Investigating Bioaccessibility of Advanced Glycation Product Precursors in Gluten-Free Foods Using In Vitro Gastrointestinal System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals
2.3. Sugar Analysis
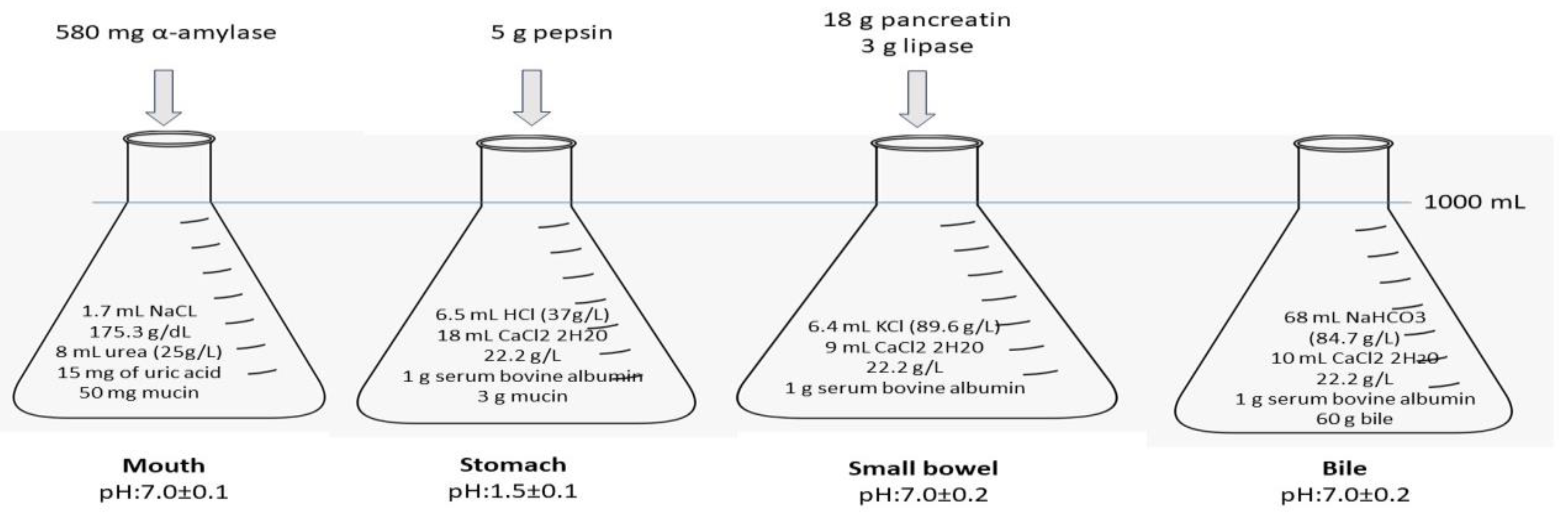
2.4. In Vitro Study
2.4.1. Extraction of GO and MGO
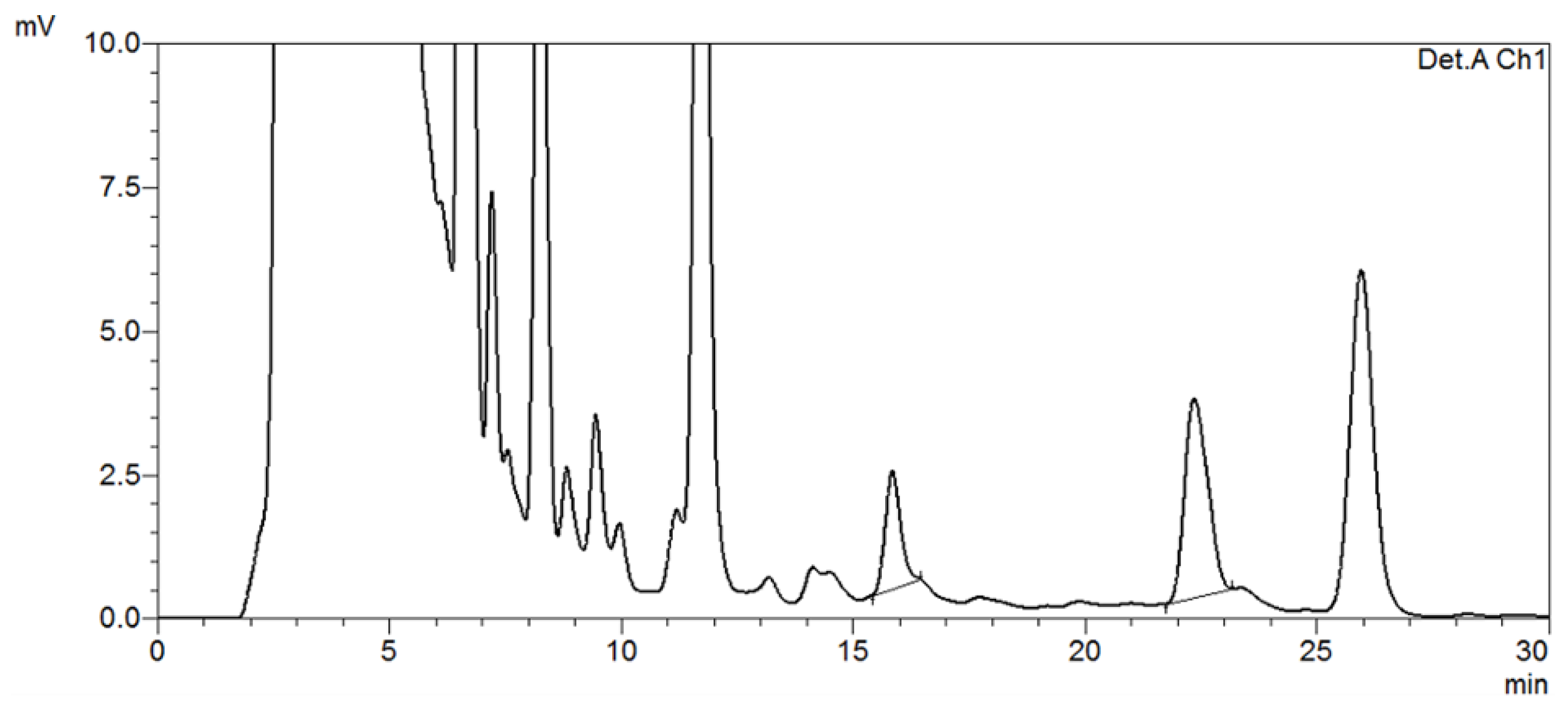
2.4.2. HPLC Analysis of Samples
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Qiao, Z.; Sang, S.; Zhang, J.; Zhan, S.; Wu, Z.; Liu, L. Research advances of advanced glycation end products in milk and dairy products: Formation, determination, control strategy and immunometabolism via gut microbiota. Food Chem. 2023, 417, 135861. [Google Scholar] [CrossRef]
- Fallavena, L.P.; Rodrigues, N.P.; Marczak, L.D.F.; Mercali, G.D. Formation of advanced glycation end products by novel food processing technologies: A review. Food Chem. 2022, 393, 133338. [Google Scholar] [CrossRef] [PubMed]
- Çatak, J.; Özdoğan, N.; Ede-Cintesun, E.; Demirci, M.; Yaman, M. Investigation of the effects of sugar type on the formation of α-dicarbonyl compounds in jams under in vitro digestive system model. J. Food Compos. Anal. 2023, 120, 105301. [Google Scholar] [CrossRef]
- Cooke, J. Dietary Reduction of Advanced Glycation End Products: An Opportunity for Improved Nutrition Care. J. Ren. Nutr. 2017, 27, e23–e26. [Google Scholar] [CrossRef][Green Version]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–27. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- DeChristopher, L.R. Perspective: The paradox in dietary advanced glycation end products research—The source of the serum and urinary advanced glycation end products is the intestines, not the food. Adv. Nutr. 2017, 8, 679–683. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; Mcdougall, G.J.; Requena, T. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Fry, L.; Madden, A.; Fallaize, R. An investigation into the nutritional composition and cost of gluten-free versus regular food products in the UK. J. Hum. Nutr. Diet. 2018, 31, 108–120. