Prenatal Diagnosis of Isolated Single Umbilical Artery: Incidence, Risk Factors and Impact on Pregnancy Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants
2.2. Measurements
2.3. Statistical Analysis
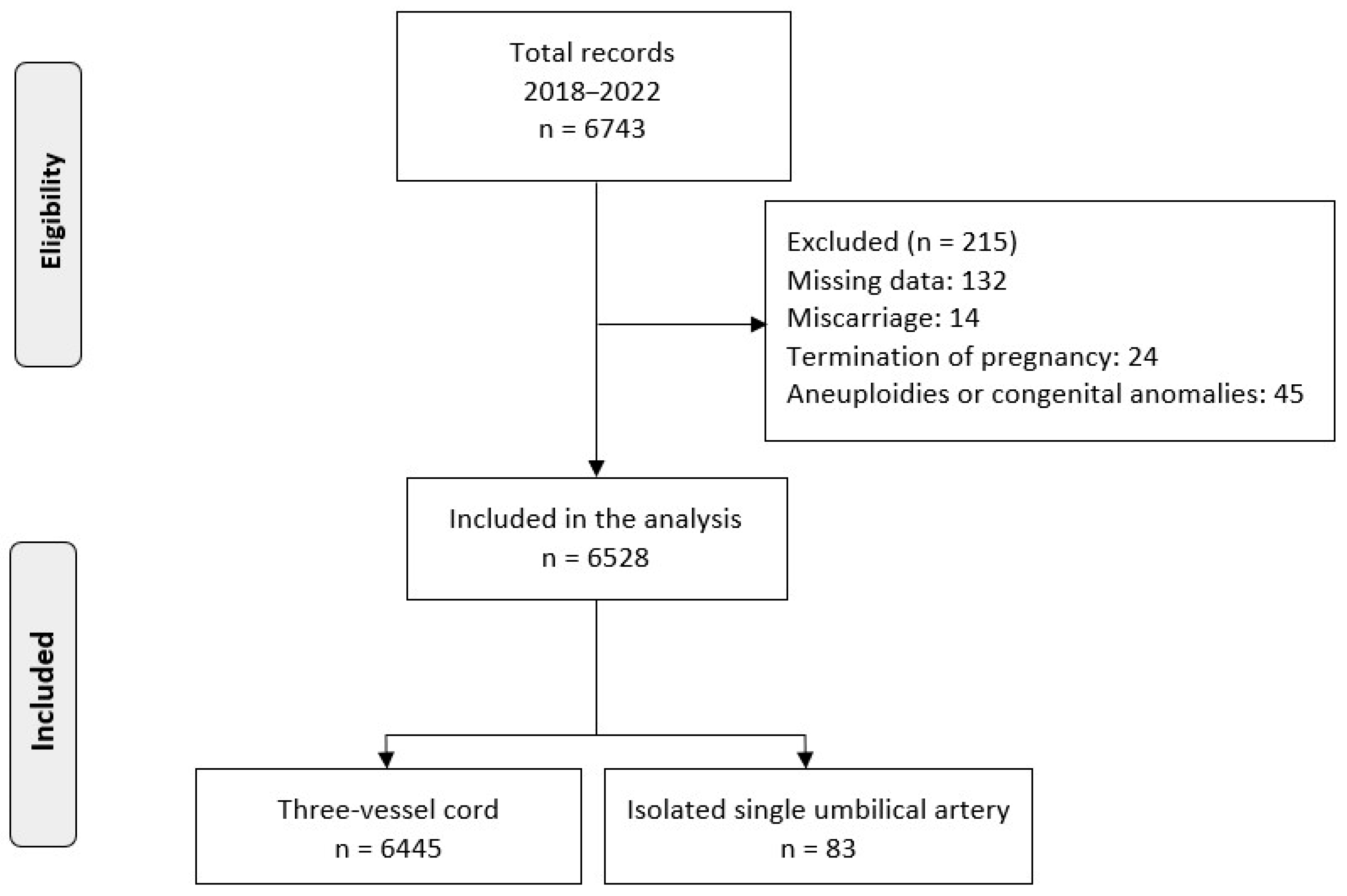
3. Results
4. Discussion
4.1. Principal Findings
4.2. Interpretation of the Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eva, T.R.; Waldo, S.; Deborah, L.; Louise, W.-H. Single Umbilical Artery; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2020. [Google Scholar]
- Fahmy, M. Anatomy of the Umbilical Cord. In Umbilicus and Umbilical Cord; Springer International Publishing: Cham, Switzerland, 2018; pp. 47–56. [Google Scholar]
- Murphy-Kaulbeck, L.; Dodds, L.; Joseph, K.S.; Van den Hof, M. Single umbilical artery risk factors and pregnancy outcomes. Obstet. Gynecol. 2010, 116, 843–850. [Google Scholar] [CrossRef]
- Dagklis, T.; Defigueiredo, D.; Staboulidou, I.; Casagrandi, D.; Nicolaides, K.H. Isolated single umbilical artery and fetal karyotype. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010, 36, 291–295. [Google Scholar] [CrossRef]
- DeFigueiredo, D.; Dagklis, T.; Zidere, V.; Allan, L.; Nicolaides, K.H. Isolated single umbilical artery: Need for specialist fetal echocardiography? Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2010, 36, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, H.; Rafeei, K.; Dalili, M.; Asadi, N.; Seirfar, N.; Akbarzadeh-Jahromi, M. Prevalence of single umbilical artery, clinical outcomes and its risk factors: A cross-sectional study. Int. J. Reprod. Biomed. 2021, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, C.; Kessler, J.; Moster, D.; Rasmussen, S. Isolated single umbilical artery and the risk of adverse perinatal outcome and third stage of labor complications: A population-based study. Acta Obstet. Gynecol. Scand. 2020, 99, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.M.; Chalouhi, G.E.; Da Silva Costa, F.; Hernandez-Andrade, E.; Malinger, G.; Munoz, H.; Paladini, D.; et al. ISUOG Practice Guidelines (updated): Performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 59, 840–856. [Google Scholar] [CrossRef]
- Bombrys, A.E.; Neiger, R.; Hawkins, S.; Sonek, J.; Croom, C.; McKenna, D.; Ventolini, G.; Habli, M.; How, H.; Sibai, B. Pregnancy outcome in isolated single umbilical artery. Am. J. Perinatol. 2008, 25, 239–242. [Google Scholar] [CrossRef]
- Hua, M.; Odibo, A.O.; Macones, G.A.; Roehl, K.A.; Crane, J.P.; Cahill, A.G. Single umbilical artery and its associated findings. Obstet. Gynecol. 2010, 115, 930–934. [Google Scholar] [CrossRef]
