Severe Vitamin D Deficiency—A Possible Cause of Resistance to Treatment in Psychiatric Pathology
Abstract
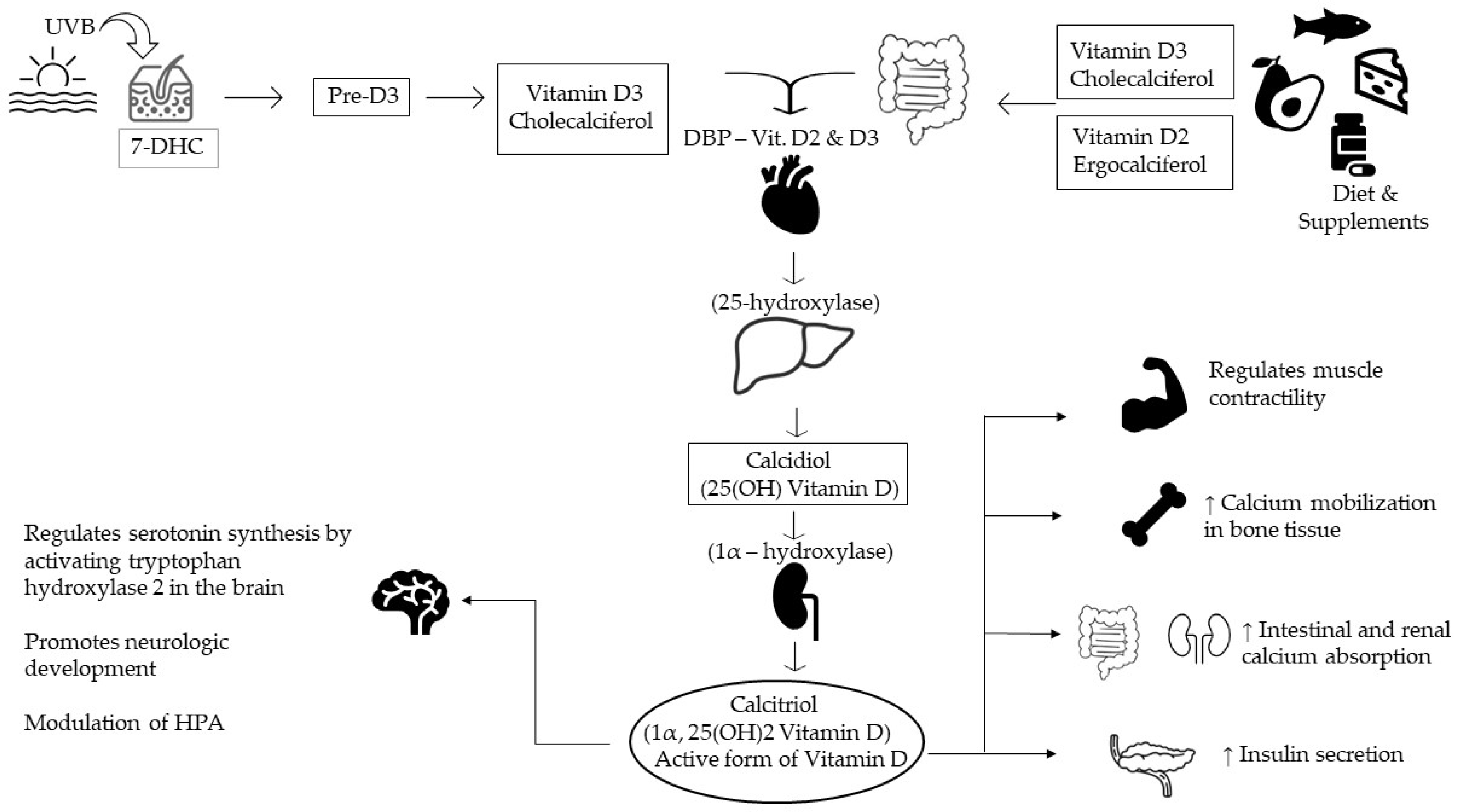
:1. Introduction
2. Materials and Methods
3. Results
3.1. Description of the Included Articles
3.2. Psychiatric Diseases and Vitamin D
3.3. Neurodevelopmental Disorders
3.4. Psychotic Spectrum Diseases
3.5. Bipolar Disorders
3.6. Depression
3.7. Anxiety Disorder
3.8. Obsessive-Compulsive Disorder
3.9. Trauma and Stress-Related Disorders
3.10. Eating Disorders
3.11. Elimination Disorders
3.12. Sleep-Wake Disorders
3.13. Sexual Disorders
3.14. Neurocognitive Diseases
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, G. 100 Years of Vitamin D: Historical aspects of vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D Deficiency in Adults: When to Test and How to Treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef]
- DeLuca, H.F. The Metabolism and Functions of Vitamin D. In Steroid Hormone Resistance: Mechanisms and Clinical Aspects; Springer: Berlin/Heidelberg, Germany, 1986; pp. 361–375. [Google Scholar] [CrossRef]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Houghton, L.; Reeder, A.; Harper, M.; Conner, T. Serum 25-Hydroxyvitamin D Concentrations and Depressive Symptoms among Young Adult Men and Women. Nutrients 2014, 6, 4720–4730. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, R.N.; Altunsoy, N.; Tikir, B.; Cingi Külük, M.; Unal, K.; Goka, S.; Aydemir, C.; Goka, E. Correlation between total vitamin D levels and psychotic psychopathology in patients with schizophrenia: Therapeutic implications for add-on vitamin D augmentation. Ther. Adv. Psychopharmacol. 2014, 4, 268–275. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 12–19. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Willner, P.; Scheel-Krüger, J.; Belzung, C. The neurobiology of depression and antidepressant action. Neurosci. Biobehav. Rev. 2013, 37, 2331–2371. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Yannakoulia, M.; Scarmeas, N. Vitamin D and Cognition: An Update of the Current Evidence. J. Alzheimer’s Dis. 2014, 42, S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The ‘sunshine’ vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Jiang, W.; Wu, D.-B.; Xiao, G.-B.; Ding, B.; Chen, E.-Q. An epidemiology survey of vitamin D deficiency and its influencing factors. Med. Clin. 2020, 154, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of All-Cause Mortality According to Serum 25-Hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, K.B.; Trigoboff, E.; Opler, L.; Demler, T.L. Physician Prescribing Practices of Vitamin D in a Psychiatric Hospital. Innov. Clin. Neurosci. 2016, 13, 21–27. [Google Scholar]
- Buscemi, S.; Buscemi, C.; Corleo, D.; De Pergola, G.; Caldarella, R.; Meli, F.; Randazzo, C.; Milazzo, S.; Barile, A.M.; Rosafio, G.; et al. Obesity and Circulating Levels of Vitamin D before and after Weight Loss Induced by a Very Low-Calorie Ketogenic Diet. Nutrients 2021, 13, 1829. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Nameni, G.; Hajiluian, G.; Shahabi, P. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci. 2017, 18, 81. [Google Scholar] [CrossRef]
- Máčová, L.; Bičíková, M.; Ostatníková, D.; Hill, M.; Stárka, L. Vitamin D, Neurosteroids and Autism. Physiol. Res. 2017, 66 (Suppl. S3), S333–S340. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Dixon, B.M.; Schneider, E.D.; Henriksen, V.T.; Weaver, L.K. Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur. J. Appl. Physiol. 2013, 113, 1523–1534. [Google Scholar] [CrossRef]
- Mayne, P.E.; Burne, T.H.J. Vitamin D in Synaptic Plasticity, Cognitive Function, and Neuropsychiatric Illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Roche, N.; Lacerre, F.; Dealberto, M.-J. Vitamin D deficiency in a psychiatric population and correlation between vitamin D and CRP. Encephale 2019, 45, 376–383. [Google Scholar] [CrossRef]
