LARIAT or AtriClip: Complications Profile and Comparison in Patients with Atrial Fibrillations Based on Manufacturer and User Facility Device Experience Database
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Exclusion Criteria
2.3. Evaluation of Reports
2.4. Adverse Effects and Cause of Death
2.5. Number of Patientsanalyzed
2.6. Statistical Analysis
3. Results
3.1. Prevalence of Device Problems and Adverse Effects in Patients
3.2. Adverse Events in Patients with Device Problems
3.3. Adverse Side Effects in Patients without Device Problems
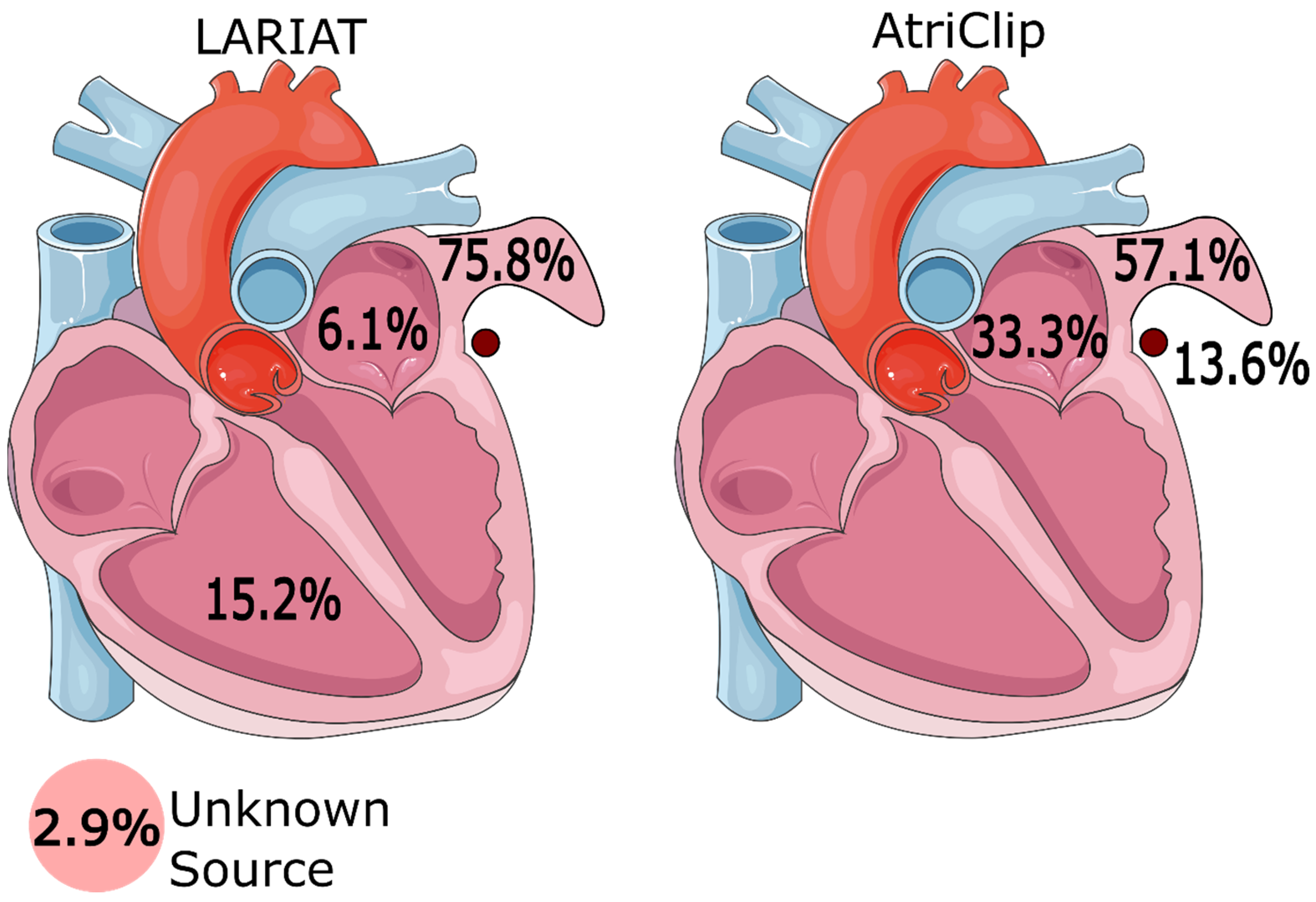
3.4. Perforation
3.5. Pericardial Effusion
3.6. Thrombus
3.7. Cause of Death
4. Discussion
4.1. MAUDE Database Results Compared to Historical Findings
4.2. Potential Prevention of the Surgical Complications
4.3. Left Atrial Appendage Closure Procedure Complications
4.4. Treatment of Atrial Fibrillation
4.5. The Clinical Anatomy of the Left Atrial Appendage
4.6. Roles of the Left Atrial Appendage
4.7. The Left Atrial Appendage Occlusion Procedures
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Eur. 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Grygier, M.; Wojakowski, W.; Smolka, G.; Demkow, M.; Wąsek, W.; Sorysz, D.; Kralisz, P.; Bartuś, K.; Sukiennik, A.; Pracoń, R.; et al. Left atrial appendage occlusion: Consensus document of Association of Cardiovascular Interventions and Heart Rhythm Section of Polish Cardiac Society. Kardiol. Pol. 2018, 76, 677–697. [Google Scholar] [CrossRef]
- Litwinowicz, R.; Bartus, M.; Malec-Litwinowicz, M.; Michalski, M.; Banaszkiewicz, K.; Kapelak, B.; Lakkireddy, D.; Bartus, K. Left Atrial Appendage Occlusion for Secondary Stroke Prevention in Patients with Atrial Fibrillation: Long-Term Results. Cerebrovasc. Dis. 2019, 47, 188–195. [Google Scholar] [CrossRef]
- Burysz, M.; Litwinowicz, R.; Bryndza, M.; Skowronek, R.; Ogorzeja, W.; Bartus, K. Percutaneous left atrial appendage closure using the LAmbre device. First Clin. Results Poland Adv. Interv. Cardiol. 2019, 15, 251–254. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12, Erratum in J. Am. Coll. Cardiol. 2014, 64, 1186. [Google Scholar] [CrossRef]
- Fountain, R.B.; Holmes, D.R.; Chandrasekaran, K.; Packer, D.; Asirvatham, S.; Van Tassel, R.; Turi, Z. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am. Heart J. 2006, 151, 956–961. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef]
- Ailawadi, G.; Gerdisch, M.W.; Harvey, R.L.; Hooker, R.L.; Damiano, R.J.; Salamon, T.; Mack, M.J. Exclusion of the left atrial appendage with a novel device: Early results of a multicenter trial. J. Thorac. Cardiovasc. Surg. 2011, 142, 1002–1009.e1. [Google Scholar] [CrossRef]
- Stone, D.; Byrne, T.; Pershad, A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation. Catheter. Cardiovasc. Interv. 2015, 86, 121–127. [Google Scholar] [CrossRef]
