Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment
2.3. Outcomes and Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Fauci, A.S.; Lane, H.C.; Redfield, R.R. Covid-19—Navigating the Uncharted. N. Engl. J. Med. 2020, 382, 1268–1269. [Google Scholar] [CrossRef]
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; Sinn, B.V.; Gerhold, M.; Hauptmann, K.; Ingold-Heppner, B.; et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 4263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Bai, X.; Wang, J.; Yu, Q.; Liu, W.; Pu, J.; Wang, X.; Hu, J.; Xu, D.; Li, X.; et al. Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann. Palliat. Med. 2020, 9, 1404–1412. [Google Scholar] [CrossRef]
- Teimury, A.; Khameneh, M.T.; Khaledi, E.M. Major coagulation disorders and parameters in COVID-19 patients. Eur. J. Med. Res. 2022, 27, 25. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yan, H.; Chen, H.; He, C.; Lin, C.; He, H.; Zhang, S.; Shi, S.; Lin, K. COVID-19 and coagulation dysfunction in adults: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 934–944. [Google Scholar] [CrossRef]
- Dorgalaleh, A. Bleeding and Bleeding Risk in COVID-19. Semin. Thromb. Hemost. 2020, 46, 815–818. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Chen, A.; Wang, C.; Zhu, W.; Chen, W. Coagulation Disorders and Thrombosis in COVID-19 Patients and a Possible Mechanism Involving Endothelial Cells: A Review. Aging Dis. 2022, 13, 144–156. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Levi, M. Disseminated intravascular coagulation. Crit. Care Med. 2007, 35, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Ten Cate, H. Disseminated intravascular coagulation. N. Engl. J. Med. 1999, 341, 586–592. [Google Scholar] [CrossRef]
- Ji, H.-L.; Zhao, R.; Matalon, S.; Matthay, M.A. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol. Rev. 2020, 100, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Nisio, M.D.; Levy, J.H.; Kitamura, N.; Thachil, J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open 2017, 7, e017046. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Tang, N.; Liu, H.; Cao, W. Coagulation Dysfunction. Arch. Pathol. Lab. Med. 2020, 144, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, S.W.; Bolig, T.C.; Molling, D.J.; Sjoding, M.W.; Flanders, S.A.; Prescott, H.C. Causes and Circumstances of Death among Patients Hospitalized with COVID-19: A Retrospective Cohort Study. Ann. ATS 2021, 18, 1076–1079. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J.; Song, Q.; Jia, Q.; Wang, J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE 2020, 15, e0235458. [Google Scholar] [CrossRef]
- Valek, V.; Husty, J. Quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage. Cardiovasc Interv. Radiol. 2013, 36, 608–612. [Google Scholar] [CrossRef]
- Chakraverty, S.; Flood, K.; Kessel, D.; McPherson, S.; Nicholson, T.; Ray, C.E.; Robertson, I.; van Delden, O.M. CIRSE guidelines: Quality improvement guidelines for endovascular treatment of traumatic hemorrhage. Cardiovasc Interv. Radiol. 2012, 35, 472–482. [Google Scholar] [CrossRef]