- Cuomo, A.; Maina, G.; Bolognesi, S.; Rosso, G.; Beccarini Crescenzi, B.; Zanobini, F.; Goracci, A.; Facchi, E.; Favaretto, E.; Baldini, I.; et al. Prevalence and Correlates of Vitamin D Deficiency in a Sample of 290 Inpatients with Mental Illness. Front. Psychiatry 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, H.; Palaniswamy, S.; Nordström, T.; Järvelin, M.R.; Herzig, K.H.; Jääskeläinen, E.; Seppälä, J.; Miettunen, J.; Sebert, S. Vitamin D status and correlates of low vitamin D in schizophrenia, other psychoses and non-psychotic depression–The Northern Finland Birth Cohort 1966 study. Psychiatry Res. 2019, 279, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Jegede, O.; Gayam, V.; Gunasekara, R.; Tiongson, B.; Ishola, A.; Sidhu, J.; Virk, J.; Virk, I.; Ahmed, S.; Ojo, O.; et al. Patterns of Vitamin D Deficiency in a Community Outpatient Psychiatric Practice: A Real-World Evaluation of Treatment Gaps. Psychiatr. Q. 2020, 91, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Goluza, I.; Borchard, J.; Wijesinghe, N.; Wijesinghe, K.; Pai, N. To screen or not to screen? Vitamin D deficiency in chronic mental illness. Australas. Psychiatry 2018, 26, 56–59. [Google Scholar] [CrossRef]
- Suri, T.; Suri, S.; Poremski, D.; Fang, T.; Su, A. Vitamin D deficiency in long-term hospitalization psychiatric wards in an equatorial nation. Asia-Pac. Psychiatry 2020, 12, e12390. [Google Scholar] [CrossRef]
- Ristic, S. Vitamin D Status in Patients with Mental Disorders: A Cross-Sectional Analysis of Single Cohort from Routine Practice. Acta Endocrinol. 2017, 13, 40–46. [Google Scholar] [CrossRef]
- Woodward, G.; Wan, J.C.M.; Viswanath, K.; Zaman, R. Serum Vitamin D and Magnesium levels in a psychiatric cohort. Psychiatr. Danub. 2019, 31 (Suppl. S3), 221–226. [Google Scholar]
- Silva, M.R.M.; Barros, W.M.A.; Silva, M.L.D.; Silva, J.M.L.D.; Souza, A.P.D.S.; Silva, A.B.J.D.; Fernandes, M.S.D.S.; Souza, S.L.D.; Souza, V.D.O.N. Relationship between vitamin D deficiency and psychophysiological variables: A systematic review of the literature. Clinics 2021, 76, e3155. [Google Scholar]
- Lerner, P.P.; Sharony, L.; Miodownik, C. Association between mental disorders, cognitive disturbances and vitamin D serum level: Current state. Clin. Nutr. ESPEN 2017, 23, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, H.; Amirani, E.; Milajerdi, A.; Kolahdooz, F.; Mirzaei, H.; Zaroudi, M.; Ghaderi, A.; Asemi, Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109651. [Google Scholar] [CrossRef]
- Terock, J.; Hannemann, A.; Van der Auwera, S.; Janowitz, D.; Spitzer, C.; Bonk, S.; Völzke, H.; Grabe, H.J. Posttraumatic stress disorder is associated with reduced vitamin D levels and functional polymorphisms of the vitamin D binding-protein in a population-based sample. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109760. [Google Scholar] [CrossRef] [PubMed]
- Chester, V.; Simmons, H.; Henriksen, M.; Alexander, R.T. Vitamin D deficiency in an inpatient forensic intellectual disability service. J. Intellect. Disabil. 2017, 21, 134–143. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, I.; Lewis, T.; Mehta, N.; Imrit, S.; Thorp, J.; Ince, C. Vitamin D in patients with intellectual and developmental disability in secure in-patient services in the North of England, UK. BJPsych Bull. 2018, 42, 24–29. [Google Scholar] [CrossRef]
- Windham, G.C.; Pearl, M.; Poon, V.; Berger, K.; Soriano, J.W.; Eyles, D.; Lyall, K.; Kharrazi, M.; Croen, L.A. Maternal Vitamin D Levels During Pregnancy in Association With Autism Spectrum Disorders (ASD)or Intellectual Disability (ID) in Offspring; Exploring Non-linear Patterns and Demographic Sub-groups. Autism Res. 2020, 13, 2216–2229. [Google Scholar] [CrossRef]
- Windham, G.C.; Pearl, M.; Anderson, M.C.; Poon, V.; Eyles, D.; Jones, K.L.; Lyall, K.; Kharrazi, M.; Croen, L.A. Newborn vitamin D levels in relation to autism spectrum disorders and intellectual disability: A case–control study in California. Autism Res. 2019, 12, 989–998. [Google Scholar] [CrossRef]