- Gerdisch, M.W.; Garrett, H.E.; Mumtaz, M.A.; Grehan, J.F.; Castillo-Sang, M.; Miller, J.S.; Zorn, G.L.; Gall, S.A.; Johnkoski, J.A.; Ramlawi, B. Prophylactic Left Atrial Appendage Exclusion in Cardiac Surgery Patients with Elevated CHA2DS2-VASc Score: Results of the Randomized ATLAS Trial. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2022, 17, 463–470. [Google Scholar] [CrossRef]
- Massumi, A.; Chelu, M.G.; Nazeri, A.; May, S.A.; Afshar-Kharaghan, H.; Saeed, M.; Razavi, M.; Rasekh, A. Initial Experience with a Novel Percutaneous Left Atrial Appendage Exclusion Device in Patients with Atrial Fibrillation, Increased Stroke Risk, and Contraindications to Anticoagulation. Am. J. Cardiol. 2013, 111, 869–873. [Google Scholar] [CrossRef]
- Bartus, K.; Han, F.T.; Bednarek, J.; Myc, J.; Kapelak, B.; Sadowski, J.; Lelakowski, J.; Bartus, S.; Yakubov, S.J.; Lee, R.J. Percutaneous Left Atrial Appendage Suture Ligation Using the LARIAT Device in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 62, 108–118. [Google Scholar] [CrossRef]
- Jazayeri, M.; Vuddanda, V.; Turagam, M.K.; Parikh, V.; Lavu, M.; Atkins, D.; Earnest, M.; Di Biase, L.; Natale, A.; Wilber, D.; et al. Safety profiles of percutaneous left atrial appendage closure devices: An analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database from 2009 to 2016. J. Cardiovasc. Electrophysiol. 2018, 29, 5–13. [Google Scholar] [CrossRef]
- Batko, J.; Rams, D.; Filip, G.; Bartoszcze, A.; Kapelak, B.; Bartuś, K.; Litwinowicz, R. Left Atrial Appendage Morphology and Course of the Circumflex Artery: Anatomical Implications for Left Atrial Appendage Occlusion Procedures. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2022, 17, 424–429. [Google Scholar] [CrossRef]
- Burysz, M.; Batko, J.; Olejek, W.; Piotrowski, M.; Litwinowicz, R.; Słomka, A.; Kowalewski, M.; Suwalski, P.; Bartuś, K.; Rams, D. Morphology and Anatomical Classification of Pericardial Cavities: Oblique and Transverse Sinuses. J. Clin. Med. 2023, 12, 4320. [Google Scholar] [CrossRef]
- Kuzmin, B.; Staack, T.; Wippermann, J.; Wacker, M. Left atrial appendage occlusion device causing coronary obstruction: A word of caution. J. Card. Surg. 2021, 36, 723–725. [Google Scholar] [CrossRef]
- Katona, A.; Temesvári, A.; Szatmári, A.; Nemes, A.; Forster, T.; Fontos, G. Left circumflex coronary artery occlusion due to a left atrial appendage closure device. Adv. Interv. Cardiol. 2015, 1, 69–70. [Google Scholar] [CrossRef]
- Langenaeken, T.; van den Berg, M.; Kaya, A.; Yilmaz, A. Thoracoscopic management of iatrogenic cardiac perforations. J. Cardiovasc. Electrophysiol. 2022, 33, 1366–1370. [Google Scholar] [CrossRef]