- Angle, J.F.; Siddiqi, N.H.; Wallace, M.J.; Kundu, S.; Stokes, L.; Wojak, J.C.; Cardella, J.F. Society of Interventional Radiology Standards of Practice Committee Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2010, 21, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, T.; Kawai, N.; Sato, M.; Tanihata, H.; Takasaka, I.; Nakai, M.; Minamiguchi, H.; Sahara, S.; Iwasaki, Y.; Shima, Y.; et al. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J. Vasc. Interv. Radiol. 2009, 20, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Tipaldi, M.A.; Orgera, G.; Krokidis, M.; Rebonato, A.; Maiettini, D.; Vagnarelli, S.; Ambrogi, C.; Rossi, M. Trans Arterial Embolization of Non-variceal Upper Gastrointestinal Bleeding: Is the Use of Ethylene-Vinyl Alcohol Copolymer as Safe as Coils? Cardiovasc Interv. Radiol. 2018, 41, 1340–1345. [Google Scholar] [CrossRef]
- Loffroy, R.; Guiu, B.; D’Athis, P.; Mezzetta, L.; Gagnaire, A.; Jouve, J.-L.; Ortega-Deballon, P.; Cheynel, N.; Cercueil, J.-P.; Krausé, D. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: Predictors of early rebleeding. Clin. Gastroenterol. Hepatol. 2009, 7, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, T.; Kawai, N.; Sato, M.; Sonomura, T.; Takasaka, I.; Nakai, M.; Minamiguchi, H.; Sahara, S.; Iwasaki, Y.; Naka, T.; et al. Comparison of hemostatic durability between N-butyl cyanoacrylate and gelatin sponge particles in transcatheter arterial embolization for acute arterial hemorrhage in a coagulopathic condition in a swine model. Cardiovasc Interv. Radiol. 2010, 33, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, L.; Molina-Nuevo, J.D.; Pedrosa-Jiménez, M.J.; Juliá-Mollá, E. Spontaneous Haematomas in Anticoagulated Covid-19 Patients: Diagnosis and Treatment by Embolization. Cardiovasc Interv. Radiol. 2022, 45, 1001–1006. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Del Giudice, C.; Coppola, A.; Carnevale, A.; Giganti, M.; Renzulli, M.; Tacher, V.; Urbano, J.; Kobeiter, H.; Loffroy, R.; et al. Gastrointestinal Hemorrhages in Patients with COVID-19 Managed with Transarterial Embolization. Am. J. Gastroenterol. 2021, 116, 838–840. [Google Scholar] [CrossRef]
- Lalatović, J.; Golubović, J.; Miljević, M.; Lukić, F.; Jeličić, S.; Kraišnik, N.; Aleksić, A.; Kalaba, Z.; Vidović, B.; Tošković, B.; et al. Transcatheter Arterial Embolization as a Treatment for Life-Threatening Retroperitoneal Hemorrhage in COVID-19 Patients on Anticoagulant Therapy. Open J. Emerg. Med. 2021, 9, 209–215. [Google Scholar] [CrossRef]
- Lucatelli, P.; De Rubeis, G.; Citone, M.; Lucarelli, N.M.; Pasqualini, V.; Sturiale, M.; Giuliani, S.; Rosati, M.; Ceccherini, C.; Corona, M.; et al. Heparin-Related Major Bleeding in Covid-19-Positive Patient: Perspective from the Outbreak. Cardiovasc Interv. Radiol. 2020, 43, 1216–1217. [Google Scholar] [CrossRef]
- Lucatelli, P.; Rocco, B.; Nardis, P.G.; Cannavale, A.; Bezzi, M.; Catalano, C.; Corona, M. Bleeding in COVID Patients: What We Have Understood So Far. Cardiovasc Interv. Radiol. 2021, 44, 666–668. [Google Scholar] [CrossRef]
