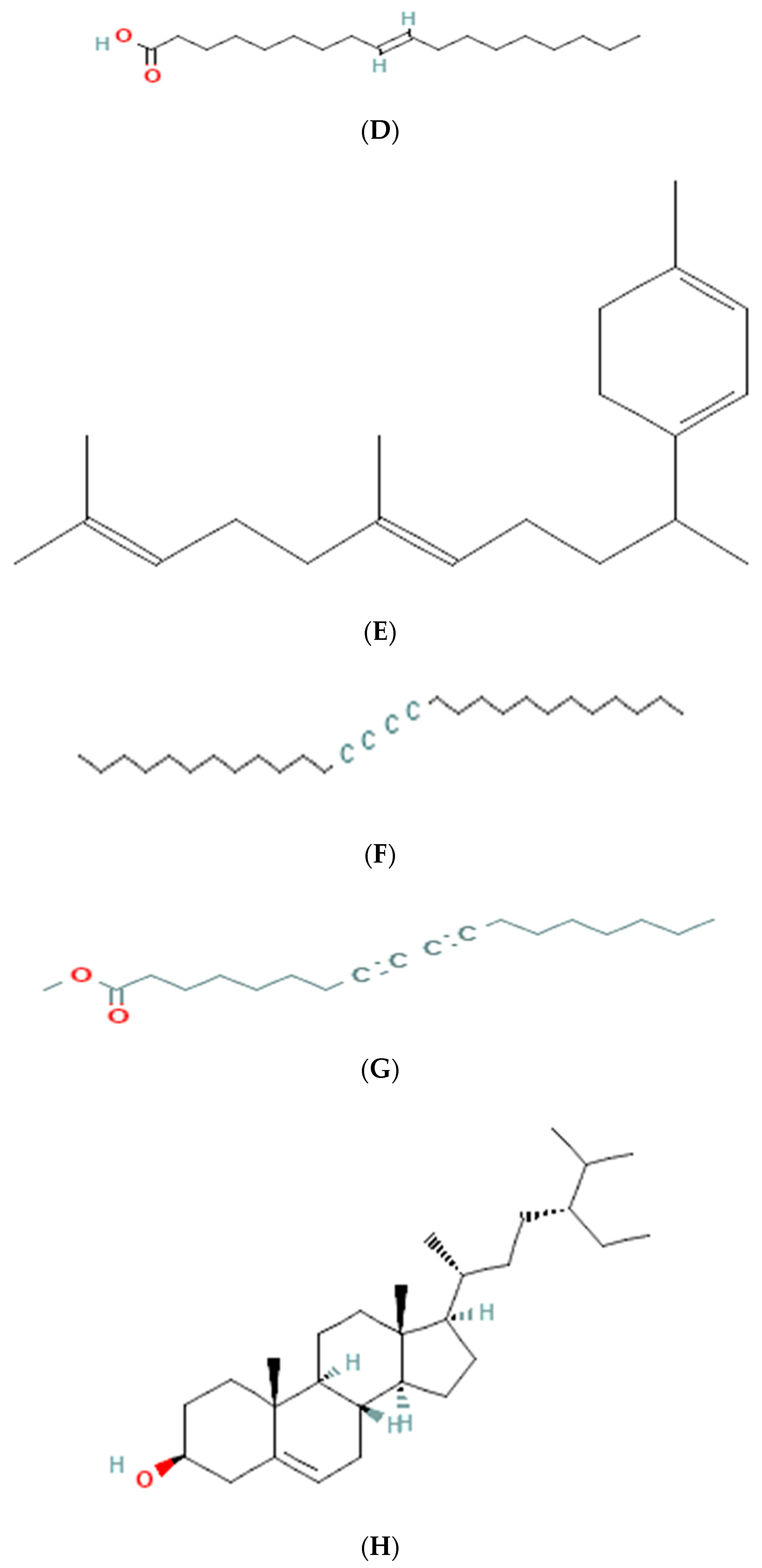
3.6. GC-MS Analysis of Ethanolic Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Monographs on Selected Medicinal Plants; World Health Organization: Ravello-Salerno, Italy, 1999; Volume 2.
