A Determination of p97/VCP (Valosin Containing Protein) and SVIP (Small VCP Interacting Protein) Expression Patterns in Human Testis
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. H-SCORE and Statistical Analysis
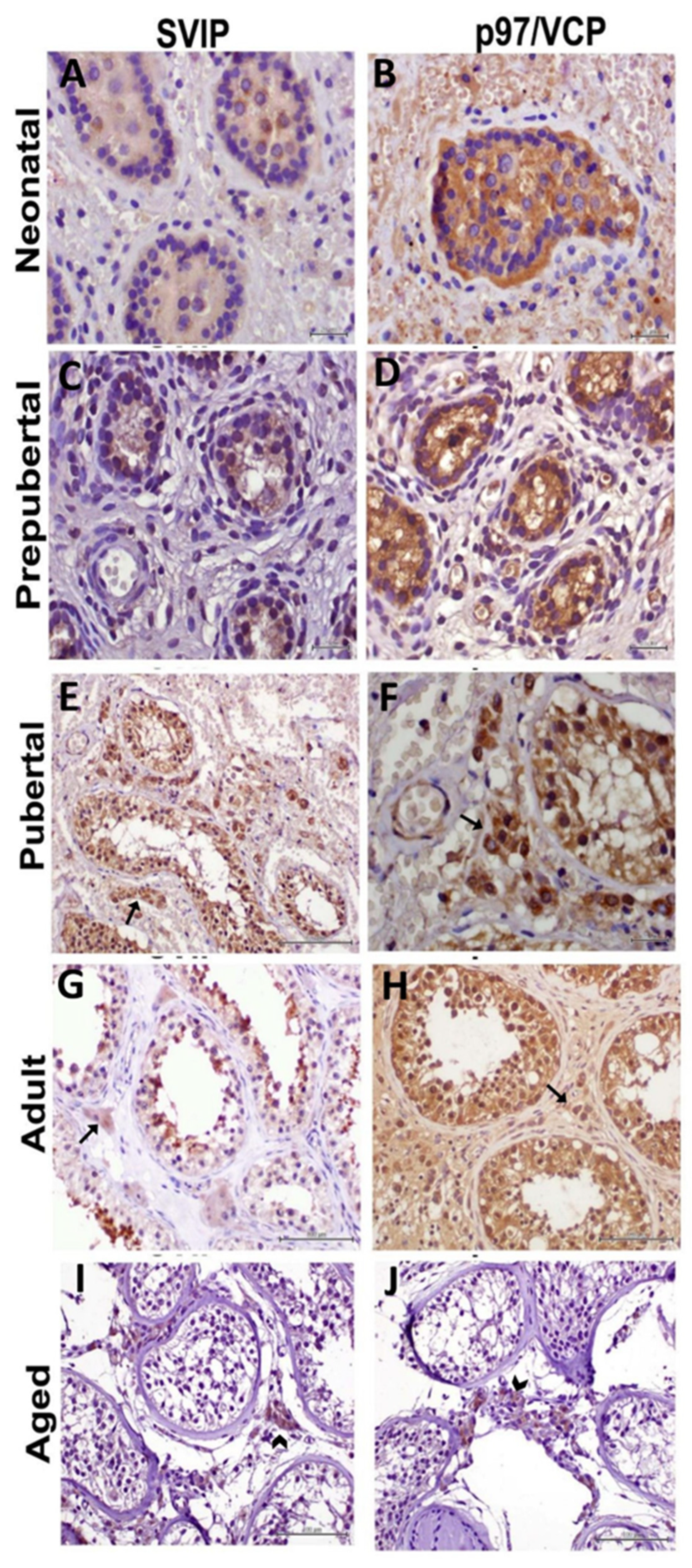
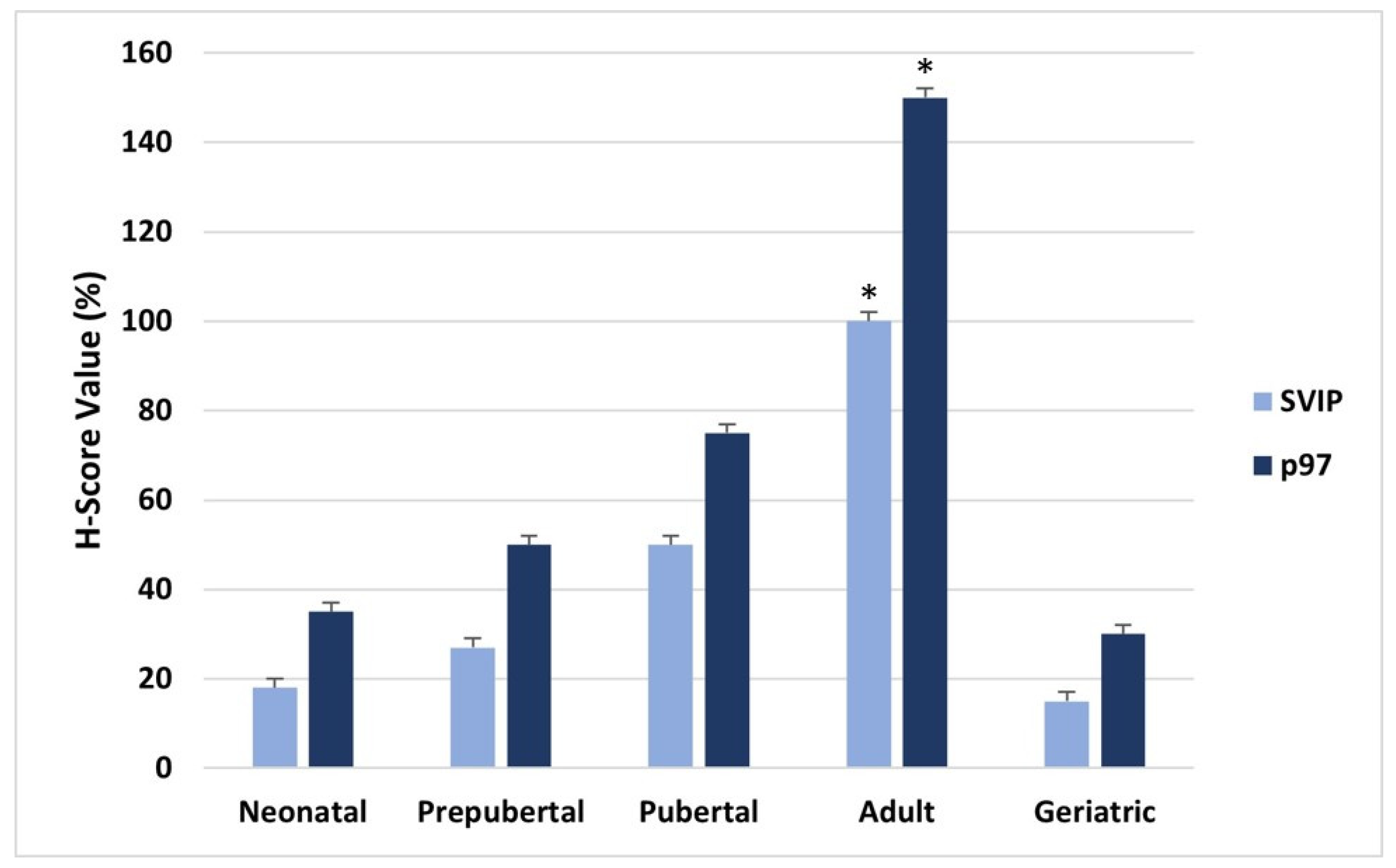
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nandi, D.; Tahiliani, P.; Kumar, A.; Chandu, D. The ubiquitin-proteasome system. J. Biosci. 2006, 31, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, G.; Mayor, T. Perilous journey: A tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014, 24, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Ciechanover, A. The ubiquitin code in the ubiquitin-proteasome system and autophagy. Tends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef]
- Meyer, H.; Weihl, C.C. The VCP/p97 system at glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 2014, 127, 3877–3883. [Google Scholar] [CrossRef]
- Meyer, H.; Bug, M.; Bremer, S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 2012, 14, 117–123. [Google Scholar] [CrossRef]
- Buchberger, A.; Schindelin, H.; Hanzelmann, P. Control of p97 function by cofactor binding. FEEBS Lett. 2015, 589, 2578–2589. [Google Scholar] [CrossRef]
- Costantini, S.; Capone, F.; Polo, A.; Bagnara, P.; Budillon, A. Valosin-containing protein (VCP)/p97: A prognostik biomarker and therapeutic target in cancer. Int. J. Mol. Sci. 2021, 22, 10177. [Google Scholar] [CrossRef]
- Yamanaka, K.; Sasagawa, Y.; Ogura, T. Recent advances in p97/VCP/Cdc48 cellular functions. Biochim. Biophys. Acta 2012, 1823, 130–137. [Google Scholar] [CrossRef]
- Ye, Y.; Tang, W.K.; Zhang, T.; Xia, D. A mighty “protein extractor” of the cell: Structure and function of the p97/CDC48 ATPase. Front. Mol. Biosci. 2017, 4, 39. [Google Scholar] [CrossRef]
- Alimogulları, E.; Akcan, G.; Ari, O.; Cayli, S. The determination of the relationship between p97/VCP, small VCP-interacting protein, two ERAD proteins and steroidogenesis in Leydig cell lines. Mol. Biol. Rep. 2022, 49, 9159–9170. [Google Scholar] [CrossRef] [PubMed]
- Ballar, P.; Zhong, Y.; Nagahama, M.; Tagaya, M.; Shen, Y.; Fang, S. Identification of SVIP as an endogenous inhibitor of endoplasmic reticulum-associated degradation. J. Biol. Chem. 2007, 282, 33908–33914. [Google Scholar] [CrossRef]