- Kang, K.-W.; Lee, J.Y.; Lee, B.-H.; Jeon, M.J.; Yu, E.S.; Kim, D.S.; Lee, S.R.; Choi, C.W.; Park, Y.; Sung, H.J.; et al. Postoperative Thromboembolism According to the Type of Surgery: A Nationwide Study in the Republic of Korea. J. Clin. Med. 2022, 11, 1477. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Ashikhmina, E.A.; Schaff, H.V.; Sinak, L.J.; Li, Z.; Dearani, J.A.; Suri, R.M.; Park, S.J.; Orszulak, T.A.; Sundt, T.M. Pericardial Effusion After Cardiac Surgery: Risk Factors, Patient Profiles, and Contemporary Management. Ann. Thorac. Surg. 2010, 89, 112–118. [Google Scholar] [CrossRef]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Whiteman, S.; Saker, E.; Courant, V.; Salandy, S.; Gielecki, J.; Zurada, A.; Loukas, M. An anatomical review of the left atrium. Transl. Res. Anat. 2019, 17, 100052. [Google Scholar] [CrossRef]
- Słodowska, K.; Szczepanek, E.; Dudkiewicz, D.; Hołda, J.; Bolechała, F.; Strona, M.; Lis, M.; Batko, J.; Koziej, M.; Hołda, M.K. Morphology of the Left Atrial Appendage: Introduction of a New Simplified Shape-Based Classification System. Heart Lung Circ. 2021, 30, 1014–1022. [Google Scholar] [CrossRef]
- Slodowska, K.M.; Batko, J.; Holda, J.P.; Dudkiewicz, D.; Koziej, M.; Litwinowicz, R.; Bartus, K.; Holda, M.K. Morphometrical features of left atrial appendage in the AF patients subjected to left atrial appendage closure. Folia Morphol. 2023, 82, 814–821. [Google Scholar] [CrossRef]
- Litwinowicz, R.; Natorska, J.; Zabczyk, M.; Kapelak, B.; Lakkireddy, D.; Vuddanda, V.; Bartus, K. Changes in fibrinolytic activity and coagulation factors after epicardial left atrial appendage closure in patients with atrial fibrillation. J. Thorac. Dis. 2022, 14, 4226–4235. [Google Scholar] [CrossRef]
- Bartus, K.; Litwinowicz, R.; Natorska, J.; Zabczyk, M.; Undas, A.; Kapelak, B.; Lakkireddy, D.; Lee, R.J. Coagulation factors and fibrinolytic activity in the left atrial appendage and other heart chambers in patients with atrial fibrillation: Is there a local intracardiac prothrombotic state? (HEART-CLOT study). Int. J. Cardiol. 2020, 301, 103–107. [Google Scholar] [CrossRef]
- Alkhouli, M.; Di Biase, L.; Natale, A.; Rihal, C.S.; Holmes, D.R.; Asirvatham, S.; Bartus, K.; Lakkireddy, D.; Friedman, P.A. Nonthrombogenic Roles of the Left Atrial Appendage. J. Am. Coll. Cardiol. 2023, 81, 1063–1075. [Google Scholar] [CrossRef]
- Bartus, K.; Podolec, J.; Lee, R.J.; Kapelak, B.; Sadowski, J.; Bartus, M.; Oles, K.; Ceranowicz, P.; Trabka, R.; Litwinowicz, R. Atrial natriuretic peptide and brain natriuretic peptide changes after epicardial percutaneous left atrial appendage suture ligation using LARIAT device. J. Physiol. Pharmacol. 2017, 68, 117–123. [Google Scholar]