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.; Gomez, F.J.; Espino, M.; Boiteux, J.; Fernández, M.D.; Silva, M.F.; Dias, F.D. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem. J. 2022, 2022, 107184. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Zabermawi, N.M.; Zabermawi, N.M.; Burollus, M.A.; Shafi, M.E.; Alagawany, M.; Yehia, N.; Askar, A.M.; Alsafy, S.A.; Noreldin, A.E.; et al. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: A review. Food Rev. Int. 2021, 39, 1–23. [Google Scholar] [CrossRef]
- Shankar, J.; Satyabrata, M.; Kumar, P. Seedling raisingtechniques of medicinal herb kalmegh. Directorate ofmedicinal and aromatic plants. Res. Boriavi Anand Gujrat India 2012, 25, 338–342. [Google Scholar]
- Sanjutha, S.; Subramanian, S.; Rani, I.M. Integrated nutrient management in Andrographispaniculata. Res. J. Agric. Biol. Sci. 2008, 4, 141–145. [Google Scholar]
- Zhao, Y.; Kao, C.-P.; Wu, K.-C.; Liao, C.-R.; Ho, Y.-L.; Chang, Y.-S. Chemical Compositions, Chromatographic Fingerprints and Antioxidant Activities of Andrographis Herba. Molecules 2014, 19, 18332–18350. [Google Scholar] [CrossRef]
- Sharma, A.; Lal, K.; Handa, S.S. Standardization of the Indian crude drug Kalmegh by high pressure liquid chromatographic determination of andrographolide. Phytochem. Anal. 1992, 3, 129–131. [Google Scholar] [CrossRef]
- Salsabila, S.; Salam, D.A.; Ishak, S.S.O. Development of analysis method of andrographolide from Andrographis paniculata using UPLC-PDA. Curr. Res. Bioscences Biotechnol. 2023, 4, 283–288. [Google Scholar] [CrossRef]
- Patil, R.; Jain, V. Andrographolide: A Review of Analytical Methods. J. Chromatogr. Sci. 2021, 59, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis paniculata (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.A.; Ramli, F.; Wahab, R. Antioxidant Activity of Andrographolide from Andrographis panicu-lata leaf and Its Extraction Optimization by using Accelerated Solvent Extraction: Antioxidant Activity of Andro-grapholide from Andrographis paniculata leaf. J. Trop. Life Sci. 2023, 13, 157–170. [Google Scholar]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- Gupta, D.; Kumar, M. Evaluation of in vitro antimicrobial potential and GC–MS analysis of Camellia sinensis and Terminalia arjuna. Biotechnol. Rep. 2017, 13, 19–25. [Google Scholar] [CrossRef]
- Dulara, B.K.; Godara, P.; Barwer, N. In-vivo and In-vitro phytochemical GC-MS analysis of volatile con-stituents of Andrographis paniculata (Burm. f.) Nees. Pharma Innov. J. 2019, 8, 255–261. [Google Scholar]
- Antony, A.; Nagella, P. Heavy metal stress influence the andrographolide content, phytochemicals and antioxidant activity of Andrographis paniculata. Plant Sci. Today 2021, 8, 324–330. [Google Scholar] [CrossRef]
- Mundada, P.; Gurme, S.; Jadhav, S.; Patil, D.; Gore, N.; Shaikh, S.; Mali, A.; Umdale, S.; Ahire, M. Andrographis paniculata (Creat or Green Chiretta) and Bacopa monnieri (Water Hyssop). In Herbs, Shrubs, and Trees of Potential Medicinal Benefits; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2022; pp. 27–44. [Google Scholar] [CrossRef]
- Brindha, P.; Sasikala, B.; Prushothaman, K.K. Pharmacological studies on Merugan kizhangu. Bull. Med. Etho Baotanical Res. 1981, 3, 84–96. [Google Scholar]
- Alam, M.F.; Safhi, M.M.; Anwer, T.; Siddiqui, R.; Khan, G.; Moni, S.S. Therapeutic potential of Vanillylacetone against CCl4 induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines and apoptosis in Swiss albino mice. Exp. Mol. Pathol. 2018, 105, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.; Devi, A.F.; Syafitri, U.D.; Heryanto, R.; Suparto, I.H.; Amran, M.B.; Rohman, A.; Prajogo, B.; Lim, L.W. Classification of Andrographis paniculata extracts by solvent extraction using HPLC fingerprint and chemometric analysis. BMC Res. Notes 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Saini, V.K.; Raja, W. Evaluation of phytochemical analysis of Andrographis paniculata leaf and stem extract. World J. Pharm. Life Sci. 2019, 5, 188–190. [Google Scholar]