- Ballar, P.; Shen, Y.; Yang, H.; Fang, S. The role of a novel p97/valosin-containing protein-interacting motif of gp78 in endoplasmic reticulum-associated degradation. J. Biol. Chem. 2006, 281, 35359–35368. [Google Scholar] [CrossRef]
- Stapf, C.; Cartwright, E.; Bycroft, M.; Hofmann, K.; Buchberger, A. The general definition of the p97/valosin-containing protein (VCP)-interacting motif (VIM) delineates a new family of p97 cofactors. J. Biol. Chem. 2011, 286, 38670–38678. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peng, D.; Voehler, M.; Sanders, C.R.; Li, J. Structure and expression of a novel compact myelin protein-small VCP-interacting protein (SVIP). Biochem. Biophys. Res. Commun. 2013, 440, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Cayli, S.; Ocaklı, S.; Erdemir, F.; Taş, U.; Aslan, H.; Yener, T.; Karaca, Z. Developmental expression of p97/VCP (Valosin-containing protein) and Jab1/CSN5 in the rat testis and epididymis. Reprod. Biol. Endocrinol. 2011, 9, 117. [Google Scholar] [CrossRef]
- Cayli, S.; Erdemir, F.; Ocaklı, S.; Ungor, B.; Kesici, H.; Yener, T.; Aslan, H. Interaction between Smad1 and p97/VCP in rat testis and epididymis during the postnatal development. Reprod. Sci. 2012, 19, 190–201. [Google Scholar] [CrossRef]
- Moir, D.; Stewart, S.E.; Osmond, B.C.; Botstein, D. Cold-sensitive cell-division-cycle mutants of yeast: Isolation, properties, and pseudoreversion studies. Genetics 1982, 100, 547–563. [Google Scholar] [CrossRef]
- Dai, R.M.; Li, C.C. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat. Cell Biol. 2001, 3, 740–744. [Google Scholar] [CrossRef]
- Yi, L.; Donsante, A.; Kennerson, M.L.; Mercer, J.F.B.; Garbern, J.Y.; Kaler, S.G. Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Hum. Mol. Genet. 2012, 21, 1794–1807. [Google Scholar] [CrossRef]