- Tiralongo, F.; Seminatore, S.; Di Pietro, S.; Distefano, G.; Galioto, F.; Vacirca, F.; Giurazza, F.; Palmucci, S.; Venturini, M.; Scaglione, M.; et al. Spontaneous Retroperitoneal Hematoma Treated with Percutaneous Transarterial Embolization in COVID-19 Era: Diagnostic Findings and Procedural Outcome. Tomography 2022, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Trentadue, M.; Calligaro, P.; Lazzarini, G.; Boseggia, F.B.; Residori, E.; Hu, J.; Vanti, S.; Lillo, L.; Varischi, G.; Cerini, R. Spontaneous bleeding in COVID-19: A retrospective experience of an Italian COVID-19 hospital. SA J. Radiol. 2022, 26, 2509. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.J.; Rahim, S.; Davidson, J.C.; Hanks, S.E.; Tam, A.L.; Walker, T.G.; Wilkins, L.R.; Sarode, R.; Weinberg, I. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions—Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2019, 30, 1168–1184.e1. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Stahel, P.F.; Montori, G.; Biffl, W.; Horer, T.M.; Catena, F.; Kluger, Y.; Moore, E.E.; Peitzman, A.B.; Ivatury, R.; et al. Pelvic trauma: WSES classification and guidelines. World J. Emerg. Surg. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Guillen, K.; Chevallier, O. Is CT scan needed before transcatheter arterial embolisation of upper gastrointestinal bleeding following endoscopic resection? Br. J. Radiol. 2022, 95, 20210573. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Wood, B.J.; Gaudino, C.; Angileri, S.A.; Jones, E.C.; Hausegger, K.; Carrafiello, G. How to Handle a COVID-19 Patient in the Angiographic Suite. Cardiovasc. Interv. Radiol. 2020, 43, 820. [Google Scholar] [CrossRef]
- Dariushnia, S.R.; Redstone, E.A.; Heran, M.K.S.; Cramer, H.R.; Ganguli, S.; Gomes, A.S.; Hogan, M.J.; Himes, E.A.; Patel, S.; Schiro, B.J.; et al. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Transcatheter Embolization. J. Vasc. Interv. Radiol. 2021, 32, 476.e1–476.e33. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology Clinical Practice Guidelines. J. Vasc. Interv. Radiol. 2003, 14, S199–S202. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Ammendola, M.; Manti, F.; Siciliano, M.A.; Minici, M.; Komaei, I.; Currò, G.; Laganà, D. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization (DSM-TACE) in the Downstaging of Intermediate-Stage Hepatocellular Carcinoma (HCC) in Patients With a Child-Pugh Score of 8-9. Front. Pharm. 2021, 12, 634087. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Ammendola, M.; Manti, F.; Siciliano, M.A.; Giglio, E.; Minici, M.; Melina, M.; Currò, G.; Laganà, D. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization as a Bridging Therapy in Patients with Early Stage Hepatocellular Carcinoma and Child-Pugh Stage B Eligible for Liver Transplant. Front. Pharm. 2021, 12, 634084. [Google Scholar] [CrossRef]
- Costa, D.; Ielapi, N.; Minici, R.; Peluso, A.; Bracale, U.M.; Andreucci, M.; Serra, R. Risk Factors for Bleeding Varicose Veins in Patients with Chronic Venous Disease. Medicina 2023, 59, 1034. [Google Scholar] [CrossRef]
