Mesenchymal Stem Cells for Enhanced Healing of the Medial Collateral Ligament of the Knee Joint
Abstract
1. Introduction
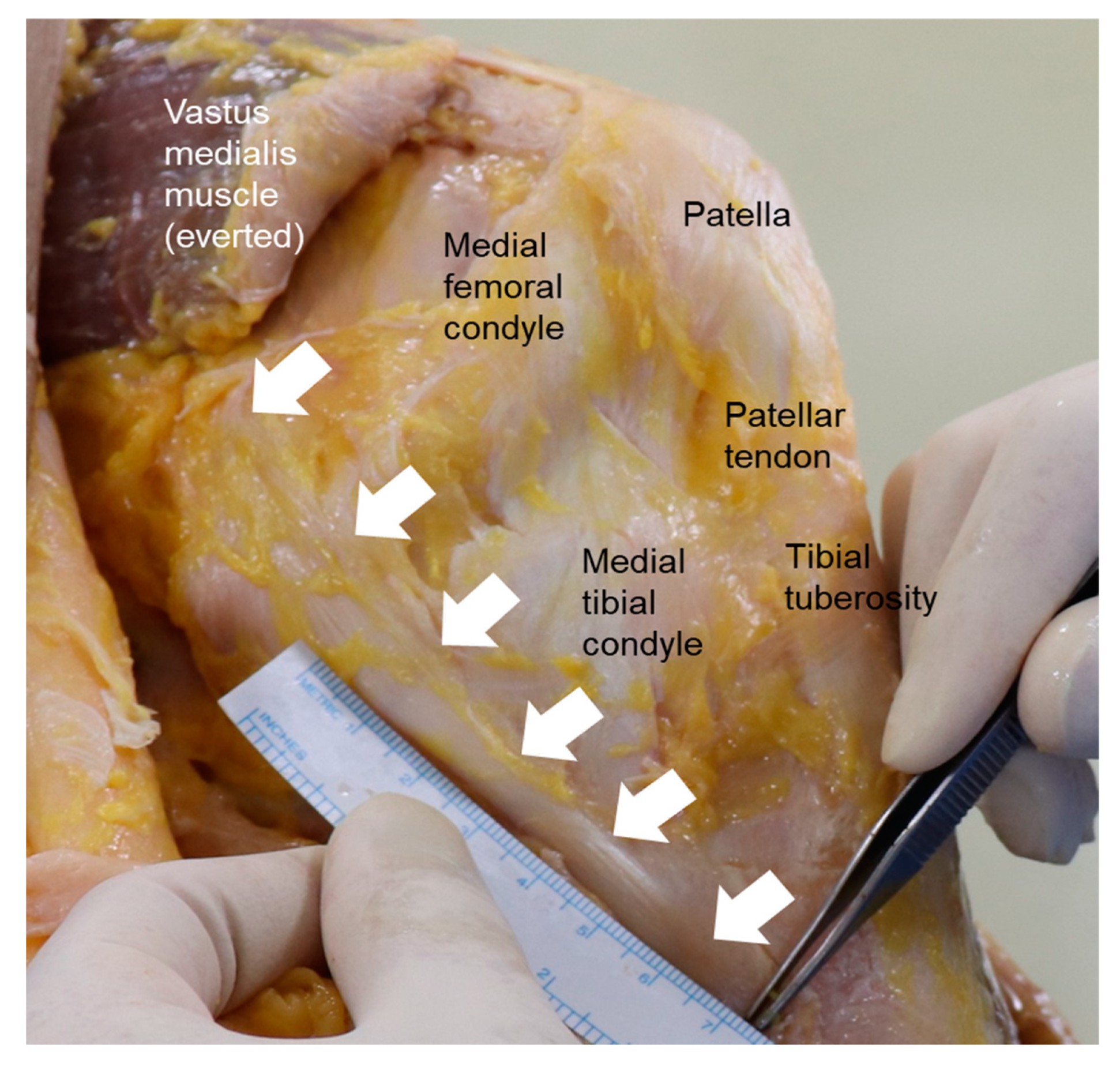
2. Anatomy of the MCL
3. Biomechanics of the MCL
4. Etiology and Mechanism of MCL Injury
5. Diagnosis of MCL Injury
6. Treatment of MCL Injury
6.1. Non-Operative Treatment
6.2. Operative Treatment
7. Advent of MSCs as Cell Therapy for Enhanced MCL Healing
8. Recent Research on MSC Applications for MCL Injury
| Ref. | Cell Source | Model | Results |
|---|---|---|---|
| Watanabe N. et al. [60] | BM-MSCs | Rat | At 3 days, transplanted MSCs from transgenic rats were evident in injured sites as well as the mid-substance of the recipient MCL. The MSCs continued to survive and were similar to recipient MCL fibroblasts morphologically at 28 days. |
| Tei K. et al. [61] | Peripheral blood CD34-positive cells | Rat | Local transplantation of human CD34+ cells in the ruptured MCL of immunodeficient rats revealed a higher expression of human-specific markers for endothelial cells, increased neovascularization and mRNA expression of VEGF, as well as higher gene expression of ligament-specific marker. In addition, histological and biomechanical properties were significantly enhanced in the CD34+ group compared to the other groups. |
| Nishimori M. et al. [62] | Muscle-derived stem cells | Rat | A total of 5 × 10⁵ MDSCs transduced with the VEGF gene was transplanted into the MCL injured site of immunodeficient rats. At 2 weeks, increased capillary density was observed in the MDSC-VEGF group compared with the other groups. In contrast, in a group transplanted with MDSCs transduced with a VEGF-specific antagonist (MDSC-sFLT1), decreased capillary density and significantly lower biomechanical properties were identified. |
| Saether E.E. et al. [63] | BM-MSCs | Rat | The higher-dose MSC group with 4 × 10⁶ cells showed decreased M2 macrophage level compared with the control groups on day 5 and 14 and increased level of several pro-inflammatory cytokines on day 5. On day 14, the lower-dose MSC group with 1 × 10⁶ cells showed lower M1 macrophage level than the higher-dose group. In a mechanical test on day 14, the lower-dose group showed an increased failure strength and stiffness compared to the higher-dose group. |
| Jiang D. et al. [64] | MCL-MSCs/CD34+cells | Rat | At 2 weeks, transplantation of combined MCL-MSCs and CD34+ cells in the MCL injured site showed a significant increase in capillary density than in the other groups. Failure load of the healing ligament was also superior in the combination treatment. |
| Saether E.E. et al. [65] | BM-MSCs | Rat | The primed MSCs for 48 h using polyinosinic acid and polycytidylic acid showed increased early endothelization, M2 macrophages, IL-1 receptor antagonists and procollagen 1a level compared with the other groups. |