- Luo, X.; Zhai, S.; Na Shi, N.; Li, M.; Cui, S.; Xu, Y.; Ran, L.; Ren, L.; Hong, T.; Liu, R. The Risk Factors and Neonatal outcomes of Isolated Single Umbilical Artery in Singleton Pregnancy: A Meta-analysis. Sci. Rep. 2017, 7, 7396. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Ciobanu, A.; Formuso, C.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of small-for-gestational-age neonates at 35-37 weeks’ gestation: Contribution of maternal factors and growth velocity between 20 and 36 weeks. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 53, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; van der Windt, D.; Croft, P.M.K. Prognosis Research in Healthcare: Concepts, Methods, and Impact; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Lang, J.M.; Lieberman, E.; Cohen, A. A Comparison of Risk Factors for Preterm Labor and Term Small-for-Gestational-Age Birth. Epidemiology 1996, 7, 369–376. [Google Scholar] [CrossRef]
- Rodriguez, M.; Couve-Perez, C.; San Martin, S.; Martinez, F.; Lozano, C.; Sepulveda-Martinez, A. Perinatal outcome and placental apoptosis in patients with late-onset pre-eclampsia and abnormal uterine artery Doppler at diagnosis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 51, 775–782. [Google Scholar] [CrossRef]
- Siargkas, A.; Tsakiridis, I.; Pachi, C.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Impact of velamentous cord insertion on perinatal outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100812. [Google Scholar] [CrossRef]
- Siargkas, A.; Tsakiridis, I.; Pachi, C.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Impact of marginal cord insertion on perinatal outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100876. [Google Scholar] [CrossRef]
- Yang, J.; Hartmann, K.; Savitz, D.A.; Herring, A.H.; Dole, N.; Olshan, A.F.; Thorp, J.M. Vaginal bleeding during pregnancy and preterm birth. Am. J. Epidemiol. 2004, 160, 118–125. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Dagklis, T.; Siargkas, A.; Apostolopoulou, A.; Tsakiridis, I.; Mamopoulos, A.; Athanasiadis, A.; Sotiriadis, A. Adverse perinatal outcomes following the prenatal diagnosis of isolated single umbilical artery in singleton pregnancies: A systematic review and meta-analysis. J. Perinat. Med. 2022, 50, 244–252. [Google Scholar] [CrossRef]
- Lin, L.; Lu, C.; Chen, W.; Li, C.; Guo, V.Y. Parity and the risks of adverse birth outcomes: A retrospective study among Chinese. BMC Pregnancy Childbirth 2021, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Goetzinger, K.R.; Cahill, A.G.; Macones, G.A.; Odibo, A.O. The relationship between maternal body mass index and tobacco use on small-for-gestational-age infants. Am. J. Perinatol. 2012, 29, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Fadigas, C.; Guerra, L.; Garcia-Tizon Larroca, S.; Poon, L.C.; Nicolaides, K.H. Prediction of small-for-gestational-age neonates: Screening by uterine artery Doppler and mean arterial pressure at 35–37 weeks. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, J.H.; Chay, D.B.; Park, J.H.; Kim, M.A. Association of isolated single umbilical artery with perinatal outcomes: Systemic review and meta-analysis. Obstet. Gynecol. Sci. 2017, 60, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Battarbee, A.N.; Palatnik, A.; Ernst, L.M.; Grobman, W.A. Association of Isolated Single Umbilical Artery With Small for Gestational Age and Preterm Birth. Obstet. Gynecol. 2015, 126, 760–764. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Worda, K.; Schmid, M.; Polterauer, S.; Bettelheim, D. Isolated single umbilical artery: Evaluating the risk of adverse pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 80–83. [Google Scholar] [CrossRef]
- Ashwal, E.; Melamed, N.; Hiersch, L.; Edel, S.; Bardin, R.; Wiznitzer, A.; Yogev, Y. The impact of isolated single umbilical artery on labor and delivery outcome. Prenat. Diagn. 2014, 34, 581–585. [Google Scholar] [CrossRef]
- Contro, E.; Larcher, L.; Lenzi, J.; Valeriani, M.; Farina, A.; Jauniaux, E. Changes in Artery Diameters and Fetal Growth in Cases of Isolated Single Umbilical Artery. Diagnostics 2023, 13, 571. [Google Scholar] [CrossRef]
- Gornall, A.S.; Kurinczuk, J.J.; Konje, J.C. Antenatal detection of a single umbilical artery: Does it matter? Prenat. Diagn. 2003, 23, 117–123. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Palatnik, A.; Ernst, L.M.; Grobman, W.A. Placental abnormalities associated with isolated single umbilical artery in small-for-gestational-age births. Placenta 2017, 59, 9–12. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, L.; Zhai, S.; Luo, X.; Hong, T.; Liu, R.; Ran, L.; Zhang, Y. Association Between Isolated Single Umbilical Artery and Perinatal Outcomes: A Meta-Analysis. Med. Sci. Monit. 2016, 22, 1451–1459. [Google Scholar] [CrossRef]
- Voskamp, B.J.; Fleurke-Rozema, H.; Oude-Rengerink, K.; Snijders RJ, M.; Bilardo, C.M.; Mol BW, J.; Pajkrt, E. Relationship of isolated single umbilical artery to fetal growth, aneuploidy and perinatal mortality: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 42, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.; McKenna, D.S.; Croom, C.; Ventolini, G.; Sonek, J.D.; Neiger, R. Serial sonographic growth assessment in pregnancies complicated by an isolated single umbilical artery. Am. J. Perinatol. 2008, 25, 149–152. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sagr, E.R.; Elrifaei, R.M.; Abdelbasit, O.B.; Halouly, T.A. Outcomes of an isolated single umbilical artery in singleton pregnancy: A large study from the Middle East and Gulf region. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Sebikari, D.; Farhad, M.; Fenton, T.; Owor, M.; Stringer, J.S.A.; Qin, M.; Chakhtoura, N.; Chi, B.H.; Saidi, F.; Nevrekar, N.; et al. Risk Factors for Adverse Birth Outcomes in the PROMISE 1077BF/1077FF Trial. J. Acquir. Immune Defic. Syndr. 2019, 81, 521–532. [Google Scholar] [CrossRef]