- Minici, R.; Mercurio, M.; Iannò, B.; Galasso, O.; Gasparini, G.; Laganà, D. Advantages of the Use of Axial Traction Magnetic Resonance Imaging (MRI) of the Shoulder in Patients with Suspected Rota-Tor Cuff Tears: An Exploratory Pilot Study. Healthcare 2023, 11, 724. [Google Scholar] [CrossRef]
- Ammendola, M.; Filice, F.; Battaglia, C.; Romano, R.; Manti, F.; Minici, R.; de’Angelis, N.; Memeo, R.; Laganà, D.; Navarra, G.; et al. Left hemicolectomy and low anterior resection in colorectal cancer patients: Knight-griffen vs. transanal purse-string suture anastomosis with no-coil placement. Front. Surg. 2023, 10, 1093347. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Talarico, M.; Schepis, F.; Coppi, F.; Sgura, F.A.; Monopoli, D.E.; Minici, R.; Boriani, G. Effects of sildenafil on right ventricle remodelling in Portopulmonary hypertension. Pulm. Pharmacol. Ther. 2021, 70, 102071. [Google Scholar] [CrossRef]
- Rossi, R.; Talarico, M.; Pascale, A.; Pascale, V.; Minici, R.; Boriani, G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics 2022, 12, 2572. [Google Scholar] [CrossRef]
- Cernigliaro, M.; Stanca, C.; Galbiati, A.; Spinetta, M.; Coda, C.; Negroni, D.; Laganà, D.; Minici, R.; Airoldi, C.; Carriero, A.; et al. Innovation in Acute Ischemic Stroke Patients over 80 y/o—A Retrospective Monocentric Study on Mechanical Thrombectomy of Consecutive Patients: Is Age an Adequate Selection Criterion? J. Clin. Med. 2023, 12, 3688. [Google Scholar] [CrossRef]
- Abate, V.; Casoria, A.; Rendina, D.; Muscariello, R.; Nuzzo, V.; Vargas, M.; Servillo, G.; Venetucci, P.; Conca, P.; Tufano, A.; et al. Spontaneous Muscle Hematoma in Patients with COVID-19: A Systematic Literature Review with Description of an Additional Case Series. Semin. Thromb. Hemost. 2022, 48, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Sapoval, M.; Chousterman, B.G.; di Primio, M.; Guerot, E.; Pellerin, O. Spontaneous Soft-Tissue Hemorrhage in Anticoagulated Patients: Safety and Efficacy of Embolization. Am. J. Roentgenol. 2015, 204, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Sapoval, M.; Vidal, V.; Déan, C.; Del Giudice, C.; Tradi, F.; Chevallier, O.; Charles-Nelson, A.; Pellerin, O.; Loffroy, R. Safety and Efficacy of Peripheral Embolization with EASYX Liquid Embolic Agent: A Multicenter Prospective Study. J. Vasc. Interv. Radiol. 2021, 32, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Corona, M.; Teodoli, L.; Nardis, P.; Cannavale, A.; Rocco, B.; Trobiani, C.; Cipollari, S.; Zilahi de Gyurgyokai, S.; Bezzi, M.; et al. Use of Phil Embolic Agent for Bleeding in Non-Neurological Interventions. J. Clin. Med. 2021, 10, 701. [Google Scholar] [CrossRef]
- Loffroy, R.; Guiu, B. Role of transcatheter arterial embolization for massive bleeding from gastroduodenal ulcers. World J. Gastroenterol. 2009, 15, 5889–5897. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Venturini, M.; Fontana, F.; Guzzardi, G.; Pingitore, A.; Piacentino, F.; Serra, R.; Coppola, A.; Santoro, R.; Laganà, D. Efficacy and Safety of Ethylene-Vinyl Alcohol (EVOH) Copolymer-Based Non-Adhesive Liquid Embolic Agents (NALEAs) in Transcatheter Arterial Embolization (TAE) of Acute Non-Neurovascular Bleeding: A Multicenter Retrospective Cohort Study. Medicina 2023, 59, 710. [Google Scholar] [CrossRef]