9. Summary and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bollen, S. Epidemiology of knee injuries: Diagnosis and triage. Br. J. Sports Med. 2000, 34, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of athletic knee injuries: A 10-year study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Hanhan, S.; Ejzenberg, A.; Goren, K.; Saba, F.; Suki, Y.; Sharon, S.; Shilo, D.; Waxman, J.; Spitzer, E.; Shahar, R.; et al. Skeletal ligament healing using the recombinant human amelogenin protein. J. Cell. Mol. Med. 2016, 20, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kanamori, A.; Washio, T.; Aoto, K.; Uemura, K.; Sakane, M.; Ochiai, N. The effects of plasma rich in growth factors (PRGF-Endoret) on healing of medial collateral ligament of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1763–1769. [Google Scholar] [CrossRef]
- Costa, E.L.D.; Teixeira, L.E.M.; Pádua, B.J.; Araújo, I.D.; Vasconcellos, L.S.; Dias, L.S.B. Biomechanical study of the effect of platelet rich plasma on the treatment of medial collateral ligament lesion in rabbits. Acta Cir. Bras. 2017, 32, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.F.; Marshall, J.L. The supporting structures and layers on the medial side of the knee: An anatomical analysis. J. Bone Jt. Surg. Am. 1979, 61, 56–62. [Google Scholar] [CrossRef]
- Wymenga, A.B.; Kats, J.J.; Kooloos, J.; Hillen, B. Surgical anatomy of the medial collateral ligament and the posteromedial capsule of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 229–234. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Engebretsen, A.H.; Ly, T.V.; Johansen, S.; Wentorf, F.A.; Engebretsen, L. The anatomy of the medial part of the knee. J. Bone Jt. Surg. Am. 2007, 89, 2000–2010. [Google Scholar] [CrossRef]
- Marchant, M.J., Jr.; Tibor, L.M.; Sekiya, J.K.; Hardaker, W.T., Jr.; Garrett, W.E., Jr.; Taylor, D.C. Management of medial-sided knee injuries, part 1: Medial collateral ligament. Am. J. Sports Med. 2011, 39, 1102–1113. [Google Scholar] [CrossRef]
- Warren, L.A.; Marshall, J.L.; Girgis, F. The prime static stabilizer of the medical side of the knee. J. Bone Jt. Surg. Am. 1974, 56, 665–674. [Google Scholar] [CrossRef]
- Levy, I.M.; Torzilli, P.A.; Warren, R.F. The effect of medial meniscectomy on anterior-posterior motion of the knee. J. Bone Jt. Surg. Am. 1982, 64, 883–888. [Google Scholar] [CrossRef]
- Grood, E.S.; Noyes, F.R.; Butler, D.L.; Suntay, W.J. Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J. Bone Jt. Surg. Am. 1981, 63, 1257–1269. [Google Scholar] [CrossRef]
- Gardiner, J.C.; Weiss, J.A.; Rosenberg, T.D. Strain in the human medial collateral ligament during valgus loading of the knee. Clin. Orthop. Relat. Res. 2001, 391, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Fetto, J.F.; Marshall, J.L. Medial collateral ligament injuries of the knee: A rationale for treatment. Clin. Orthop. Relat. Res 1978, 132, 206–218. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Carr, D.R. Combined anterior and posterior cruciate and medial collateral ligament injury: Nonsurgical and delayed surgical treatment. Instr. Course Lect. 2003, 52, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Rachun, A. Standard Nomenclature of Athletic Injuries, 1st ed.; Medical Association: Chicago, IL, USA, 1966; p. 157. [Google Scholar]