- Chtourou, M.; Naifar, M.; Grayaa, S.; Hajkacem, I.; Touhemi, D.B.; Ayadi, F.; Moalla, Y. Vitamin d status in TUNISIAN children with autism spectrum disorders. Clin. Chim. Acta 2019, 493, S619–S620. [Google Scholar] [CrossRef]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A., Jr.; Meyer, B.J.; Jones, B.; von Hurst, P.R. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J. Steroid Biochem. Mol. Biol. 2019, 187, 9–16. [Google Scholar] [CrossRef]
- Kotsi, E.; Kotsi, E.; Perrea, D.N. Vitamin D levels in children and adolescents with attention-deficit hyperactivity disorder (ADHD): A meta-analysis. ADHD Atten. Deficit Hyperact. Disord. 2019, 11, 221–232. [Google Scholar] [CrossRef]
- Hemamy, M.; Pahlavani, N.; Amanollahi, A.; Islam, S.M.S.; McVicar, J.; Askari, G.; Malekahmadi, M. The effect of vitamin D and magnesium supplementation on the mental health status of attention-deficit hyperactive children: A randomized controlled trial. BMC Pediatr. 2021, 21, 178. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.-M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e1760. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Eyles, D.W. Vitamin D and schizophrenia: 20 years on. Mol. Psychiatry 2021, 26, 2708–2720. [Google Scholar] [CrossRef]
- Adamson, J.; Lally, J.; Gaughran, F.; Krivoy, A.; Allen, L.; Stubbs, B. Correlates of vitamin D in psychotic disorders: A comprehensive systematic review. Psychiatry Res. 2017, 249, 78–85. [Google Scholar] [CrossRef]
- Salavert, J.; Grados, D.; Ramiro, N.; Carrión, M.I.; Fadeuilhe, C.; Palma, F.; López, L.; Erra, A.; Ramírez, N. Association Between Vitamin D Status and Schizophrenia. J. Nerv. Ment. Dis. 2017, 205, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.M. Impact of vitamin D on neurocognitive function in dementia, depression, schizophrenia and ADHD. Front. Biosci. 2021, 26, 4908. [Google Scholar] [CrossRef]
- Van der Leeuw, C.; de Witte, L.D.; Stellinga, A.; van der Ley, C.; Bruggeman, R.; Kahn, R.S.; van Os, J.; Marcelis, M.; for G.R.O.U.P. Vitamin D concentration and psychotic disorder: Associations with disease status, clinical variables and urbanicity. Psychol. Med. 2020, 50, 1680–1686. [Google Scholar] [CrossRef]
- Krivoy, A.; Onn, R.; Vilner, Y.; Hochman, E.; Weizman, S.; Paz, A.; Hess, S.; Sagy, R.; Kimhi-Nesher, S.; Kalter, E.; et al. Vitamin D Supplementation in Chronic Schizophrenia Patients Treated with Clozapine: A Randomized, Double-Blind, Placebo-controlled Clinical Trial. EBioMedicine 2017, 26, 138–145. [Google Scholar] [CrossRef]
- Neriman, A.; Hakan, Y.; Ozge, U. The psychotropic effect of vitamin D supplementation on schizophrenia symptoms. BMC Psychiatry 2021, 21, 309. [Google Scholar] [CrossRef]
- Sheikhmoonesi, F.; Zarghami, M.; Mamashli, S.; Yazdani Charati, J.; Hamzehpour, R.; Fattahi, S.; Azadbakht, R.; Kashi, Z.; Ala, S.; Moshayedi, M.; et al. Effectiveness of Vitamin D Supplement Therapy in Chronic Stable Schizophrenic Male Patients: A Randomized Controlled Trial. Iran. J. Pharm. Res. 2016, 15, 941–950. [Google Scholar]
- Altunsoy, N.; Yüksel, R.N.; Cingi Yirun, M.; Kılıçarslan, A.; Aydemir, Ç. Exploring the relationship between vitamin D and mania: Correlations between serum vitamin D levels and disease activity. Nord. J. Psychiatry 2018, 72, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Naifar, M.; Maalej Bouali, M.; Guidara, W.; Ellouze, A.S.; Jmal, K.; Omri, S.; Messedi, M.; Zouari, L.; Elleuch, A.; Maalej, M.; et al. Vulnérabilité au Trouble Bipolaire: La Piste de la Vitamine D. Can. J. Psychiatry 2020, 65, 184–192. [Google Scholar] [CrossRef]
- Marsh, W.K.; Penny, J.L.; Rothschild, A.J. Vitamin D supplementation in bipolar depression: A double blind placebo controlled trial. J. Psychiatr. Res. 2017, 95, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Köhnke, C.; Herrmann, M.; Berger, K. Associations of major depressive disorder and related clinical characteristics with 25-hydroxyvitamin D levels in middle-aged adults. Nutr. Neurosci. 2022, 25, 1209–1218. [Google Scholar] [CrossRef]