- Hirabayashi, M.; Inoue, K.; Tanaka, K.; Nakadate, K.; Ohsawa, Y.; Kamei, Y.; Popiel, A.H.; Sinohara, A.; Iwamatsu, A.; Kimura, Y.; et al. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001, 8, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Bodas, M.; Mazur, S.; Vij, N. Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J. Mol. Med. 2011, 89, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Vij, N. AAA ATPase p97/VCP: Cellular functions, disease and therapeutic potential. J. Cell Mol. Med. 2008, 12, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Niu, M.; Zhang, X.; Zhong, Z.; Wang, J.; Pang, D. High expression of valosin-vontaining protein predicts poor prognosis in patients with breast carcinoma. Tumor Biol. 2015, 36, 9919–9927. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Sakon, M.; Kameyama, M.; Imaoka, S.; Sekimoto, M.; Nakamori, S.; Monden, M.; Aozasa, K. Expression of valosin-containing protein in colorectal Carcinomas as a predictor for disease recurrence and prognosis. Clin. Cancer Res. 2004, 10, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Uruno, T.; Hoshida, Y.; Qiu, Y.; Iizuka, N.; Nakamichi, I.; Miyauchi, A.; Aozasa, K. Increased expression of valosin-containing protein (p97) is correlated with disease recurrence in follicular thyroid cancer. Ann. Surg. Oncol. 2005, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Suzuki, M.; Hamada, Y.; Hatsuzawa, K.; Tani, K.; Yamamoto, A.; Tagaya, M. SVIP is a novel VCP/p97-interacting protein whose expression causes cell vacuolation. Mol. Biol. Cell 2003, 14, 262–273. [Google Scholar] [CrossRef]
- Itman, C.; Mendis, S.; Barakat, B.; Loveland, K.T. All in the family: TGF-β family action in testis development. Reproduction 2006, 132, 233–246. [Google Scholar] [CrossRef]
- Schlatt, H.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef]
- Moreno, S.G.; Attali, M.; Allemand, I.; Messiaen, S.; Fouchet, P.; Coffigny, H.; Romeo, P.H.; Habert, R. TGFβ signaling in male germ cells regulates gonocyte quiescence and fertility in mice. Dev. Biol. 2010, 342, 74–84. [Google Scholar] [CrossRef]
- Hai, Y.; Hou, J.; Liu, Y.; Liu, Y.; Yang, H.; Li, Z.; He, Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Horibe, A.; Eid, N.; Ito, Y.; Otsuki, Y.; Kondo, Y. Thanol-induced autophagy in Sertoli cells is specifially marked at androgen-dependent stages of the spermatogenic cycle: Potential mechanisms and implications. Int. J. Mol. Sci. 2019, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Buschiazzo, A.; Burel, A.; Lavault, M.T.; Pimentel, C.; Jouve, G.; Jaillard, S.; Jegou, B.; Bourmeyster, N.; Ravel, C. Autophagy is increased in cryptorchid testis resulting in abnormal spermatozoa. Asian J. Androl. 2019, 21, 570–576. [Google Scholar]