- Hughston, J.C. The importance of the posterior oblique ligament in repairs of acute tears of the medial ligaments in knees with and without an associated rupture of the anterior cruciate ligament. Results of long-term follow-up. J. Bone Jt. Surg. Am. 1994, 76, 1328–1344. [Google Scholar] [CrossRef] [PubMed]
- Bergfeld, J. Symposium: Functional rehabilitation of isolated medial collateral ligament sprains. First-, second-, and third-degree sprains. Am. J. Sports Med. 1979, 7, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Osborne, J.R.; Gordon, W.T.; Hinkin, D.T.; Brinker, M.R. The natural history of bone bruises. A prospective study of magnetic resonance imaging-detected trabecular microfractures in patients with isolated medial collateral ligament injuries. Am. J. Sports Med. 1998, 26, 15–19. [Google Scholar] [CrossRef]
- Creighton, R.A.; Spang, J.T.; Dahners, L.E. Basic Science of Ligament Healing: Medial Collateral Ligament Healing With and Without Treatment. Sport. Med. Arthrosc. Rev. 2005, 13, 145–150. [Google Scholar] [CrossRef]
- Thornton, G.M.; Johnson, J.C.; Maser, R.V.; Marchuk, L.L.; Shrive, N.G.; Frank, C.B. Strength of medial structures of the knee joint are decreased by isolated injury to the medial collateral ligament and subsequent joint immobilization. J. Orthop. Res. 2005, 23, 1191–1198. [Google Scholar] [CrossRef]
- Walsh, W.R.; Wiggins, M.E.; Fadale, P.D.; Ehrlich, M.G. Effects of a delayed steroid injection on ligament healing using a rabbit medial collateral ligament model. Biomaterials 1995, 16, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Mackie, J.W.; Goldin, B.; Foss, M.L.; Cockrell, J.L. Mechanical properties of rabbit tendons after repeated anti-inflammatory steroid injections. Med. Sci. Sports 1974, 6, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Khan, N.K. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. Inflamm. Res 2005, 54, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Dahners, L.E.; Mullis, B.H. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J. Am. Acad. Orthop. Surg. 2004, 12, 139–143. [Google Scholar] [CrossRef]
- Mehallo, C.J.; Drezner, J.A.; Bytomski, J.R. Practical management: Nonsteroidal antiinflammatory drug (NSAID) use in athletic injuries. Clin. J. Sport. Med. 2006, 16, 170–174. [Google Scholar] [CrossRef]
- Fredberg, U. Local corticosteroid injection in sport: Review of literature and guidelines for treatment. Scand. J. Med. Sci. Sports 1997, 7, 131–139. [Google Scholar] [CrossRef]
- Fadale, P.D.; Wiggins, M.E. Corticosteroid Injections: Their Use and Abuse. J. Am. Acad. Orthop. Surg. 1994, 2, 133–140. [Google Scholar] [CrossRef]
- Derscheid, G.L.; Garrick, J.G. Medial collateral ligament injuries in football. Nonoperative management of grade I and grade II sprains. Am. J. Sports Med. 1981, 9, 365–368. [Google Scholar] [CrossRef]