- Obradovic, D.; Gronemeyer, H.; Lutz, B.; Rein, T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J. Neurochem. 2006, 96, 500–509. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes. Nutr. 2018, 13, 19. [Google Scholar] [CrossRef]
- Sedaghat, K.; Yousefian, Z.; Vafaei, A.A.; Rashidy-Pour, A.; Parsaei, H.; Khaleghian, A.; Choobdar, S. Mesolimbic dopamine system and its modulation by vitamin D in a chronic mild stress model of depression in the rat. Behav. Brain Res. 2019, 356, 156–169. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J. Affect. Disord. 2020, 269, 28–35. [Google Scholar] [CrossRef] [PubMed]
- El-Salem, K.; Khalil, H.; Al-Sharman, A.; Al-Mistarehi, A.-H.; Yassin, A.; Alhayk, K.A.; Qawasmeh, M.A.; Bashayreh, S.Y.; Kofahi, R.M.; Obeidat, A.Z. Serum vitamin d inversely correlates with depression scores in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 48, 102732. [Google Scholar] [CrossRef]
- Alavi, N.M.; Khademalhoseini, S.; Vakili, Z.; Assarian, F. Effect of vitamin D supplementation on depression in elderly patients: A randomized clinical trial. Clin. Nutr. 2019, 38, 2065–2070. [Google Scholar] [CrossRef]
- McFarland, D.C.; Fernbach, M.; Breitbart, W.S.; Nelson, C. Prognosis in metastatic lung cancer: Vitamin D deficiency and depression—A cross-sectional analysis. BMJ Support. Palliat. Care 2022, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, Z.; Luan, X.; He, J. Vitamin D status and its association with season, depression in stroke. Neurosci. Lett. 2019, 690, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Högberg, G.; Gustafsson, S.A.; Hällström, T.; Gustafsson, T.; Klawitter, B.; Petersson, M. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012, 101, 779–783. [Google Scholar] [CrossRef]
- Hansen, J.P.; Pareek, M.; Hvolby, A.; Schmedes, A.; Toft, T.; Dahl, E.; Nielsen, C.T. Vitamin D3 supplementation and treatment outcomes in patients with depression (D3-vit-dep). BMC Res. Notes 2019, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.-M.; Zhao, W.; Zhang, B.; Zhang, Y.; Yang, Y.; Zhang, C.; Wang, Y.; Zhu, J.; Yu, Y. The Relationship Between Serum Concentration of Vitamin D, Total Intracranial Volume, and Severity of Depressive Symptoms in Patients with Major Deprewithe Disorder. Front. Psychiatry 2019, 10, 322. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Edmondson, D.; Wasson, L.T.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K.W. Vitamin D Supplementation for Depressive Symptoms. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, J.-Y.; Illias, A.M.; Chiu, C.-C.; Chang, Y.-J.; Liao, S.-W.; Wang, K.-F.; Chen, I.-W.; Sun, C.-K. Association of a low vitamin D status with risk of post-stroke depression: A meta-analysis and systematic review. Front. Nutr. 2023, 10, 1142035. [Google Scholar] [CrossRef]
- Eid, A.; Khoja, S.; AlGhamdi, S.; Alsufiani, H.; Alzeben, F.; Alhejaili, N.; Tayeb, H.O.; Tarazi, F.I. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab. Brain Dis. 2019, 34, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- GCasseb, A.S.; Kaster, M.P.; Rodrigues, A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef] [PubMed]
- De Koning, E.J.; Verweij, L.; Lips, P.; Beekman, A.T.F.; Comijs, H.C.; van Schoor, N.M. The relationship between serum 25(OH)D levels and anxiety symptoms in older persons: Results from the Longitudinal Aging Study Amsterdam. J. Psychosom. Res. 2017, 97, 90–95. [Google Scholar] [CrossRef]
- Berridge, M.J. Vitamin D and Depression: Cellular and Regulatory Mechanisms. Pharmacol. Rev. 2017, 69, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Esnafoğlu, E.; Yaman, E. Vitamin B12, folic acid, homocysteine and vitamin D levels in children and adolescents with obsessive compulsive disorder. Psychiatry Res. 2017, 254, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Tas, D.; Tahiroglu, A.; Avci, A.; Yuksel, B.; Cam, P. Vitamin D Deficiency in Obsessive–Compulsive Disorder Patients with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections: A Case Control Study. Noro Psikiyatr. Ars. 2016, 53, 33–37. [Google Scholar] [CrossRef]