- Bartus, K.; Kanuri, S.H.; Litwinowicz, R.; Elbey, M.A.; Natorska, J.; Zabczyk, M.; Bartus, M.; Kapelak, B.; Gopinnathannair, R.; Garg, J.; et al. Long Term Impact of Epicardial Left Atrial Appendage Ligation on Systemic Hemostasis: LAA HOMEOSTASIS-2. J. Clin. Med. 2022, 11, 1495. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Turagam, M.; Afzal, M.R.; Rajasingh, J.; Atkins, D.; Dawn, B.; Di Biase, L.; Bartus, K.; Kar, S.; Natale, A.; et al. Left Atrial Appendage Closure and Systemic Homeostasis. J. Am. Coll. Cardiol. 2018, 71, 135–144. [Google Scholar] [CrossRef]
- Litwinowicz, R.; Bartus, M.; Ceranowicz, P.; Brzezinski, M.; Kapelak, B.; Lakkireddy, D.; Bartus, K. Left atrial appendage occlusion for stroke prevention in diabetes mellitus patients with atrial fibrillation: Long-term results. J. Diabetes 2019, 11, 75–82. [Google Scholar] [CrossRef]
- Dar, T.; Afzal, M.R.; Yarlagadda, B.; Kutty, S.; Shang, Q.; Gunda, S.; Samanta, A.; Thummaluru, J.; Arukala, K.S.; Kanmanthareddy, A.; et al. Mechanical function of the left atrium is improved with epicardial ligation of the left atrial appendage: Insights from the LAFIT-LARIAT Registry. J. HeartRhythm 2018, 15, 955–959. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left Atrial Appendage. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Mohanty, S.; Sanchez, J.E.; Trivedi, C.; Güneş, M.; Gökoğlan, Y.; Gianni, C.; Horton, R.P.; et al. Left Atrial Appendage Isolation in Patients with Longstanding Persistent AF Undergoing Catheter Ablation. J. Am. Coll. Cardiol. 2016, 68, 1929–1940. [Google Scholar] [CrossRef]
- Fanton, Y.; Robic, B.; Rummens, J.-L.; Daniëls, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Possibilities and limitations for co-transplantation of cardiac atrial appendage stem cells and mesenchymal stem cells for myocardial repair. Int. J. Cardiol. 2016, 203, 1155–1156. [Google Scholar] [CrossRef]
- Fanton, Y.; Robic, B.; Rummens, J.-L.; Daniëls, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Cardiac atrial appendage stem cells engraft differentiate into cardiomyocytes in vivo: A new tool for cardiac repair after MI. Int. J. Cardiol. 2015, 201, 10–19. [Google Scholar] [CrossRef]
- Litwinowicz, R.; Burysz, M.; Mazur, P.; Kapelak, B.; Bartus, M.; Lakkireddy, D.; Lee, R.J.; Malec-Litwinowicz, M.; Bartus, K. Endocardial versus epicardial left atrial appendage exclusion for stroke prevention in patients with atrial fibrillation: Midterm follow-up. J. Cardiovasc. Electrophysiol. 2021, 32, 93–101. [Google Scholar] [CrossRef]
| Parameter | LARIAT n (%) (n = 29) | AtriClip n (%) (n = 16) | p-Value |
|---|---|---|---|
| Arrythmia | 0 (0.0%) | 1 (6.3%) | 0.356 |
| Cardiac arrest | 0 (0.0%) | 1 (6.3%) | 0.356 |
| Postoperative bleeding | 14 (48.3%) | 4 (25.0%) | 0.127 |
| Hypotension | 3 (10.3%) | 1 (6.3%) | 1.000 |
| Inflammation | 1 (3.4%) | 0 (0.0%) | 1.000 |
| Thrombus | 2 (6.9%) | 2 (12.5%) | 0.608 |