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Quan, C.V.F.; Ferreiro, S.E.R.; Cantón, O.S. Gluten-free diet: Always as easy, useful, and healthy as people think? J. Child Sci. 2018, 8, e75–e81. [Google Scholar]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Lamacchia, C.; Camarca, A.; Picascia, S.; Di Luccia, A.; Gianfrani, C. Cereal-based gluten-free food: How to reconcile nutritional and technological properties of wheat proteins with safety for celiac disease patients. Nutrients 2014, 6, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Finglas, P.M.; Berry, R.; Astley, S. Assessing and improving the quality of food composition databases for nutrition and health applications in Europe: The contribution of EuroFIR. Adv. Nutr. 2014, 5, 608S–614S. [Google Scholar] [CrossRef] [PubMed]
- Richmond, M.L.; Brandao, S.C.; Gray, J.I.; Markakis, P.; Stine, C.M. Analysis of simple sugars and sorbitol in fruit by high-performance liquid chromatography. J. Agric. Food Chem. 1981, 29, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Demirci, M.; Ede-Cintesun, E.; Kurt, E.; Mızrak, Ö.F. Investigation of formation of well-known AGEs precursors in cookies using an in vitro simulated gastrointestinal digestive system. Food Chem. 2022, 373, 131451. [Google Scholar] [CrossRef]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; Volume 1219. [Google Scholar]
- Cengiz, S.; Kişmiroğlu, C.; Cebi, N.; Çatak, J.; Yaman, M. Determination of the most potent precursors of advanced glycation end products (AGEs) in chips, crackers, and breakfast cereals by high performance liquid chromatography (HPLC) using precolumn derivatization with 4-nitro-1, 2-phenlenediamine. Microchem. J. 2020, 158, 105170. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A. The Effects of Ultra-Processed Food Consumption—Is There Any Action Needed? Nutrients 2020, 12, 2556. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Crespo-Escobar, P.; Martínez-Barona, S.; Fornés-Ferrer, V.; Donat, E.; Ribes-Koninckx, C. Differences in the macronutrient and dietary fibre profile of gluten-free products as compared to their gluten-containing counterparts. Eur. J. Clin. Nutr. 2019, 73, 930–936. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Paul Singh, R. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annu. Rev. Food Sci. Technol. 2014, 5, 111–132. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Vrolijk, M.; Bovee, T.; Van Leeuwen, S.; Vonsovic, S.; Hamers, A.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts increases their pro-inflammatory potential. Food Funct. 2021, 12, 6691–6696. [Google Scholar] [CrossRef] [PubMed]