- Cayli, S.; Sahin, C.; Sanci, T.O.; Nakkas, H. Inhibition of p97/VCP function leads to defective autophagosome maturation, cell cycle arrest and apoptosis in mouse Sertoli cells. Theriogenology 2020, 158, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, A.Z.; Cayli, S.; Sahin, C.; Ocaklı, S.; Sanci, T.O.; Delibas, I.B. Altered expression of p97/Valosin containing protein and impaired autophagy in preeclamptic human placenta. Placenta 2018, 67, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, S.W.J.; Beld, A.W.; Lely, A.J. The endocrinology of aging. Science 1997, 278, 419–424. [Google Scholar] [CrossRef]
- Romanuik, T.L.; Wang, G.; Holt, R.A.; Jones, S.J.M.; Marra, M.A.; Sadar, M.D. Identification of novel androgen-responsive genes by sequencing of longSAGE libraries. BMC Genom. 2009, 10, 476. [Google Scholar] [CrossRef]
- Erzurumlu, Y.; Ballar, P. Androgen mediated regulation of endoplaplazmic reticulum-associated degradation and its effects on prostate cancer. Sci. Rep. 2016, 7, 40719. [Google Scholar] [CrossRef]
- Bao, D.; Cheng, C.; Lan, X.; Xing, R.; Chen, Z.; Zhao, H.; Sun, J.; Wang, Y.; Niu, C.; Zhang, B.; et al. Regulation of p53 glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget 2017, 8, 23142–23154. [Google Scholar] [CrossRef]
- Chang, S.; Chen, Y.T.; Yeh, S.D.; Xu, Q.; Wang, R.S.; Guillou, F.; Lardy, H.; Yeh, S. Infertility with defective spermatogenesis and hypotestostronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 6876–6881. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. Lipid droplets and steroidogenic cells. Exp. Cell Res. 2016, 340, 209–2014. [Google Scholar] [CrossRef] [PubMed]
- Akcan, G.; Alimogulları, E.; Abu Issa, R.; Cayli, S. Analysis of the developmental expression of small VCP-interacting protein and its interaction with steroidogenic acute regulatory protein in Leydig cells. Reprod. Biol. 2020, 20, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, S.; Li, L.; Su, X.; Du, C.; Li, F.; Geng, B.; Liu, P.; Xu, G. Proteomic analysis of murine testes lipid droplets. Sci. Rep. 2015, 5, 12070. [Google Scholar] [CrossRef]