- Cobo, T.; Kacerovsky, M.; Jacobsson, B. Risk factors for spontaneous preterm delivery. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 150, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shevell, T.; Malone, F.D.; Vidaver, J.; Porter, T.F.; Luthy, D.A.; Comstock, C.H.; Hankins, G.D.; Eddleman, K.; Dolan, S.; Dugoff, L.; et al. Assisted reproductive technology and pregnancy outcome. Obstet. Gynecol. 2005, 106, 1039–1045. [Google Scholar] [CrossRef]
- Giouleka, S.M.; Tsakiridis, I.; Kostakis, N.; Koutsouki, G.; Kalogiannidis, I.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Preterm Labor: A Comprehensive Review of Guidelines on Diagnosis, Management, Prediction and Prevention. Obstet. Gynecol. Surv. 2022, 77, 302–317. [Google Scholar] [CrossRef]
- Osuchukwu, O.O.; Reed, D.J. Small for Gestational Age. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
| Characteristics | Overall (n = 6528) | Normal Cord (n = 6445) | iSUA (n = 83) | p Value |
|---|---|---|---|---|
| Maternal age, mean (SD) | 31.7 (5.1) | 31.7 (5.1) | 32.4 (5.4) | 0.205 |
| No smoking, n (%) | 4168 (63.8) | 4112 (63.8) | 56 (67.5) | 0.358 |
| Quit in pregnancy | 1670 (25.6) | 1654 (25.7) | 16 (19.2) | |
| Current smoking | 690 (10.6) | 679 (10.5) | 11 (13.3) | |
| Multiparous, n (%) | 2620 (40.1) | 2584 (40.1) | 36 (43.4) | 0.622 |
| ART, n (%) | 323 (4.9) | 314 (4.9) | 9 (10.8) | 0.025 a |
| BMI, median (IQR) | 23.0 (21.0, 26.0) | 23.0 (21.0, 26.0) | 23.0 (21.1, 26.9) | 0.767 |
| Bleeding in 1st trimester, n (%) | 331 (5.1) | 327 (5.1) | 4 (4.8) | 1.000 |
| Previous history of miscarriage in 1st trimester, n (%) | 1030 (15.8) | 1011 (15.7) | 19 (22.9) | 0.101 |
| Previous history of PTD, n (%) | 155 (2.4) | 151 (2.3) | 4 (4.8) | 0.267 |
| Abnormal cord insertion, n (%) | 667 (10.2) | 657 (10.2) | 10 (12.0) | 0.710 |
| UtA PI z-score, mean (SD) | 0.03 (1.10) | 0.03 (1.10) | 0.14 (1.07) | 0.381 |
| PE, n (%) | 486 (7.4) | 480 (7.4) | 6 (7.2) | 1.000 |
| PTD, n (%) | 558 (8.5) | 544 (8.4) | 14 (16.9) | 0.011 a |
| SGA, n (%) | 1066 (16.3) | 1043 (16.2) | 23 (27.7) | 0.008 a |
| Gestational age at delivery, median (QR) | 39.0 (38.1, 39.9) | 39.0 (38.1, 39.9) | 38.3 (37.6, 38.8) | <0.001 a |
| BW, median (IQR) | 3250 (2970, 3540) | 3250 (2978, 3550) | 3000 (2725, 3330) | <0.001 a |
| SGA | PTD | |||
|---|---|---|---|---|
| Variables | aOR | 95% CI | aOR | 95% CI |
| Maternal age (years) | - | - | - | - |