- Yazici, K.U.; Yazici, I.P.; Ustundag, B. Vitamin D levels in children and adolescents with obsessive compulsive disorder. Nord. J. Psychiatry 2018, 72, 173–178. [Google Scholar] [CrossRef]
- Celik, G.; Tas, D.A.; Varmıs, D.A.; Tahiroglu, A. Avci. Vitamin D insufficiency in a boy with obsessive–compulsive disorder. Pediatr. Int. 2016, 58, 646–648. [Google Scholar] [CrossRef]
- Brady, K.T.; Killeen, T.K.; Brewerton, T.; Lucerini, S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J. Clin. Psychiatry 2000, 61 (Suppl. S7), 22–32. [Google Scholar]
- Furtado, M.; Katzman, M.A. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015, 229, 37–48. [Google Scholar] [CrossRef]
- Kaneko, I.; Sabir, M.S.; Dussik, C.M.; Whitfield, G.K.; Karrys, A.; Hsieh, J.-C.; Haussler, M.R.; Meyer, M.B.; Pike, J.W.; Jurutka, P.W. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: Implication for behavioral influences of vitamin D. FASEB J. 2015, 29, 4023–4035. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Ambroziak, G.; Thornton, D.; Mundt, J.C.; Kahn, R.E.; Dahl, L.; Waage, L.; Kattenbraker, D.; Araujo, P.; Murison, R.; et al. Vitamin D Supplementation during Winter: Effects on Stress Resilience in a Randomized Control Trial. Nutrients 2020, 12, 3258. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Rizza, W.; Manzato, E.; Sergi, G.; Santonastaso, P.; Caregaro, L.; Favaro, A.; Correll, C.U. Vitamin D status in anorexia nervosa: A meta-analysis. Int. J. Eat. Disord. 2015, 48, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, M.; Dicembre, M.; Rives-Lange, C.; Ropers, J.; Bemer, P.; Zazzo, J.-F.; Poupon, J.; Dauvergne, A.; Melchior, J.-C. Micronutrients Deficiencies in 374 Severely Malnourished Anorexia Nervosa Inpatients. Nutrients 2019, 11, 792. [Google Scholar] [CrossRef]
- Tasegian, A.; Curcio, F.; Dalla Ragione, L.; Rossetti, F.; Cataldi, S.; Codini, M.; Ambesi-Impiombato, F.S.; Beccari, T.; Albi, E. Hypovitaminosis D3, Leukopenia, and Human Serotonin Transporter Polymorphism in Anorexia Nervosa and Bulimia Nervosa. Mediat. Inflamm. 2016, 2016, 8046479. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Levy-Shraga, Y.; Pinhas-Hamiel, O.; Kochavi, B.; Enoch-Levy, A.; Vered, I.; Stein, D. High prevalence of vitamin D deficiency and insufficiency in adolescent inpatients diagnosed with eating disorders. Int. J. Eat. Disord. 2015, 48, 607–614. [Google Scholar] [CrossRef]
- Velickovic, K.M.C.; Makovey, J.; Abraham, S.F. Vitamin D, bone mineral density and body mass index in eating disorder patients. Eat. Behav. 2013, 14, 124–127. [Google Scholar] [CrossRef]
- Crescioli, C.; Morelli, A.; Adorini, L.; Ferruzzi, P.; Luconi, M.; Vannelli, G.B.; Marini, M.; Gelmini, S.; Fibbi, B.; Donati, S.; et al. Human Bladder as a Novel Target for Vitamin D Receptor Ligands. J. Clin. Endocrinol. Metab. 2005, 90, 962–972. [Google Scholar] [CrossRef]
- Vaughan, C.P.; Tangpricha, V.; Motahar-Ford, N.; Goode, P.S.; Burgio, K.L.; Allman, R.M.; Daigle, S.G.; Redden, D.T.; Markland, A.D. Vitamin D and incident urinary incontinence in older adults. Eur. J. Clin. NutR 2016, 70, 987–989. [Google Scholar] [CrossRef]
- Stumpf, E.; O’Brien, L.P. 1,25(OH)2 vitamin D3 sites of action in the brain. Histochemistry 1987, 87, 393–406. [Google Scholar] [CrossRef]
- Shiue, I. Low vitamin D levels in adults with longer time to fall asleep: US NHANES, 2005–2006. Int. J. Cardiol. 2013, 168, 5074–5075. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, H.; Yoon, I.-Y. Impacts of serum vitamin D levels on sleep and daytime sleepiness according to working conditions. J. Clin. Sleep. Med. 2020, 16, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawwa, B.; Ehsan, Z.; Ingram, D.G. Vitamin D and sleep in children. J. Clin. Sleep. Med. 2020, 16, 1119–1123. [Google Scholar] [CrossRef]