- Baysal, A. Beslenme, 12th ed.; Hatiboğlu Yayıncılık: Ankara, Turkey, 2009. [Google Scholar]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Taetzsch, A.; Das, S.K.; Brown, C.; Krauss, A.; Silver, R.E.; Roberts, S.B. Are gluten-free diets more nutritious? An evaluation of self-selected and recommended gluten-free and gluten-containing dietary patterns. Nutrients 2018, 10, 1881. [Google Scholar] [CrossRef]
- Ferrara, P.; Cicala, M.; Tiberi, E.; Spadaccio, C.; Marcella, L.; Gatto, A.; Calzolari, P.; Castellucci, G. High fat consumption in children with celiac disease. Acta Gastro-Enterol. Belg. 2009, 72, 296–300. [Google Scholar]
- Wild, D.; Robins, G.; Burley, V.; Howdle, P. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kadan, R.; Robinson, M.; Thibodeaux, D.; Pepperman, A., Jr. Texture and other physicochemical properties of whole rice bread. J. Food Sci. 2001, 66, 940–944. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Strategies to extend bread and GF bread shelf-life: From Sourdough to antimicrobial active packaging and nanotechnology. Fermentation 2018, 4, 9. [Google Scholar] [CrossRef]
- Shipar, M. A General Review on Maillard Reactions in Foods. Trends Food Sci. Technol. 2011, 11, 64–373. [Google Scholar]
- Li, L.; Zhuang, Y.; Zou, X.; Chen, M.; Cui, B.; Jiao, Y.; Cheng, Y. Advanced Glycation End Products: A Comprehensive Review of Their Detection and Occurrence in Food. Foods 2023, 12, 2103. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Datta, A.K. Biscuit baking: A review. LWT 2020, 131, 109726. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigations on the reactions of α-dicarbonyl compounds with amino acids and proteins during in vitro digestion of biscuits. Food Funct. 2016, 7, 2544–2550. [Google Scholar] [CrossRef]
- Çintesun, E.E.; Tanyıldız, S.N.; Yıldırım, H.; Mızrak, Ö.F.; Yaman, M. Investigation of the α-Dicarbonyl Compounds in Some Snack Foods by HPLC Using Precolumn Derivatization with 4-Nitro-1, 2-Phenylenediamine. Biointerface Res. Appl. Chem. 2022, 12, 2242–2250. [Google Scholar]