- Pathi, R.; Voyvodic, F.; Thompson, W.R. Spontaneous extraperitoneal haemorrhage: Computed tomography diagnosis and treatment by selective arterial embolization. Australas. Radiol. 2004, 48, 123–128. [Google Scholar] [CrossRef]
- Kalinowski, E.A.; Trerotola, S.O. Postcatheterization retroperitoneal hematoma due to spontaneous lumbar arterial hemorrhage. Cardiovasc Interv. Radiol. 1998, 21, 337–339. [Google Scholar] [CrossRef]
- Isokangas, J.-M.; Perälä, J.M. Endovascular embolization of spontaneous retroperitoneal hemorrhage secondary to anticoagulant treatment. Cardiovasc Interv. Radiol. 2004, 27, 607–611. [Google Scholar] [CrossRef]
- Choo, I.W.; Sproat, I.A.; Cho, K.J. Transcatheter embolization of the marginal artery of Drummond as treatment for life-threatening retroperitoneal hemorrhage complicating heparin therapy. Cardiovasc Interv. Radiol. 1994, 17, 161–163. [Google Scholar] [CrossRef]
- Zissin, R.; Gayer, G.; Kots, E.; Ellis, M.; Bartal, G.; Griton, I. Transcatheter arterial embolisation in anticoagulant-related haematoma--a current therapeutic option: A report of four patients and review of the literature. Int. J. Clin. Pract. 2007, 61, 1321–1327. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chang, C.-C.; Liou, J.-M.; Jaw, F.-S.; Liu, K.-L. Transcatheter arterial embolization with N-butyl cyanoacrylate for nonvariceal upper gastrointestinal bleeding in hemodynamically unstable patients: Results and predictors of clinical outcomes. J. Vasc. Interv. Radiol. 2014, 25, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalak, G.; Chevallier, O.; Falvo, N.; Di Marco, L.; Bertaut, A.; Moulin, B.; Abi-Khalil, C.; Gehin, S.; Charles, P.-E.; Latournerie, M.; et al. Safety and efficacy of transcatheter embolization with Glubran®2 cyanoacrylate glue for acute arterial bleeding: A single-center experience with 104 patients. Abdom. Radiol. 2018, 43, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Darnige, L.; Sapoval, M.; Pellerin, O. Spontaneous soft tissue hematomas. Diagn. Interv. Imaging 2015, 96, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ouchi, G.; Miyagi, K.; Higure, Y.; Otsuki, M.; Nishiyama, N.; Kinjo, T.; Nakamatsu, M.; Tateyama, M.; Kukita, I.; et al. Case Report: Iliopsoas Hematoma during the Clinical Course of Severe COVID-19 in Two Male Patients. Am. J. Trop. Med. Hyg. 2021, 104, 1018–1021. [Google Scholar] [CrossRef]
- Barral, M.; Pellerin, O.; Tran, V.T.; Gallix, B.; Boucher, L.-M.; Valenti, D.; Sapoval, M.; Soyer, P.; Dohan, A. Predictors of Mortality from Spontaneous Soft-Tissue Hematomas in a Large Multicenter Cohort Who Underwent Percutaneous Transarterial Embolization. Radiology 2019, 291, 250–258. [Google Scholar] [CrossRef]
- Riu, P.; Albarello, F.; Di Stefano, F.; Vergori, A.; D’Abramo, A.; Cerini, C.; Nocioni, M.; Morucci, M.; Tetaj, N.; Cristofaro, M.; et al. Management of Spontaneous Bleeding in COVID-19 Inpatients: Is Embolization Always Needed? J. Clin. Med. 2021, 10, 4119. [Google Scholar] [CrossRef] [PubMed]