- Lundberg, M.; Messner, K. Long-term prognosis of isolated partial medial collateral ligament ruptures. A ten-year clinical and radiographic evaluation of a prospectively observed group of patients. Am. J. Sports Med. 1996, 24, 160–163. [Google Scholar] [CrossRef]
- Indelicato, P.A.; Hermansdorfer, J.; Huegel, M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin. Orthop. Relat. Res. 1990, 256, 174–177. [Google Scholar] [CrossRef]
- Reider, B.; Sathy, M.R.; Talkington, J.; Blyznak, N.; Kollias, S. Treatment of isolated medial collateral ligament injuries in athletes with early functional rehabilitation. A five-year follow-up study. Am. J. Sports Med. 1994, 22, 470–477. [Google Scholar] [CrossRef]
- Jones, R.E.; Henley, M.B.; Francis, P. Nonoperative management of isolated grade III collateral ligament injury in high school football players. Clin. Orthop. Relat. Res 1986, 213, 137–140. [Google Scholar] [CrossRef]
- Tibor, L.M.; Marchant, M.H., Jr.; Taylor, D.C.; Hardaker, W.T., Jr.; Garrett, W.E., Jr.; Sekiya, J.K. Management of medial-sided knee injuries, part 2: Posteromedial corner. Am. J. Sports Med. 2011, 39, 1332–1340. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Pedroza, A.D.; Parker, R.D.; Spindler, K.P.; McCarty, E.C.; Andrish, J.T. Intra-articular findings in the reconstructed multiligament-injured knee. Arthroscopy 2005, 21, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Muratsu, H.; Harada, T.; Hino, T.; Takayama, H.; Miwa, M.; Sakai, H.; Yoshiya, S.; Kurosaka, M. Avulsion fracture of the posterior oblique ligament associated with acute tear of the medial collateral ligament. Arthroscopy 2003, 19, E18. [Google Scholar] [CrossRef] [PubMed]
- Delamarter, R.B.; Hohl, M.; Hopp, E., Jr. Ligament injuries associated with tibial plateau fractures. Clin. Orthop. Relat. Res 1990, 250, 226–233. [Google Scholar] [CrossRef]
- Wilson, T.C.; Satterfield, W.H.; Johnson, D.L. Medial collateral ligament “tibial” injuries: Indication for acute repair. Orthopedics 2004, 27, 389–393. [Google Scholar] [CrossRef]
- Sims, W.F.; Jacobson, K.E. The posteromedial corner of the knee: Medial-sided injury patterns revisited. Am. J. Sports Med. 2004, 32, 337–345. [Google Scholar] [CrossRef]
- Frank, C.; McDonald, D.; Shrive, N. Collagen Fibril Diameters in the Rabbit Medial Collateral Ligament Scar. Connect. Tissue Res. 1997, 36, 261–269. [Google Scholar] [CrossRef]
- Woo, S.L.; Gomez, M.A.; Sites, T.J.; Newton, P.O.; Orlando, C.A.; Akeson, W.H. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J. Bone Jt. Surg. Am. 1987, 69, 1200–1211. [Google Scholar] [CrossRef]
- Thornton, G.M.; Leask, G.P.; Shrive, N.G.; Frank, C.B. Early medial collateral ligament scars have inferior creep behaviour. J. Orthop. Res. 2000, 18, 238–246. [Google Scholar] [CrossRef]
- Niyibizi, C.; Kavalkovich, K.; Yamaji, T.; Woo, S.L. Type V collagen is increased during rabbit medial collateral ligament healing. Knee. Surg. Sports Traumatol. Arthrosc. 2000, 8, 281–285. [Google Scholar] [CrossRef]