- Lange, J.N.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Glyoxal formation and its role in endogenous oxalate synthesis. Adv. Urol. 2012. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Sun, D.-W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Smit, A.J.; Lutgers, H. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr. Med. Chem. 2004, 11, 2767–2784. [Google Scholar] [CrossRef] [PubMed]
- Jariyapamornkoon, N.; Yibchok-anun, S.; Adisakwattana, S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement. Altern. Med. 2013, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harsha, P.S.S.; Lavelli, V. Use of grape pomace phenolics to counteract endogenous and exogenous formation of advanced glycation end-products. Nutrients 2019, 11, 1917. [Google Scholar] [CrossRef]
- Įintesun, E.E.; Yaman, M.; Aslan, R.; Mizrak, Ö.F.; Nur, S. Effects of Different Herbal Teas on Reducing the Bioaccessibility of Methylglyoxal in Crackers under Stimulated Gastrointestinal Digestive System. Lett. Appl. NanoBioSci. 2022, 11, 3421–3429. [Google Scholar]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef]
- Li, Y.; Shi, R.; Qin, C.; Zhang, Y.; Liu, L.; Wu, Z. Gluten-free and prebiotic oat bread: Optimization formulation by transglutaminase improvement dough structure. J. Food Process. Preserv. 2021, 45, e15684. [Google Scholar] [CrossRef]
| Fructose | Glucose | Sucrose | ||||
|---|---|---|---|---|---|---|
| Variables | Mean ± SD | Median (IQR) Min–Max | Mean ± SD | Median (IQR) Min–Max | Mean ± SD | Median (IQR) Min–Max |
| Gluten-free bread (n = 10) | 0.4 ± 0.2 | 0.3 (0.5) 0.0–0.8 | 0.4 ± 0.4 | 0.3 (0.7) 0.0–1.0 | 1.9 ± 5 | 0.0 (0.7) 0.0–17.0 |
| Control bread (n = 9) | 0.2 ± 0.3 | 0.0–0.87 0.0 (0.6) | 0.1 ± 0.3 | 0.0–0.7 0.0 (0.5) | 0.0 ± 0 | 0.0–0.0 0.0 (0.0) |
| p value | 0.11 | 0.23 | 0.54 | |||
| Gluten-free biscuit (n = 5) | 0.3 ± 0.2 | 0.3 (0.6) 0.0–0.7 | 3.9 ± 5.1 | 0.6 (9.0) 0.0–11.2 | 12.9 ± 12 | 18.5 (23.2) 0.0–23.9 |
| Control biscuit (n = 5) | 1.2 ± 1.4 | 0.7 (1.6) 0.0–3.5 | 1.0 ± 0.9 | 0.8 (1.7) 0.0–2.3 | 22.1 ± 15 | 23.6 (23.5) 0.0–39.9 |