- McCarty, D.E.; Reddy, A.; Keigley, Q.; Kim, P.Y.; Marino, A.A. Vitamin D, Race, and Excessive Daytime Sleepiness. J. Clin. Sleep. Med. 2012, 8, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Carlander, B.; Puech-Cathala, A.M.; Jaussent, I.; Scholz, S.; Bayard, S.; Cochen, V.; Dauvilliers, Y. Low Vitamin D in Narcolepsy with Cataplexy. PLoS ONE 2011, 6, e20433. [Google Scholar] [CrossRef]
- Zhao, K.; Luan, X.; Liu, Y.; Tu, X.; Chen, H.; Shen, H.; Qiu, H.; Zhu, Z.; He, J. Low serum 25-hydroxyvitamin D concentrations in chronic insomnia patients and the association with poor treatment outcome at 2 months. Clin. Chim. Acta 2017, 475, 147–151. [Google Scholar] [CrossRef]
- Bouloukaki, I.; Tsiligianni, I.; Mermigkis, C.; Bonsignore, M.R.; Markakis, M.; Pataka, A.; Steiropoulos, P.; Ermidou, C.; Alexaki, I.; Tzanakis, N.; et al. Vitamin D deficiency in patients evaluated for obstructive sleep apnea: Is it associated with disease severity? Sleep Breath. 2021, 25, 1109–1117. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Peris, E.; Gozal, D. Vitamin D levels and obstructive sleep apnoea in children. Sleep Med. 2014, 15, 459–463. [Google Scholar] [CrossRef]
- Fan, Z.; Cao, B.; Long, H.; Feng, L.; Li, Q.; Zhang, Y.; Li, T. Independent association of vitamin D and insulin resistance in obstructive sleep apnea. Ann. Endocrinol. 2019, 80, 319–323. [Google Scholar] [CrossRef]
- Kerley, C.P.; Hutchinson, K.; Bolger, K.; McGowan, A.; Faul, J.; Cormican, L. Serum Vitamin D Is Significantly Inversely Associated with Disease Severity in Caucasian Adults with Obstructive Sleep Apnea Syndrome. Sleep 2016, 39, 293–300. [Google Scholar] [CrossRef]
- Liguori, C.; Izzi, F.; Mercuri, N.B.; Romigi, A.; Cordella, A.; Tarantino, U.; Placidi, F. Vitamin D status of male OSAS patients improved after long-term CPAP treatment mainly in obese subjects. Sleep Med. 2017, 29, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Canguven, O.; Talib, R.A.; El Ansari, W.; Yassin, D.-J.; Al Naimi, A. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male 2017, 20, 9–16. [Google Scholar] [CrossRef]
- Jalali-Chimeh, F.; Gholamrezaei, A.; Vafa, M.; Nasiri, M.; Abiri, B.; Darooneh, T.; Ozgoli, G. Effect of Vitamin D Therapy on Sexual Function in Women with Sexual Dysfunction and Vitamin D Deficiency: A Randomized, Double-Blind, Placebo Controlled Clinical Trial. J. Urol. 2019, 201, 987–993. [Google Scholar] [CrossRef]
- Krysiak, R.; Szwajkosz, A.; Okopień, B. The effect of low vitamin D status on sexual functioning and depressive symptoms in apparently healthy men: A pilot study. Int. J. Impot. Res. 2018, 30, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.M.K.; Guallar, E.; Zhao, D.; Kalyani, R.R.; Blaha, M.J.; Feldman, D.I.; Martin, S.S.; Lutsey, P.L.; Billups, K.L.; Michos, E.D. Vitamin D deficiency is independently associated with greater prevalence of erectile dysfunction: The National Health and Nutrition Examination Survey (NHANES) 2001–2004. Atherosclerosis 2016, 252, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Crafa, A.; Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Is There an Association Between Vitamin D Deficiency and Erectile Dysfunction? A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Cooper-Brown, L.A.; Allali, G. Vitamin D Supplementation and Cognition in Adults: A Systematic Review of Randomized Controlled Trials. CNS Drugs 2021, 35, 1249–1264. [Google Scholar] [CrossRef]
- Bivona, G.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; Scazzone, C.; Agnello, L.; Ciaccio, M. The Role of Vitamin D as a Biomarker in Alzheimer’s Disease. Brain Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Landel, V.; Annweiler, C.; Millet, P.; Morello, M.; Féron, F. Vitamin D, Cognition and Alzheimer’s Disease: The Therapeutic Benefit is in the D-Tails. J. Alzheimer’s Dis. 2016, 53, 419–444. [Google Scholar] [CrossRef]
- Da Rosa, M.I.; Beck, W.O.; Colonetti, T.; Budni, J.; Falchetti, A.C.B.; Colonetti, L.; Coral, A.S.; Meller, F.O. Association of vitamin D and vitamin B12 with cognitive impairment in elderly aged 80 years or older: A cross-sectional study. J. Hum. Human. Nutr. Diet. 2019, 32, 518–524. [Google Scholar] [CrossRef]