- Frank, C.; McDonald, D.; Bray, D.; Bray, R.; Rangayyan, R.; Chimich, D.; Shrive, N. Collagen fibril diameters in the healing adult rabbit medial collateral ligament. Connect. Tissue. Res. 1992, 27, 251–263. [Google Scholar] [CrossRef]
- Frank, C.; Woo, S.L.; Amiel, D.; Harwood, F.; Gomez, M.; Akeson, W. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am. J. Sports Med. 1983, 11, 379–389. [Google Scholar] [CrossRef]
- Dean, R.S.; DePhillipo, N.N.; LaPrade, R.F. Use of Biologics for Knee Collateral Ligament Injuries. Can We Heal Them Faster? Oper. Tech. Sports Med. 2020, 28, 150760. [Google Scholar] [CrossRef]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kopka, M.; Bradley, J.P. The Use of Biologic Agents in Athletes with Knee Injuries. J. Knee Surg. 2016, 29, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Buckwalter, J.A. AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18-20, 1987. J. Orthop. Res. 1988, 6, 907–931. [Google Scholar] [CrossRef]
- Hildebrand, K.A.; Deie, M.; Allen, C.R.; Smith, D.W.; Georgescu, H.I.; Evans, C.H.; Robbins, P.D.; Woo, S.L. Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: The use of different viral vectors and the effects of injury. J. Orthop. Res. 1999, 17, 37–42. [Google Scholar] [CrossRef]
- Day, C.S.; Kasemkijwattana, C.; Menetrey, J.; Floyd, S.S., Jr.; Booth, D.; Moreland, M.S.; Fu, F.H.; Huard, J. Myoblast-mediated gene transfer to the joint. J. Orthop. Res. 1997, 15, 894–903. [Google Scholar] [CrossRef]
- Nakamura, N.; Shino, K.; Natsuume, T.; Horibe, S.; Matsumoto, N.; Kaneda, Y.; Ochi, T. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998, 5, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Letson, A.K.; Dahners, L.E. The effect of combinations of growth factors on ligament healing. Clin. Orthop. Relat. Res. 1994, 308, 207–212. [Google Scholar] [CrossRef]
- Kuroda, R.; Kurosaka, M.; Yoshiya, S.; Mizuno, K. Localization of growth factors in the reconstructed anterior cruciate ligament: Immunohistological study in dogs. Knee Surg. Sports Traumatol. Arthrosc. 2000, 8, 120–126. [Google Scholar] [CrossRef]
- Batten, M.L.; Hansen, J.C.; Dahners, L.E. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J. Orthop. Res. 1996, 14, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, K.A.; Woo, S.L.; Smith, D.W.; Allen, C.R.; Deie, M.; Taylor, B.J. Schmidt CC. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am. J. Sports Med. 1998, 26, 549–554. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Fong, E.L.; Chan, C.K.; Goodman, S.B. Stem cell homing in musculoskeletal injury. Biomaterials 2011, 32, 395–409. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In search of the in vivo identity of mesenchymal stem cells. Stem. Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef]
- Watanabe, N.; Woo, S.L.; Papageorgiou, C.; Celechovsky, C.; Takai, S. Fate of donor bone marrow cells in medial collateral ligament after simulated autologous transplantation. Microsc. Res. Tech. 2002, 58, 39–44. [Google Scholar] [CrossRef]