| p value | 0.30 | 0.84 | 0.31 | |||
| Gluten-free cookie (n = 11) | 0.4 ± 0.9 | 0.0 (0.5) 0.0–3.2 | 0.5 ± 1.3 | 0.0 (0.0) 0.0–4.3 | 19.5 ± 8 | 20.0 (10.3) 2.3–29.7 |
| Control cookie (n = 9) | 0.5 ± 0.5 | 0.3 (1.1) 0.0–1.3 | 0.5 ± 0.6 | 0.2 (1.5) 0.0–1.7 | 25.3 ± 6 | 0.3 (1.9) 0.0–3.9 |
| p value | 0.12 | 0.10 | 0.14 | |||
| Gluten-Free | GO (µg/100 g) Mean ± SD | MGO (µg/100 g) Mean ± SD | Bioaccessibility (%) | |||
|---|---|---|---|---|---|---|
| Pre-Digestion | Post-Digestion | Pre-Digestion | Post-Digestion | GO | MGO | |
| 1 | 17 ± 2 a | 223 ± 7 b | 72 ± 4 a | 537 ± 25 b | 1288 | 746 |
| 2 | 22 ± 1 a | 154 ± 4 b | 81 ± 4 a | 230 ± 7 b | 691 | 282 |
| 3 | 17 ± 1 a | 157 ± 5 b | 68 ± 2 a | 149 ± 5 b | 944 | 218 |
| 4 | 46 ± 2 a | 179 ± 5 b | 233 ± 11 a | 407 ± 18 b | 390 | 175 |
| 5 | 51 ± 3 a | 246 ± 12 b | 227 ± 11 a | 449 ± 20 b | 485 | 198 |
| 6 | 18 ± 2 a | 130 ± 6 b | 43 ± 2 a | 71 ± 4 b | 738 | 166 |
| 7 | 42 ± 2 a | 44 ± 2 a | 56 ± 3 a | 427 ± 19 b | 105 | 766 |
| 8 | 49 ± 2 a | 611 ± 16 b | 34 ± 2 a | 105 ± 4 b | 1247 | 305 |
| 9 | 170 ± 8 a | 222 ± 6 b | 442 ± 10 a | 502 ± 23 b | 130 | 114 |
| 10 | 147 ± 3 b | 279 ± 13 b | 378 ± 10 a | 914 ± 42 b | 189 | 242 |
| * Median (IQR) Min–Max | 44.0 (32.8) 16.0–178.0 | 199.5 (97.8) 42.0–626.0 | 76.0 (182.5) 33.0–444.8 | 416.0 (358.8) 68.8–954.0 | ||
| Control | ||||||
| 1, 2, and 3 | 209 ± 8 a | 161 ± 3 b | 18 ± 1 a | 161 ± 5 b | 77 | 878 |
| 4 | 31 ± 2 a | 166 ± 8 b | 130 ± 4 a | 304 ± 14 b | 540 | 234 |
| 5 | 17 ± 1 a | 43 ± 4 b | 22 ± 1 a | 98 ± 6 b | 255 | 444 |
| 6 | 41 ± 3 a | 182 ± 8 b | 257 ± 5 a | 544 ± 25 b | 448 | 212 |
| 7 | 11 ± 1 a | 135 ± 6 b | 211 ± 8 a | 522 ± 29 b | 1269 | 248 |
| 8 | 49 ± 3 a | 169 ± 8 b | 59 ± 2 a | 274 ± 13 b | 342 | 467 |
| 9 | 23 ± 1 a | 101 ± 6 b | 183 ± 6 a | 244 ± 66 b | 434 | 134 |
| * Median (IQR) Min–Max | 35.5 (186.3) 0.0–219.0 | 158.0 (66.3) 0.0–190.0 | 40.0 (167.0) 0.0–263.0 | 167.5 (160.0) 0.0–568.0 | ||
| Comparison of mean values of GO and MGO between groups | ||||||
| Gluten-free | 57.9 ± 53 | 224.6 ± 14 | 163 ± 143 | 379 ± 245 | ||
| Control | 80.4 ± 88 | 127.7 ± 59 | 91.5 ± 92 | 246 ± 169 | ||
| p value | 0.95 | 0.001 ** | 0.01 ** | 0.11 | ||