- Sultan, S.; Taimuri, U.; Basnan, S.A.; Ai-Orabi, W.K.; Awadallah, A.; Almowald, F.; Hazazi, A. Low Vitamin D and Its Association with Cognitive Impairment and Dementia. J. Aging Res. 2020, 2020, 6097820. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Kos, K.; Henley, W.E.; Kuma, E.; Llewellyn, D.J. Vitamin D and Dementia. J. Prev. Alzheimers Dis. 2015, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Lawlor, B.; O’Sullivan, M. Is there a role for vitamin D in supporting cognitive function as we age? Proc. Nutr. Soc. 2018, 77, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Bradford, D.; Sullivan, R.K.P.; Kurniawan, N.D.; Moon, Y.; Han, S.-H.; Zalesky, A.; Burne, T.H.J. Vitamin D deficiency is associated with reduced hippocampal volume and disrupted structural connectivity in patients with mild cognitive impairment. Hum. Brain Mapp. 2019, 40, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimer’s Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef]
- Kuźma, E.; Soni, M.; Littlejohns, T.J.; Ranson, J.M.; van Schoor, N.M.; Deeg, D.J.H.; Comijs, H.; Chaves, P.H.M.; Kestenbaum, B.R.; Kuller, L.H.; et al. Vitamin D and Memory Decline: Two Population-Based Prospective Studies. J. Alzheimer’s Dis. 2016, 50, 1099–1108. [Google Scholar] [CrossRef]
- Chouët, J.; Sacco, G.; Karras, S.N.; Llewellyn, D.J.; Sánchez-Rodríguez, D.; Annweiler, C. Vitamin D and Delirium in Older Adults: A Case-Control Study in Geriatric Acute Care Unit. Front. Neurol. 2020, 11, 34. [Google Scholar] [CrossRef]
- Kang, J.H.; Vyas, C.M.; Okereke, O.I.; Ogata, S.; Albert, M.; Lee, I.-M.; D’Agostino, D.; Buring, J.E.; Cook, N.R.; Grodstein, F.; et al. Effect of vitamin D on cognitive decline: Results from two ancillary studies of the VITAL randomized trial. Sci. Rep. 2021, 11, 23253. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef]
- Giménez-Meseguer, J.; Tortosa-Martínez, J.; Cortell-Tormo, J. The Benefits of Physical Exercise on Mental Disorders and Quality of Life in Substance Use Disorders Patients. Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3680. [Google Scholar] [CrossRef]
- Xie, F.; Huang, T.; Lou, D.; Fu, R.; Ni, C.; Hong, J.; Ruan, L. Effect of vitamin D supplementation on the incidence and prognosis of depression: An updated meta-analysis based on randomized controlled trials. Front. Public Health 2022, 10, 903547. [Google Scholar] [CrossRef]
- Penckofer, S.; Kouba, J.; Byrn, M.; Ferrans, C.E. Vitamin D and Depression: Where is all the Sunshine? Issues Ment. Health Nurs. 2010, 31, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.A.; Menculini, G.; de Filippis, R.; D’Angelo, M.; De Fazio, P.; Tortorella, A.; Steardo, L., Jr. Sleep Disturbances in Generalized Anxiety Disorder: The Role of Calcium Homeostasis Imbalance. Int. J. Environ. Res. Public Health 2023, 20, 4431. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Sun, L.H.; Yang, W.; Li, B.J.; Cui, R.J. Potential role of insulin on the pathogenesis of depression. Cell Prolif. 2020, 53, e12806. [Google Scholar] [CrossRef]
- Garcion, E.; Nataf, S.; Berod, A.; Darcy, F.; Brachet, P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Mol. Brain Res. 1997, 45, 255–267. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Yilmazer, S. Vitamin D inquiry in hippocampal neurons: Consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol. Sci. 2013, 34, 1453–1458. [Google Scholar] [CrossRef]
- Itzhaky, D.; Amital, D.; Gorden, K.; Bogomolni, A.; Arnson, Y.; Amital, H. Low serum vitamin D concentrations in patients with schizophrenia. Isr. Med. Assoc. J. 2012, 14, 88–92. [Google Scholar]
- Jamilian, H.; Bagherzadeh, K.; Nazeri, Z.; Hassanijirdehi, M. Vitamin D, parathyroid hormone, serum calcium and phosphorus in patients with schizophrenia and major depression. Int. J. Psychiatry Clin. Pract. 2013, 17, 30–34. [Google Scholar] [CrossRef]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