- Berná, J.D.; Garcia-Medina, V.; Guirao, J.; Garcia-Medina, J. Rectus sheath hematoma: Diagnostic classification by CT. Abdom. Imaging 1996, 21, 62–64. [Google Scholar] [CrossRef]
- Qanadli, S.D.; El Hajjam, M.; Mignon, F.; Bruckert, F.; Chagnon, S.; Lacombe, P. Life-threatening spontaneous psoas haematoma treated by transcatheter arterial embolization. Eur. Radiol. 1999, 9, 1231–1234. [Google Scholar] [CrossRef]
- Di Pietro, S.; Tiralongo, F.; Desiderio, C.M.; Vacirca, F.; Palmucci, S.; Giurazza, F.; Venturini, M.; Basile, A. Efficacy of Percutaneous Transarterial Embolization in Patients with Spontaneous Abdominal Wall Hematoma and Comparison between Blind and Targeted Embolization Approaches. J. Clin. Med. 2022, 11, 1270. [Google Scholar] [CrossRef]
- Cavaliere, K.; Levine, C.; Wander, P.; Sejpal, D.V.; Trindade, A.J. Management of upper GI bleeding in patients with COVID-19 pneumonia. Gastrointest. Endosc. 2020, 92, 454–455. [Google Scholar] [CrossRef]
- Minici, R.; Ammendola, M.; Talarico, M.; Luposella, M.; Minici, M.; Ciranni, S.; Guzzardi, G.; Laganà, D. Endovascular recanalization of chronic total occlusions of the native superficial femoral artery after failed femoropopliteal bypass in patients with critical limb ischemia. CVIR Endovasc. 2021, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Né, R.; Chevallier, O.; Falvo, N.; Facy, O.; Berthod, P.-E.; Galland, C.; Gehin, S.; Midulla, M.; Loffroy, R. Embolization with ethylene vinyl alcohol copolymer (Onyx®) for peripheral hemostatic and non-hemostatic applications: A feasibility and safety study. Quant. Imaging Med. Surg. 2018, 8, 280–290. [Google Scholar] [CrossRef]
- Kim, P.H.; Tsauo, J.; Shin, J.H.; Yun, S.-C. Transcatheter Arterial Embolization of Gastrointestinal Bleeding with N-Butyl Cyanoacrylate: A Systematic Review and Meta-Analysis of Safety and Efficacy. J. Vasc. Interv. Radiol. 2017, 28, 522–531.e5. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Paone, S.; Talarico, M.; Zappia, L.; Abdalla, K.; Petullà, M.; Laganà, D. Percutaneous treatment of vascular access-site complications: A ten years’ experience in two centres. CVIR Endovasc. 2020, 3, 29. [Google Scholar] [CrossRef]
- Regine, R.; Palmieri, F.; De Siero, M.; Rescigno, A.; Sica, V.; Cantarela, R.; Villari, V. Embolization of traumatic and non-traumatic peripheral vascular lesions with Onyx. Interv. Med. Appl. Sci. 2015, 7, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.J.; Wang, C.E.; Wang, Y.H.; Xie, L.L.; Liu, T.H.; Ren, W.C. Transcatheter arterial embolization of acute gastrointestinal tumor hemorrhage with Onyx. Indian J. Cancer. 2015, 51 (Suppl. S2), e56–e59. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; Ierardi, A.M.; Petullà, M.; Bracale, U.M.; Carrafiello, G.; Laganà, D. Thoracic endovascular repair for blunt traumatic thoracic aortic injury: Long-term results. Vascular 2022, 0, 17085381221127740. [Google Scholar] [CrossRef]