| GO (µg/100 g) Mean ± SD | MGO (µg/100 g) Mean ± SD | Bioaccessibility (%) | ||||
|---|---|---|---|---|---|---|
| Gluten-Free | Pre-Digestion | Post-Digestion | Pre-Digestion | Post-Digestion | GO | MGO |
| 11 | 17 ± 1 a | 401 ± 11 b | 68 ± 2 a | 831 ± 19 b | 2406 | 1228 |
| 12 | 19 ± 2 a | 313 ± 15 b | 55 ± 1 a | 165 ± 4 b | 1617 | 301 |
| 13 | 138 ± 7 a | 394 ± 18 b | 114 ± 4 a | 182 ± 8 b | 286 | 160 |
| 14 | 12 ± 1 a | 451 ± 18 b | 97 ± 3 a | 1778 ± 80 b | 3866 | 1839 |
| 15 | 80 ± 3 a | 169 ± 7 b | 68 ± 3 a | 92 ± 6 b | 212 | 135 |
| * Median (IQR) Min–max | 19.0 (67.0) 11.0–144.0 | 391.0 (116.0) 163.0–469.0 | 69.0 (35.0) 54.0–118.0 | 183.0 (689.0) 87.0–1855.0 | ||
| Control | ||||||
| 11 | 62 ± 2 a | 832 ± 33 b | 322 ± 7 a | 1562 ± 71 b | 1335 | 486 |
| 12 | 84 ± 4 a | 525 ± 12 b | 469 ± 22 a | 1145 ± 52 b | 627 | 244 |
| 13 | 94 ± 5 a | 281 ± 10 b | 374 ± 8 a | 399 ± 13 b | 298 | 107 |
| 14 | 26 ± 1 a | 830 ± 38 b | 200 ± 4 a | 2252 ± 102 b | 3152 | 1126 |
| 15 | 63 ± 4 a | 665 ± 30 b | 214 ± 12 z | 1327 ± 60 b | 1056 | 619 |
| * Median (IQR) Min–max | 64.0 (29.0) 25.0–99.0 | 667.0 (321.0) 271.0–866.0 | 320.0 (179.0) 196.0–490.0 | 1332.0 (538.0) 387.0–2350.0 | ||
| Comparison of mean values of GO and MGO between groups | ||||||
| Gluten-free | 53.1 ± 50 | 345.6 ± 102 | 80.2 ± 22 | 609.6 ± 665 | ||
| Control | 65.9 ± 24.3 | 626.6 ± 215 | 315.8 ± 104 | 1337.2 ± 624 | ||
| p value | 0.12 | 0.001 ** | < 0.001 ** | 0.004 ** | ||
| GO (µg/100 g) Mean ± SD | MGO (µg/100 g) Mean ± SD | Bioaccessibility (%) | |||||
|---|---|---|---|---|---|---|---|
| Gluten-Free | Pre-Digestion | Post-Digestion | Pre-Digestion | Post-Digestion | GO | MGO | |
| 16 | 203 ± 9 a | 557 ± 17 b | 1346 ± 61 a | 2297 ± 104 b | 275 | 171 | |
| 17 | 28 ± 1 a | 137 ± 8 b | 85 ± 4 a | 188 ± 9 b | 482 | 220 | |
| 18 | 139 ± 7 a | 365 ± 13 b | 513 ± 24 a | 533 ± 24 a | 263 | 104 | |
| 19 | 18 ± 1 a | 76 ± 2 b | 79 ± 3 a | 381 ± 9 b | 420 | 480 | |
| 20 | 24 ± 2 a | 68 ± 2 b | 37 ± 2 a | 89 ± 2 b | 285 | 239 | |
| 21 | 12 ± 2 a | 46 ± 2 b | 68 ± 3 a | 120 ± 6 b | 370 | 176 | |
| 22 | 31 ± 2 a | 19 ± 1 b | 0 ± 0 a | 0 ± 0 a | 62 | 0 | |
| 23 | 14 ± 1 a | 51 ± 4 b | 34 ± 2 a | 125 ± 9 b | 356 | 371 | |
| 24 | 37 ± 1 a | 66 ± 2 b | 71 ± 2 a | 252 ± 8 b | 178 | 356 | |