| Perforation | 16 (55.2%) | 7 (43.8%) | 0.463 |
| Pericardial effusion | 11 (37.9%) | 0 (0.0%) | 0.004 |
| Parameter | LARIAT n (%) (n = 34) | Atriclip n (%) (n = 37) | p-Value |
|---|---|---|---|
| Arrythmia | 0 (0.0%) | 1 (2.7%) | 1.000 |
| Cardiac tamponade | 2 (5.9%) | 2 (5.4%) | 1.000 |
| Cardiomyopathy | 0 (0.0%) | 1 (2.7%) | 1.000 |
| Endocarditis | 0 (0.0%) | 1 (2.7%) | 1.000 |
| Heart block | 0 (0.0%) | 1 (2.7%) | 1.000 |
| Heart failure | 0 (0.0%) | 1 (2.7%) | 1.000 |
| Hematoma | 0 (0.0%) | 1 (2.7%) | 1.000 |
| Postoperative bleeding | 15 (44.1%) | 1 (2.7%) | <0.001 |
| Hypotension | 7 (20.6%) | 2 (5.4%) | 0.077 |
| Perforation | 15 (44.1%) | 14 (37.8%) | 0.590 |
| Pericardial effusion | 18 (52.9%) | 0 (0.0%) | <0.001 |
| Pleural effusion | 1 (2.9%) | 0 (0.0%) | 0.479 |
| Stroke | 0 (0.0%) | 7 (18.9%) | 0.012 |
| Thrombus | 2 (5.9%) | 11 (29.7%) | 0.013 |
| Vascular dissection | 0 (0.0%) | 3 (8.1%) | 1.000 |
| Cause of Death | LARIAT n (%), n = 11 | AtriClip n (%), n = 8 | p |
|---|---|---|---|
| unknown | 3 (27.3%) | 1 (12.5%) | 0.435 |
| LAA perforation | 6 (54.5%) | 1 (12.5%) | 0.060 |
| PEA | 1 (9.1%) | 0 (0%) | 1.000 |
| hemorrhage | 1 (9.1%) | 1 (12.5%) | 0.811 |
| stroke | 0 (0%) | 2 (25%) | 1.000 |
| Cx occlusion | 0 (0%) | 1 (12.5%) | 1.000 |
| sepsis | 0 (0%) | 1 (12.5%) | 1.000 |
| heart failure | 0 (0%) | 1 (12.5%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litwinowicz, R.; Batko, J.; Rusinek, J.; Olejek, W.; Rams, D.; Kowalewski, M.; Bartuś, K.; Burysz, M. LARIAT or AtriClip: Complications Profile and Comparison in Patients with Atrial Fibrillations Based on Manufacturer and User Facility Device Experience Database. Medicina 2023, 59, 2055. https://doi.org/10.3390/medicina59122055
Litwinowicz R, Batko J, Rusinek J, Olejek W, Rams D, Kowalewski M, Bartuś K, Burysz M. LARIAT or AtriClip: Complications Profile and Comparison in Patients with Atrial Fibrillations Based on Manufacturer and User Facility Device Experience Database. Medicina. 2023; 59(12):2055. https://doi.org/10.3390/medicina59122055
Chicago/Turabian StyleLitwinowicz, Radosław, Jakub Batko, Jakub Rusinek, Wojciech Olejek, Daniel Rams, Mariusz Kowalewski, Krzysztof Bartuś, and Marian Burysz. 2023. "LARIAT or AtriClip: Complications Profile and Comparison in Patients with Atrial Fibrillations Based on Manufacturer and User Facility Device Experience Database" Medicina 59, no. 12: 2055. https://doi.org/10.3390/medicina59122055
APA StyleLitwinowicz, R., Batko, J., Rusinek, J., Olejek, W., Rams, D., Kowalewski, M., Bartuś, K., & Burysz, M. (2023). LARIAT or AtriClip: Complications Profile and Comparison in Patients with Atrial Fibrillations Based on Manufacturer and User Facility Device Experience Database. Medicina, 59(12), 2055. https://doi.org/10.3390/medicina59122055