- Bracale, U.M.; Peluso, A.; Panagrosso, M.; Cecere, F.; Del Guercio, L.; Minici, R.; Giannotta, N.; Ielapi, N.; Licastro, N.; Serraino, G.F.; et al. Ankle-Brachial Index evaluation in totally percutaneous approach vs. femoral artery cutdown for endovascular aortic repair of abdominal aortic aneurysms. Chirurgia 2022, 35, 349–354. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; De Rosi, N.; Ciranni, S.; Talarico, M.; Petullà, M.; Guzzardi, G.; Fontana, F.; Laganà, D. Endovascular treatment of femoro-popliteal occlusions with retrograde tibial access after failure of the antegrade approach. Catheter. Cardiovasc. Interv. 2023, 101, 1108–1119. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; Giurdanella, M.; Talarico, M.; Siciliano, M.A.; Carrafiello, G.; Laganà, D. Efficacy and Safety of Distal Radial Access for Transcatheter Arterial Chemoembolization (TACE) of the Liver. J. Pers. Med. 2023, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Serra, R.; Maglia, C.; Guzzardi, G.; Spinetta, M.; Fontana, F.; Venturini, M.; Laganà, D. Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers. J. Pers. Med. 2023, 13, 812. [Google Scholar] [CrossRef] [PubMed]
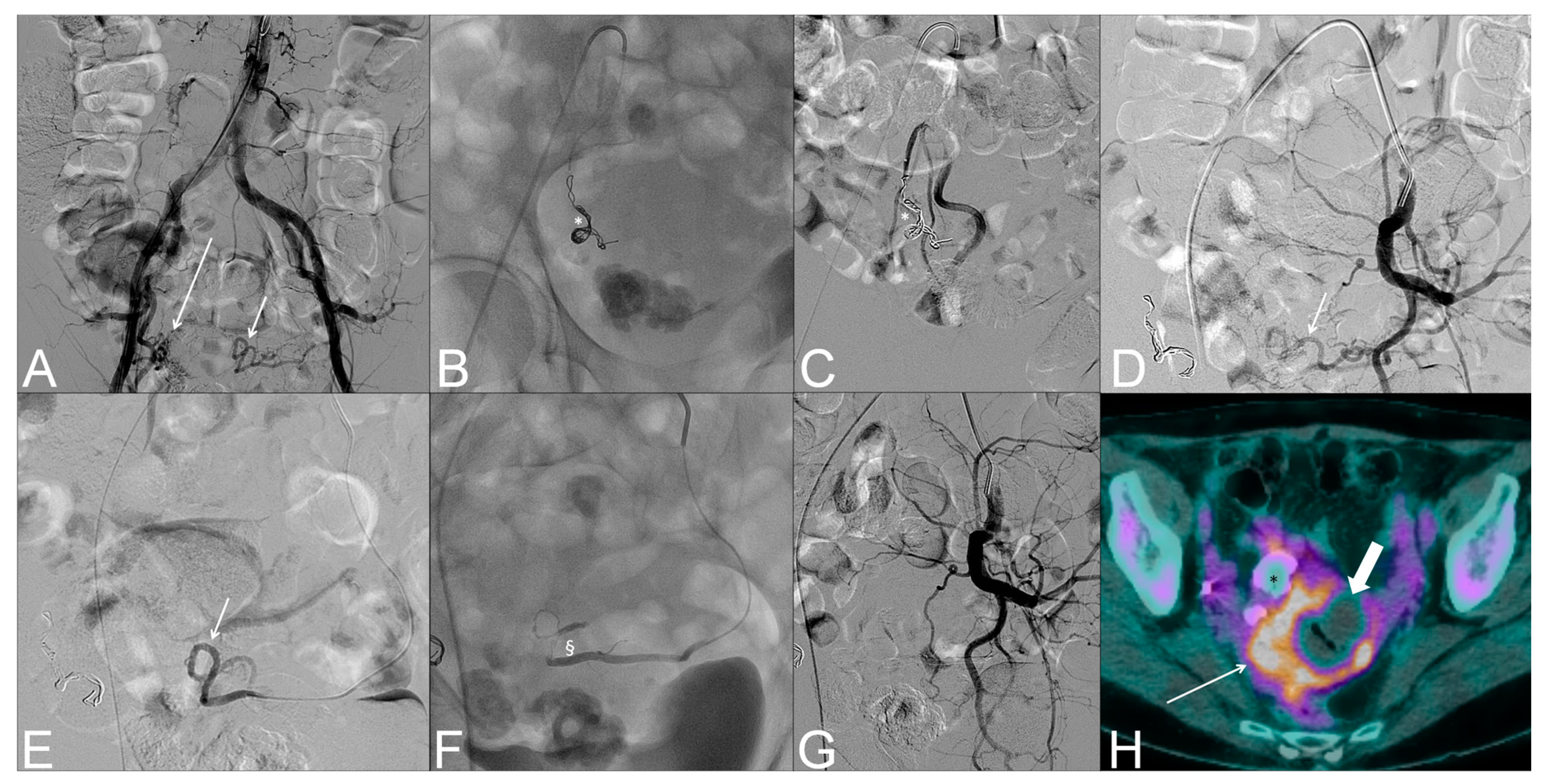
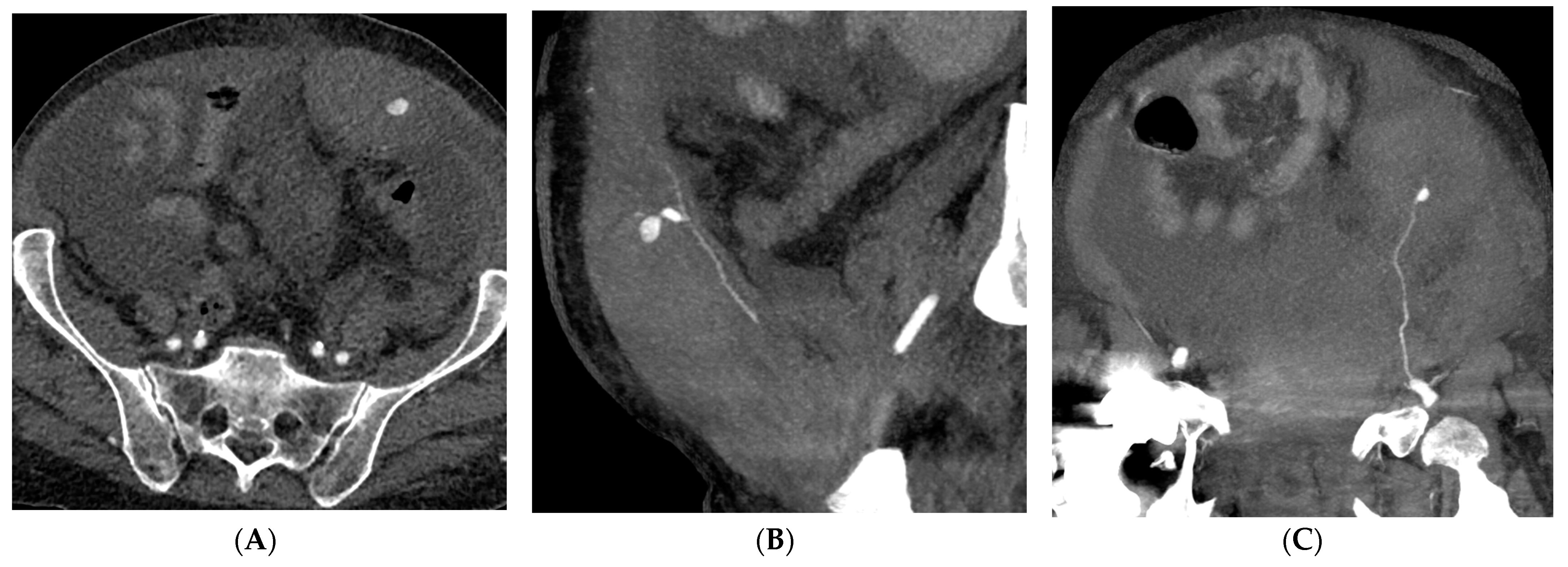
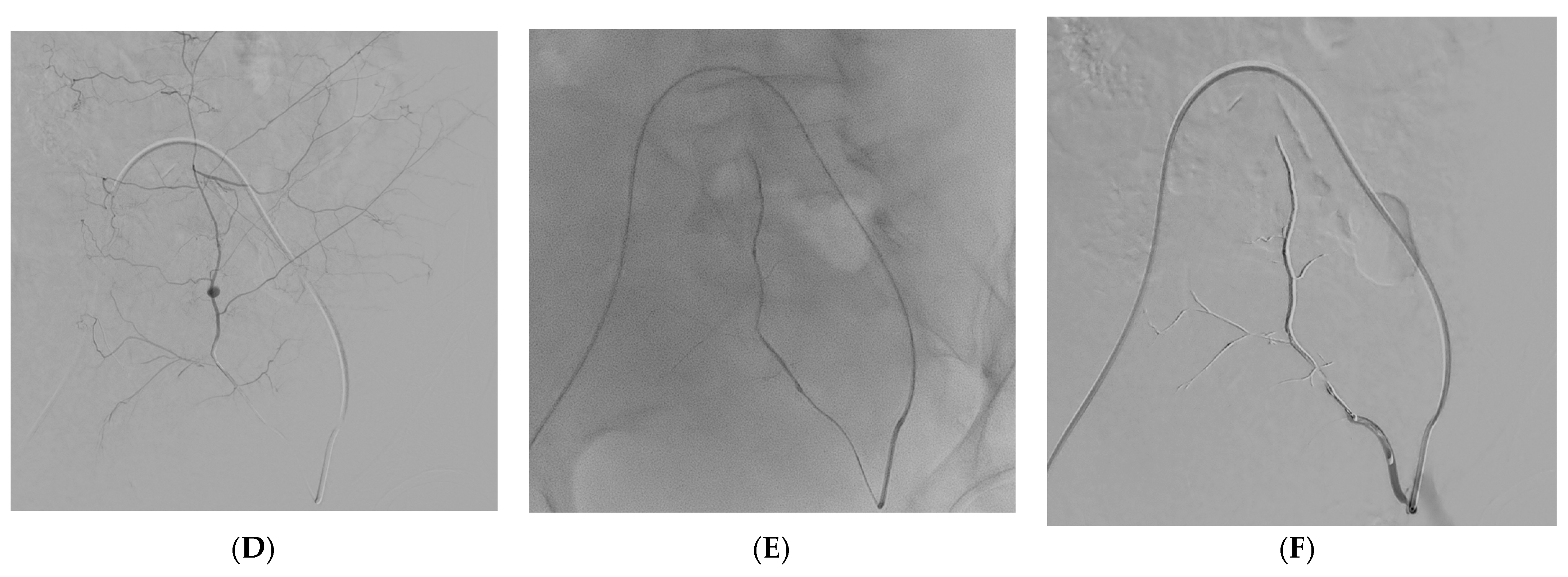
| Variables | All Patients (n = 73) |
|---|---|
| Age (years) | 65.8 (±15.4) |
| Sex (M/F) | 48 (65.8%)/25 (34.2%) |
| BMI | 26 (±3.6) |
| eGFR (mL/min) | 66.4 (±25) |
| CKD Stage | 2 (1–3) |
| INR | 1.3 (±0.3) |
| aPTT (s) | 37.5 (±4.9) |
| PT (s) | 13.9 (±2.6) |
| D-Dimer (mg/L) | 1.2 (±0.7) |
| Fibrinogen (g/L) | 1.9 (±0.6) |
| Platelet count (No. ×103/μL) | 367.4 (±99) |
| Coagulopathy (no/yes) | 29 (39.7%)/44 (60.3%) |
| Hemoglobin (g/dL) | 7.7 (±0.8) |
| CT-angiography execution | 69 (94.5%) |
| Bleeding on CT-angiography | 69 (94.5%) |
| Hematoma volume (mL) | 356.2 (±309.4) |
| Antiplatelet therapy | 12 (16.4%) |
| 9 (12.3%) |
| 3 (4.1%) |
| Anticoagulant therapy | 47 (64.4%) |
| Antiplatelet AND Anticoagulant therapy | 1 (1.4%) |
| Antiplatelet OR Anticoagulant therapy | 57 (78.1%) |
| Variables | All Patients (n = 73) |
|---|---|
| Bleeding on XA | 72 (98.6%) |
| Blind embolization | 1 (1.4%) |
| Site of bleeding | |
| 9 (12.3%) |
| 1 (1.4%) |
| 2 (2.8%) |
| 49 (67.1%) |
| 8 (11%) |
| 2 (2.7%) |
| 2 (2.7%) |
| Number of embolized vessels | 1.2 (±0.5) |
| Cause of the bleeding | |
| 18 (24.7%) |
| 46 (63.0%) |
| 9 (12.3%) |
| Embolic agent | |
| 41 (56.2%) |
| 16 (21.9%) |
| 12 (16.4%) |
| 2 (2.75%) |
| 2 (2.75%) |
| Intraoperative unfractionated heparin (IU) | |
| 67 (91.8%) |
| 4 (5.5%) |
| 2 (2.7%) |
| Intraoperative contrast medium (mL) | 35.3 (±9.5) |
| Volume of contrast to creatinine clearance ratio | 0.7 (±0.6) |
| Vascular access site | |
| 54 (74%) |
| 16 (21.9%) |
| 3 (4.1%) |
| Sheath diameter, 4F/5F/6F/≥7F | 9 (12.3%)/59 (80.8%)/5 (6.8%)/0 (0%) |
| Angiography injection technique (manual/powered) | 40 (54.8%)/33 (45.2%) |
| CT-to-groin time (min) | 41.8 (±48.9) |
| Procedure time (min) | 28.3 (±8.4) |
| CT-to-embolization time (min) | 70.1 (±48.8) |
| Fluoroscopy time (min) | 7.7 (±3) |
| Cumulative air kerma (mGy) | 162 (±60.8) |