| 25 | 15 ± 2 a | 70 ± 3 b | 34 ± 1 a | 55 ± 1 a | 477 | 162 | |
| 26 | 7 ± 1 a | 74 ± 4 b | 10 ± 2 a | 74 ± 5 b | 1110 | 762 | |
| * Median (IQR) Min–max | 25.0 (22.0) 6.0–211.0 | 70.0 (80.0) 18.0–568.0 | 68.0 (51.0) 0.0–1404.0 | 125.0 (299.0) 0.0–2397.0 | |||
| Control | |||||||
| 16 and 17 | 29 ± 1 a | 485 ± 19 b | 69 ± 4 a | 572 ± 26 b | 1672 | 825 | |
| 18 | 70 ± 2 a | 314 ± 15 b | 197 ± 9 a | 206 ± 16 b | 451 | 105 | |
| 19 | 43 ± 2 a | 139 ± 7 b | 99 ± 2 a | 293 ± 14 b | 322 | 297 | |
| 20 | 74 ± 3 a | 302 ± 14 b | 465 ± 21 a | 884 ± 35 b | 408 | 190 | |
| 21 and 22 | 140 ± 7 a | 207 ± 9 b | 556 ± 22 a | 560 ± 25 a | 148 | 101 | |
| 23 | 77 ± 3 a | 279 ± 13 b | 124 ± 4 a | 361 ± 16 b | 362 | 290 | |
| 24 | 413 ± 19 a | 110 ± 5 b | 335 ± 15 a | 101 ± 6 b | 27 | 30 | |
| * Median (IQR) Min–max | 74.0 (99.0) 27.0–431.0 | 280.0 (130.0) 106.0–504.0 | 198.0 (389.0) 65.0–578.0 | 534.0 (305.0) 96.0–919.0 | |||
| Comparison of mean values of GO and MGO between groups | |||||||
| Gluten-free | 49.7 ± 61 | 138.9 ± 162 | 207 ± 391 | 373.9 ± 636 | |||
| Control | 112.7 ± 115.4 | 281.0 ± 129 | 274 ± 198 | 456.6 ± 230 | |||
| p value | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serin, Y.; Akbulut, G.; Yaman, M. Investigating Bioaccessibility of Advanced Glycation Product Precursors in Gluten-Free Foods Using In Vitro Gastrointestinal System. Medicina 2023, 59, 1578. https://doi.org/10.3390/medicina59091578
Serin Y, Akbulut G, Yaman M. Investigating Bioaccessibility of Advanced Glycation Product Precursors in Gluten-Free Foods Using In Vitro Gastrointestinal System. Medicina. 2023; 59(9):1578. https://doi.org/10.3390/medicina59091578
Chicago/Turabian StyleSerin, Yeliz, Gamze Akbulut, and Mustafa Yaman. 2023. "Investigating Bioaccessibility of Advanced Glycation Product Precursors in Gluten-Free Foods Using In Vitro Gastrointestinal System" Medicina 59, no. 9: 1578. https://doi.org/10.3390/medicina59091578
APA StyleSerin, Y., Akbulut, G., & Yaman, M. (2023). Investigating Bioaccessibility of Advanced Glycation Product Precursors in Gluten-Free Foods Using In Vitro Gastrointestinal System. Medicina, 59(9), 1578. https://doi.org/10.3390/medicina59091578