| Psychiatric Diagnosis | Mechanism and Receptors |
|---|---|
| Psychotic spectrum diseases | Vitamin D has an important role in cell differentiation, neurotransmitter synthesis, calcium homeostasis, cognitive function, prevention of oxidative damage, and neuron function. Receptor—1,25(OH)2D3 antagonizing the effects of the glucocorticoid on hippocampal neurons [59] |
| Lower level of serum Vitamin D is caused by long periods of institutionalization, inactivity, and, therefore, less exposure to sunlight [49] | |
| Bipolar disorders | Increased 25OHD synthesis during decompensation [54] Lower level of Vitamin D in depressive patients [55] |
| Depression | Mechanisms implied are exerting influence on neuroendocrine, immunological, and neurotrophic systems [59] |
| Receptors are localized in the hypothalamus [124] | |
| Anxiety disorders | Imbalanced calcium homeostasis [125] |
| Interference in serotonin synthesis by expression of the serotonin-synthesizing gene, therefore maintaining serotonin within the normal range [126] | |
| Mediation of several pathways for insulin or serotonin which were associated with mood disorders [127] | |
| Obsessive-compulsive disorder | Elusive underlying mechanism [80] |
| Trauma- and stress-related disorders | Vitamin D regulates neuro-inflammatory and neuro-immunological mechanisms [82] |
| Brain areas with altered activity in patients with PTSD express vitamin D receptors in the prefrontal cortex, cingulate cortex, and hypothalamus [9] | |
| Vitamin D mediates the regulation of serotonin and catecholamine [83] | |
| Eating disorder | Vitamin D deficiency as a consequence [87] |
| Sleep-wake disorder | Vitamin D deficiency is associated with lower sleep duration, worse sleep quality, an increase in time of sleep onset in pediatric populations, and circadian rhythm regulation [95] |
| Elimination disorders | Prostatic cells can express a hydroxylase that can synthesize the active form of vitamin D [90] |
| Vitamin D receptors are located in the urinary bladder and pelvic floor muscles [91] | |
| Sexual disorders | Low levels of vitamin D are associated with an increased risk for atherosclerotic events through inflammation, endothelial dysfunction, atherosclerosis, and impaired glucose metabolism [104] |
| Receptors of 1,25(OH)2-D3 inhibit the expression of inducible nitric oxide NO synthetase [128] | |
| Neurocognitive disease | Important role in memory formation; the active form of vitamin D supports neurotransmission, neuroprotection, and synaptic plasticity [110] |
| Vitamin D receptors are present in the hippocampus. They are responsible for blocking calcium influx and also the toxicity in cultured mesencephalic neurons or hippocampal neurons [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, A.M.; Petrescu, C.; Anghele, C.; Manea, M.C.; Ciobanu, C.A.; Petrescu, D.M.; Antonia, M.O.; Riga, S. Severe Vitamin D Deficiency—A Possible Cause of Resistance to Treatment in Psychiatric Pathology. Medicina 2023, 59, 2056. https://doi.org/10.3390/medicina59122056
Ciobanu AM, Petrescu C, Anghele C, Manea MC, Ciobanu CA, Petrescu DM, Antonia MO, Riga S. Severe Vitamin D Deficiency—A Possible Cause of Resistance to Treatment in Psychiatric Pathology. Medicina. 2023; 59(12):2056. https://doi.org/10.3390/medicina59122056
Chicago/Turabian StyleCiobanu, Adela Magdalena, Cristian Petrescu, Cristina Anghele, Mihnea Costin Manea, Constantin Alexandru Ciobanu, Diana Mihaela Petrescu, Mihalache Oana Antonia, and Sorin Riga. 2023. "Severe Vitamin D Deficiency—A Possible Cause of Resistance to Treatment in Psychiatric Pathology" Medicina 59, no. 12: 2056. https://doi.org/10.3390/medicina59122056
APA StyleCiobanu, A. M., Petrescu, C., Anghele, C., Manea, M. C., Ciobanu, C. A., Petrescu, D. M., Antonia, M. O., & Riga, S. (2023). Severe Vitamin D Deficiency—A Possible Cause of Resistance to Treatment in Psychiatric Pathology. Medicina, 59(12), 2056. https://doi.org/10.3390/medicina59122056