- Rajesh, K.D.; Vasantha, S.; Rajesh, N.V.; Panneerselvam, A. Qualitative and quantitative phytochemical analysis in four pteridophytes. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 408–412. [Google Scholar]
- Verma, V.K.; Sarwa, K.K.; Kumar, A.; Zaman, K. Comparison of hepatoprotective activity of Swertia chirayita and Andrographis paniculata plant of North–East India against CCl4 induced hepatotoxic rats. J. Pharm. Res. 2013, 7, 647–653. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for determination of serum glutamate oxaloacetate and glutamic py-ruvate transaminase. Am. J. Clin. Path. 1957, 28, e56–e58. [Google Scholar] [CrossRef]
- Kind, P.R.N.; King, E.J. Estimation of Plasma Phosphatase by Determination of Hydrolysed Phenol with Amino-antipyrine. J. Clin. Pathol. 1954, 7, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Mallay, H.T.; Evelyn, K.A. Estimation of serum bilirubin level with the photoelectric colorimeter. J. Biol. Chem. 1951, 173, e265–e275. [Google Scholar]
- Szaszi, G. A kinetic photometric method for serum gammaglutamyl transpeptidase. Clin. Chem. 1969, 15, e124–e126. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tarachand, U. Decreased lipid peroxidation in the rat kidney during gestation. Biochem. Biophys. Res. Commun. 1987, 145, 134–138. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Shalini, V.B.; Narayanan, J.S. Characterization studies on medicinal plant of Andrographis paniculata (NEES). J. Med. Plants 2015, 3, 96–102. [Google Scholar]
- Wenzig, E.; Widowitz, U.; Kunert, O.; Chrubasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Kayaoglu, G.; Ömürlü, H.; Akca, G.; Gürel, M.; Gençay, Ö.; Sorkun, K.; Salih, B. Antibacterial activity of Propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tu-bules. J. Endod. 2011, 37, 376–381. [Google Scholar] [CrossRef]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical profile and biological activity of en-demic Sideritis sipylea Boiss. in North Aegean Greek islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef]
- Arora, S.; Kumar, G. Phytochemical screening of root, stem and leaves of Cenchrus biflorus Roxb. J. Pharmacogn. Phytochem. 2018, 7, 1445–1450. [Google Scholar]
- Das, P.; Srivastav, A.K. Phytochemical extraction and characterization of the leaves of Andrographis pa-niculata for its anti-bacterial, anti-oxidant, anti-pyretic and anti-diabetic activity. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15176–15184. [Google Scholar] [CrossRef]
- Tiwari, V. Phytochemical analysis and medicinal value of Kalmegh (Andrographis paniculata Nees). Indian J. Appl. Pure Biol. 2017, 32, 283–288. [Google Scholar]
- Chao, W.-W.; Lin, B.-F. Hepatoprotective Diterpenoids Isolated from Andrographis paniculata. Chin. Med. 2012, 3, 136–143. [Google Scholar] [CrossRef]
- Nagalekshmi, R.; Menon, A.; Chandrasekharan, D.K.; Nair, C.K.K. Hepatoprotective activity of An-drographis paniculata and Swertia chirayita. Food Chem. Toxicol. 2011, 49, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.K.; Gothalwal, R. Evaluation of Hepatoprotective action of Andrographis paniculata in in-vitro cultured hepatocytes. Int. J. Curr. Biotechnol. 2015, 3, 7–10. [Google Scholar]
- Jahangir, T.; Safhi, M.M.; Alam, M.F. Pomegranate juiceattenuate the acetamino-phen induced hepatotoxicity in rat model of experiment. Adv. Life Sci. 2014, 2, 5–9. [Google Scholar]
- Chellappann, D.K. Hepatoprotec-tive effects of aqueous extract of Andrographis panniculataagainst CCL4 induced hepatotoxicity in albino wistar rats. Asian J. Pharm. Clin. Res. 2011, 4, 93–94. [Google Scholar]
| Phytoconstituents | Organic Solvent Extracts | ||||
|---|---|---|---|---|---|
| Ethanol | Ethyl Acetate | Petroleum Ether | Hexane | DCM | |
| Flavonoids | + | + | + | + | + |