- Ji, C.H.; Kwon, Y.T. Crosstalk and interplay between the ubiquitin-proteasome system and autophagy. Mol. Cells 2017, 40, 441–449. [Google Scholar] [PubMed]
- Chou, T.F.; Brown, S.J.; Minond, D.; Nordin, B.E.; Li, K.; Jones, A.C.; Chase, P.; Porubsky, P.R.; Stoltz, B.M.; Schoenen, F.J.; et al. Reversible inhibitör of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4834–4839. [Google Scholar] [CrossRef]
- Huryn, D.M.; Kornfilt, D.J.P.; Wipf, P. P97: An emerging target for cancer, neurodegenerative diseases, and viral infections. J. Med. Chem. 2020, 63, 1892–1907. [Google Scholar] [CrossRef]
- Nakkas, H.; Ocal, B.G.; Kipel, S.; Akcan, G.; Sahin, C.; Ardicoglu, A.; Cayli, S. Ubiquitin proteasome system and autophagy associated proteins in human testicular tumors. Tissue Cell 2021, 71, 101513. [Google Scholar] [CrossRef]
- Bastola, P.; Neums, L.; Schenen, F.J.; Chien, J. VCP inhibitors induce endoplasmic reticulum stress, cause cell cycle arrest, trigger caspase-mediated cell death and synergistically kill ovarian cancer cells in combination with Salubrinal. Mol. Oncol. 2016, 10, 1559–1574. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arıcı, A.; Erdemir, F. A Determination of p97/VCP (Valosin Containing Protein) and SVIP (Small VCP Interacting Protein) Expression Patterns in Human Testis. Medicina 2023, 59, 1079. https://doi.org/10.3390/medicina59061079
Arıcı A, Erdemir F. A Determination of p97/VCP (Valosin Containing Protein) and SVIP (Small VCP Interacting Protein) Expression Patterns in Human Testis. Medicina. 2023; 59(6):1079. https://doi.org/10.3390/medicina59061079
Chicago/Turabian StyleArıcı, Akgül, and Fikret Erdemir. 2023. "A Determination of p97/VCP (Valosin Containing Protein) and SVIP (Small VCP Interacting Protein) Expression Patterns in Human Testis" Medicina 59, no. 6: 1079. https://doi.org/10.3390/medicina59061079
APA StyleArıcı, A., & Erdemir, F. (2023). A Determination of p97/VCP (Valosin Containing Protein) and SVIP (Small VCP Interacting Protein) Expression Patterns in Human Testis. Medicina, 59(6), 1079. https://doi.org/10.3390/medicina59061079