| Dose area product (DAP) (Gy/cm2) | 25.4 (±9.5) |
| Variables | All Patients (n = 73) |
|---|---|
| Technical success | 73 (100%) |
| Clinical success | 67 (91.8%) |
| Vascular access site hemostasis | |
| 68 (93.2%) |
| 5 (6.8%) |
| Units of packed red blood cells transfused per patient | 1 (±0.6) |
| Rebleeding | 6 (8.2%) |
| Non-target embolization | 0 (0%) |
| Complications | 13 (17.8%) |
| Vascular access-site complications (VASCs) | 4 (5.5%) |
| Complications, according to SIR classifications | |
| 60 (82.2%) |
| 10 (13.7%) |
| 3 (4.1%) |
| Complications, according to CIRSE classification | |
| 60 (82.2%) |
| 11 (15.1%) |
| 2 (2.7%) |
| Treatment required for complications | |
| 60 (82.2%) |
| 8 (11.0%) |
| 5 (6.8%) |
| 0 (0%) |
| 30-day bleeding-related mortality | 2 (2.7%) |
| Variables | Group 1 (n = 44) Patients with Coagulopathy | Group 2 (n = 29) Patients without Coagulopathy | p Value |
|---|---|---|---|
| BMI | 25.8 (±3.2) | 26.1 (±3.9) | 0.5856 |
| INR | 1.4 (±0.3) | 1.1 (±0.1) | <0.0001 |
| D-Dimer (mg/L) | 1.7 (±0.2) | 0.4 (±0.2) | <0.0001 |
| Platelet count (No. ×103/μL) | 344.7 (±119.9) | 401.8 (±33.2) | 0.0543 |
| Anticoagulant therapy | 43 (97.7%) | 4 (13.8%) | <0.0001 |
| Hematoma volume (mL) | 417.5 (±285.1) | 206.2 (NA) | 0.0001 |
| Cause of the bleeding | <0.0001 | ||
| 44 (100%) | 2 (6.9%) | |
| 0 (0%) | 27 (93.1%) | |
| CT-to-groin time (min) | 51.1 (±58.5) | 25.4 (NA) | 0.0316 |
| Procedure time (min) | 28.2 (±9.3) | 28.4 (±7) | 0.7432 |
| Fluoroscopy time (min) | 7.5 (±3) | 7.9 (±2.9) | 0.7236 |
| Technical success | 44 (100%) | 29 (100%) | 1 |
| Clinical success | 41 (93.2%) | 26 (97.8%) | 0.6762 |
| Rebleeding | 41 (93.2%) | 26 (89.6%) | 0.6762 |
| Complications | 6 (13.6%) | 7 (24.1%) | 0.3499 |
| 30-day bleeding-related mortality | 1 (2.3%) | 1 (3.4%) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minici, R.; Fontana, F.; Venturini, M.; Guzzardi, G.; Siciliano, A.; Piacentino, F.; Serra, R.; Coppola, A.; Guerriero, P.; Apollonio, B.; et al. Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient. Medicina 2023, 59, 1062. https://doi.org/10.3390/medicina59061062
Minici R, Fontana F, Venturini M, Guzzardi G, Siciliano A, Piacentino F, Serra R, Coppola A, Guerriero P, Apollonio B, et al. Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient. Medicina. 2023; 59(6):1062. https://doi.org/10.3390/medicina59061062
Chicago/Turabian StyleMinici, Roberto, Federico Fontana, Massimo Venturini, Giuseppe Guzzardi, Agostino Siciliano, Filippo Piacentino, Raffaele Serra, Andrea Coppola, Pasquale Guerriero, Biagio Apollonio, and et al. 2023. "Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient" Medicina 59, no. 6: 1062. https://doi.org/10.3390/medicina59061062
APA StyleMinici, R., Fontana, F., Venturini, M., Guzzardi, G., Siciliano, A., Piacentino, F., Serra, R., Coppola, A., Guerriero, P., Apollonio, B., Santoro, R., Team, M. R., Brunese, L., & Laganà, D. (2023). Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient. Medicina, 59(6), 1062. https://doi.org/10.3390/medicina59061062