| Tannin | + | − | − | − | + |
| Terpenoids | + | + | − | + | + |
| Steroids | + | − | − | − | − |
| Alkaloids | + | + | + | + | + |
| Quinone | + | − | − | − | − |
| Phenolic compounds | + | + | + | + | + |
| Solvent Fractions | Concentrations of Extracts (µg mL−1) | ||||
|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | |
| Ethanolic | 23.2 ± 3.2 | 43.1 ± 3.2 | 50.2 ± 1.3 | 55.5 ± 4.2 | 62.3 ± 2.3 |
| Ethyl acetate | 12.3 ± 2.1 | 23.3 ± 3.2 | 33.34 ± 2.4 | 42.2 ± 3.2 | 51.2 ± 4.2 |
| Petroleum ether | 9.2 ± 3.1 | 16.23 ± 4.2 | 22.34 ± 4.2 | 32.3 ± 4.1 | 45.32 ± 2.3 |
| Hexane | 16.3 ± 2.3 | 33.23 ± 1.5 | 42.12 ± 3.2 | 52.12 ± 4.1 | 61.4 ± 1.5 |
| DCM | 10.23 ± 2.1 | 18.34 ± 3.9 | 28.12 ± 3.1 | 39.2 ± 3.2 | 51.23 ± 1.3 |
| Biochemical Parameters | Animal Groups | |||
|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | |
| Vehicle Control (0.0 mg/kg b.w) | Leaf Ethanol Extract (300 mg/kg b.w) | CCl4 (0.1 mL/kg b.w) | Leaf Extract + CCl4 | |
| SGPT (IU/L) | 30.12 ± 3.1 | 35.23 ± 4.2 ns | 121.3 ± 4.5 a | 55.23 ± 1.5 b |
| SGOT (IU/L) | 41.2 ± 6.2 | 48.23 ± 4.9 ns | 119.12 ± 5.8 a | 52.23 ± 7.2 b |
| ALP (IU/L) | 9.8 ± 2.3 | 12.22 ± 4.3 ns | 38.23 ± 5.1 a | 18.23 ± 5.7 b |
| Total bilirubin (mg/dL) | 0.32 ± 0.02 | 0.41 ± 0.02 ns | 6.23 ± 0.02 a | 0.67 ± 0.02 b |
| Total protein (mg/dL) | 6.23 ± 1.2 | 6.78 ± 2.2 ns | 4.23 ± 1.4 a | 7.12 ± 2.1 b |
| GGTP (µ/L) | 71.23 ± 4.6 | 81.23 ± 5.3 ns | 160.34 ± 7.2 a | 90.23 ± 1.3 b |
| LPO (nM/mg protein) | 2.33 ± 0.03 | 2.44 ± 0.02 ns | 6.12 ± 0.05 a | 2.98 ± 0.03 b |
| Catalase (U/mg protein) | 32.12 ± 2.1 | 30.32 ± 1.9 ns | 14.23 ± 1.4 a | 27.23 ± 1.7 b |
| Peak No | R. Time | Area % | Molecular Weight | Molecular Formula | Name of the Compounds |
|---|---|---|---|---|---|
| 1 | 18.122 | 1.21 | 284 | C18H36O2 | octadecanoic acid |
| 2 | 32.211 | 3.23 | 412 | C29H48O | stigmasterol |
| 3 | 29.162 | 19.12 | 318 | C20H30O3 | phenanthrenecarboxylic acid, 7-ethenyl |
| 4 | 16.212 | 6.32 | 282 | C18H34O2 | 1-9-octadecenoic acid |
| 5 | 27.312 | 2.12 | 272 | C20H32 | geranyl-α-terpinene |
| 6 | 24.132 | 5.23 | 386 | C28H50 | 13,15-octacosadiyne |
| 7 | 27.132 | 1.23 | 290 | C19H30O2 | methyl 8,10-octadecadiynoate |
| 8 | 32.253 | 7.54 | 414 | C29H50O | stigmast-5-en-3-ol (3β) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.K.; Makeen, H.A.; Khuwaja, G.; Alhazmi, H.A.; Sharma, M.; Koty, A.; Mazahirul, I.; Parveen, H.; Mohammed, A.; Mukhtar, S.; et al. Assessment of the Phytochemical Profile, Antioxidant Capacity, and Hepatoprotective Effect of Andrographis paniculata against CCl4-Induced Liver Dysfunction in Wistar Albino Rats. Medicina 2023, 59, 1260. https://doi.org/10.3390/medicina59071260
Ali SK, Makeen HA, Khuwaja G, Alhazmi HA, Sharma M, Koty A, Mazahirul I, Parveen H, Mohammed A, Mukhtar S, et al. Assessment of the Phytochemical Profile, Antioxidant Capacity, and Hepatoprotective Effect of Andrographis paniculata against CCl4-Induced Liver Dysfunction in Wistar Albino Rats. Medicina. 2023; 59(7):1260. https://doi.org/10.3390/medicina59071260
Chicago/Turabian StyleAli, Syed Kashif, Hafiz A. Makeen, Gulrana Khuwaja, Hassan A. Alhazmi, Mukul Sharma, Afraim Koty, Islam Mazahirul, Humaira Parveen, Asaduddin Mohammed, Sayeed Mukhtar, and et al. 2023. "Assessment of the Phytochemical Profile, Antioxidant Capacity, and Hepatoprotective Effect of Andrographis paniculata against CCl4-Induced Liver Dysfunction in Wistar Albino Rats" Medicina 59, no. 7: 1260. https://doi.org/10.3390/medicina59071260
APA StyleAli, S. K., Makeen, H. A., Khuwaja, G., Alhazmi, H. A., Sharma, M., Koty, A., Mazahirul, I., Parveen, H., Mohammed, A., Mukhtar, S., & Alam, M. F. (2023). Assessment of the Phytochemical Profile, Antioxidant Capacity, and Hepatoprotective Effect of Andrographis paniculata against CCl4-Induced Liver Dysfunction in Wistar Albino Rats. Medicina, 59(7), 1260. https://doi.org/10.3390/medicina59071260