- Tei, K.; Matsumoto, T.; Mifune, Y.; Ishida, K.; Sasaki, K.; Shoji, T.; Kubo, S.; Kawamoto, A.; Asahara, T.; Kurosaka, M.; et al. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem. Cells 2008, 26, 819–830. [Google Scholar] [CrossRef]
- Nishimori, M.; Matsumoto, T.; Ota, S.; Kopf, S.; Mifune, Y.; Harner, C.; Ochi, M.; Fu, F.H.; Huard, J. Role of angiogenesis after muscle derived stem cell transplantation in injured medial collateral ligament. J. Orthop. Res. 2012, 30, 627–633. [Google Scholar] [CrossRef]
- Saether, E.E.; Chamberlain, C.S.; Leiferman, E.M.; Kondratko-Mittnacht, J.R.; Li, W.J.; Brickson, S.L.; Vanderby, R. Enhanced medial collateral ligament healing using mesenchymal stem cells: Dosage effects on cellular response and cytokine profile. Stem. Cell. Rev. Rep. 2014, 10, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yang, S.; Gao, P.; Zhang, Y.; Guo, T.; Lin, H.; Geng, H. Combined effect of ligament stem cells and umbilical-cord-blood-derived CD34+ cells on ligament healing. Cell. Tissue Res. 2015, 362, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Saether, E.E.; Chamberlain, C.S.; Aktas, E.; Leiferman, E.M.; Brickson, S.L.; Vanderby, R. Primed Mesenchymal Stem Cells Alter and Improve Rat Medial Collateral Ligament Healing. Stem. Cell. Rev. Rep. 2016, 12, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Park, H.G.; Kwon, S.M. Influence of posteromedial corner injuries on clinical outcome and second-look arthroscopic findings after allograft transtibial anterior cruciate ligament reconstruction. Knee Surg. Relat. Res. 2020, 32, 41. [Google Scholar] [CrossRef]
- LaPrade, R.F.; DePhillipo, N.N.; Dornan, G.J.; Kennedy, M.I.; Cram, T.R.; Dekker, T.J.; Strauss, M.J.; Engebretsen, L.; Lind, M. Comparative Outcomes Occur After Superficial Medial Collateral Ligament Augmented Repair vs Reconstruction: A Prospective Multicenter Randomized Controlled Equivalence Trial. Am. J. Sports Med. 2022, 50, 968–976. [Google Scholar] [CrossRef]
- Madi, S.; Acharya, K.; Pandey, V. Current concepts on management of medial and posteromedial knee injuries. J. Clin. Orthop. Trauma 2022, 18, 101807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-S.; Jeon, O.-H.; Han, S.-B.; Jang, K.-M. Mesenchymal Stem Cells for Enhanced Healing of the Medial Collateral Ligament of the Knee Joint. Medicina 2023, 59, 725. https://doi.org/10.3390/medicina59040725
Lee C-S, Jeon O-H, Han S-B, Jang K-M. Mesenchymal Stem Cells for Enhanced Healing of the Medial Collateral Ligament of the Knee Joint. Medicina. 2023; 59(4):725. https://doi.org/10.3390/medicina59040725
Chicago/Turabian StyleLee, Chul-Soo, Ok-Hee Jeon, Seung-Beom Han, and Ki-Mo Jang. 2023. "Mesenchymal Stem Cells for Enhanced Healing of the Medial Collateral Ligament of the Knee Joint" Medicina 59, no. 4: 725. https://doi.org/10.3390/medicina59040725
APA StyleLee, C.-S., Jeon, O.-H., Han, S.-B., & Jang, K.-M. (2023). Mesenchymal Stem Cells for Enhanced Healing of the Medial Collateral Ligament of the Knee Joint. Medicina, 59(4), 725. https://doi.org/10.3390/medicina59040725