| ΒΜΙ (kg/m2) | 0.957 | 0.942, 0.972 | 1.021 | 1.003, 1.039 |
| No smoking | reference | reference | ||
| Quit smoking | - | - | - | - |
| Current smoking | 1.844 | 1.507, 2.256 | 1.397 | 1.059, 1.842 |
| Multiparity | 0.634 | 0.544, 0.738 | - | - |
| ART | - | - | 2.033 | 1.436, 2.876 |
| UtA PI z-score | 1.560 | 1.470, 1.656 | 1.523 | 1.413, 1.642 |
| Bleeding in 1st trimester | - | - | - | - |
| Previous history of miscarriage in 1st trimester | - | - | 1.282 | 1.017, 1.616 |
| Previous history of PTD | - | - | 4.896 | 3.326, 7.209 |
| Abnormal cord insertion | - | - | - | - |
| iSUA | 1.909 | 1.152, 3.163 | 1.903 | 1.035, 3.498 |
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p-Value | aOR | 95% CI | p-Value |
| Maternal age (years) | 1.028 | 0.985, 1.073 | 0.205 | - | - | - |
| ΒΜΙ (kg/m2) | 0.999 | 0.955, 1.046 | 0.981 | - | - | - |
| No smoking | reference | reference | ||||
| Quit smoking | 0.710 | 0.406, 1.242 | 0.230 | - | - | - |
| Current smoking | 1.190 | 0.620, 2.282 | 0.602 | - | - | - |
| Multiparity | 1.144 | 0.739, 1.772 | 0.545 | - | - | - |
| ART | 2.375 | 1.178, 4.788 | 0.016 a | 2.234 | 1.104, 4.523 | 0.025 a |
| Bleeding in 1st trimester | 0.947 | 0.345, 2.603 | 0.916 | - | - | - |
| Previous history of 1st trimester miscarriage | 1.596 | 0.952, 2.674 | 0.076 | - | - | - |
| Previous history of PTD | 2.110 | 0.763, 5.837 | 0.150 | - | - | - |
| Male fetus | 0.757 | 0.490, 1.170 | 0.210 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siargkas, A.; Giouleka, S.; Tsakiridis, I.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Dagklis, T. Prenatal Diagnosis of Isolated Single Umbilical Artery: Incidence, Risk Factors and Impact on Pregnancy Outcomes. Medicina 2023, 59, 1080. https://doi.org/10.3390/medicina59061080
Siargkas A, Giouleka S, Tsakiridis I, Mamopoulos A, Kalogiannidis I, Athanasiadis A, Dagklis T. Prenatal Diagnosis of Isolated Single Umbilical Artery: Incidence, Risk Factors and Impact on Pregnancy Outcomes. Medicina. 2023; 59(6):1080. https://doi.org/10.3390/medicina59061080
Chicago/Turabian StyleSiargkas, Antonios, Sonia Giouleka, Ioannis Tsakiridis, Apostolos Mamopoulos, Ioannis Kalogiannidis, Apostolos Athanasiadis, and Themistoklis Dagklis. 2023. "Prenatal Diagnosis of Isolated Single Umbilical Artery: Incidence, Risk Factors and Impact on Pregnancy Outcomes" Medicina 59, no. 6: 1080. https://doi.org/10.3390/medicina59061080
APA StyleSiargkas, A., Giouleka, S., Tsakiridis, I., Mamopoulos, A., Kalogiannidis, I., Athanasiadis, A., & Dagklis, T. (2023). Prenatal Diagnosis of Isolated Single Umbilical Artery: Incidence, Risk Factors and Impact on Pregnancy Outcomes. Medicina, 59(6), 1080. https://doi.org/10.3390/medicina59061080









