Associations between Selected ADRB1 and CYP2D6 Gene Polymorphisms in Children with Ventricular and Supraventricular Arrhythmias
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Population
2.2. Serum Collection and Storage
2.3. Genomic DNA Isolation
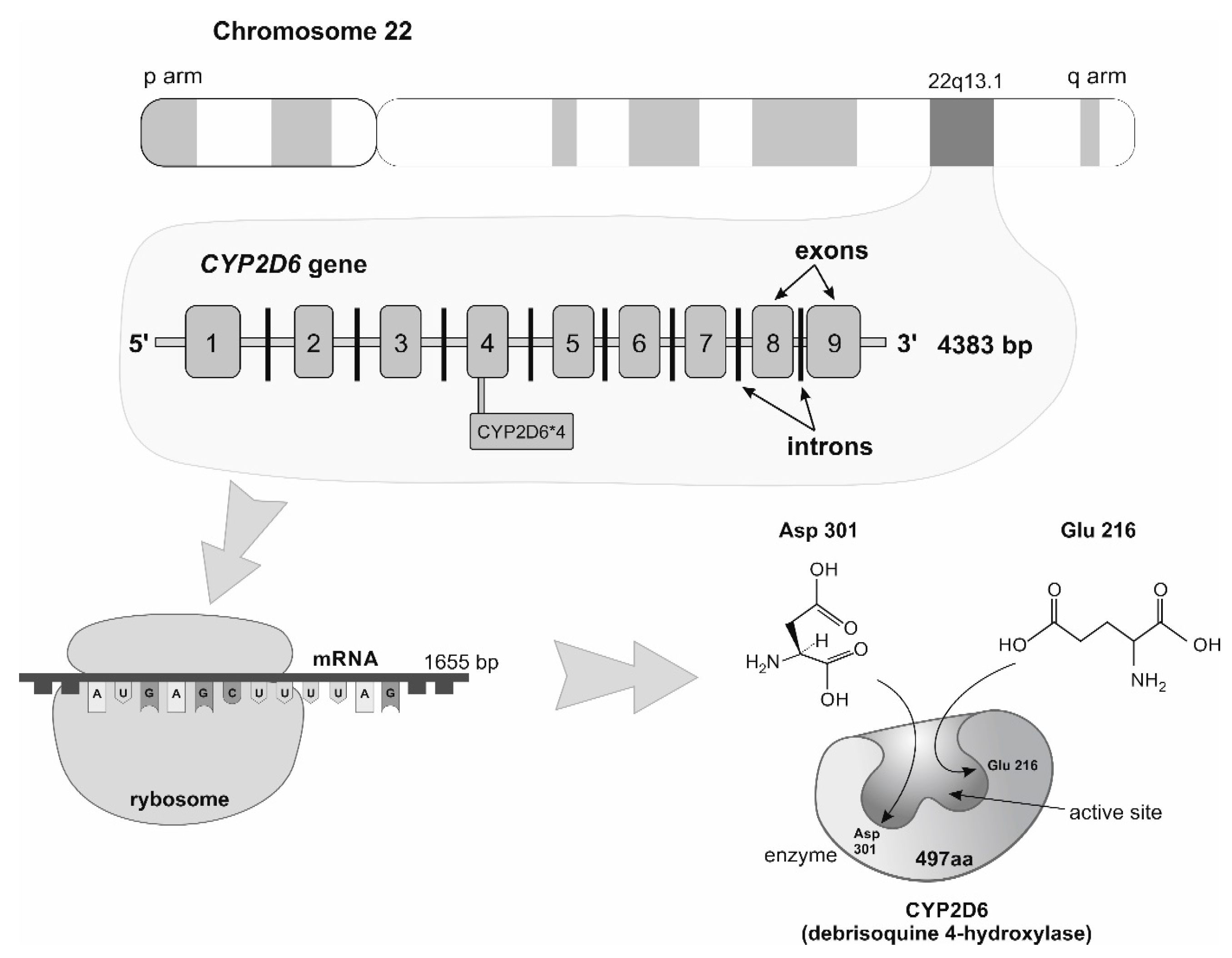
2.4. PCR-HRM Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
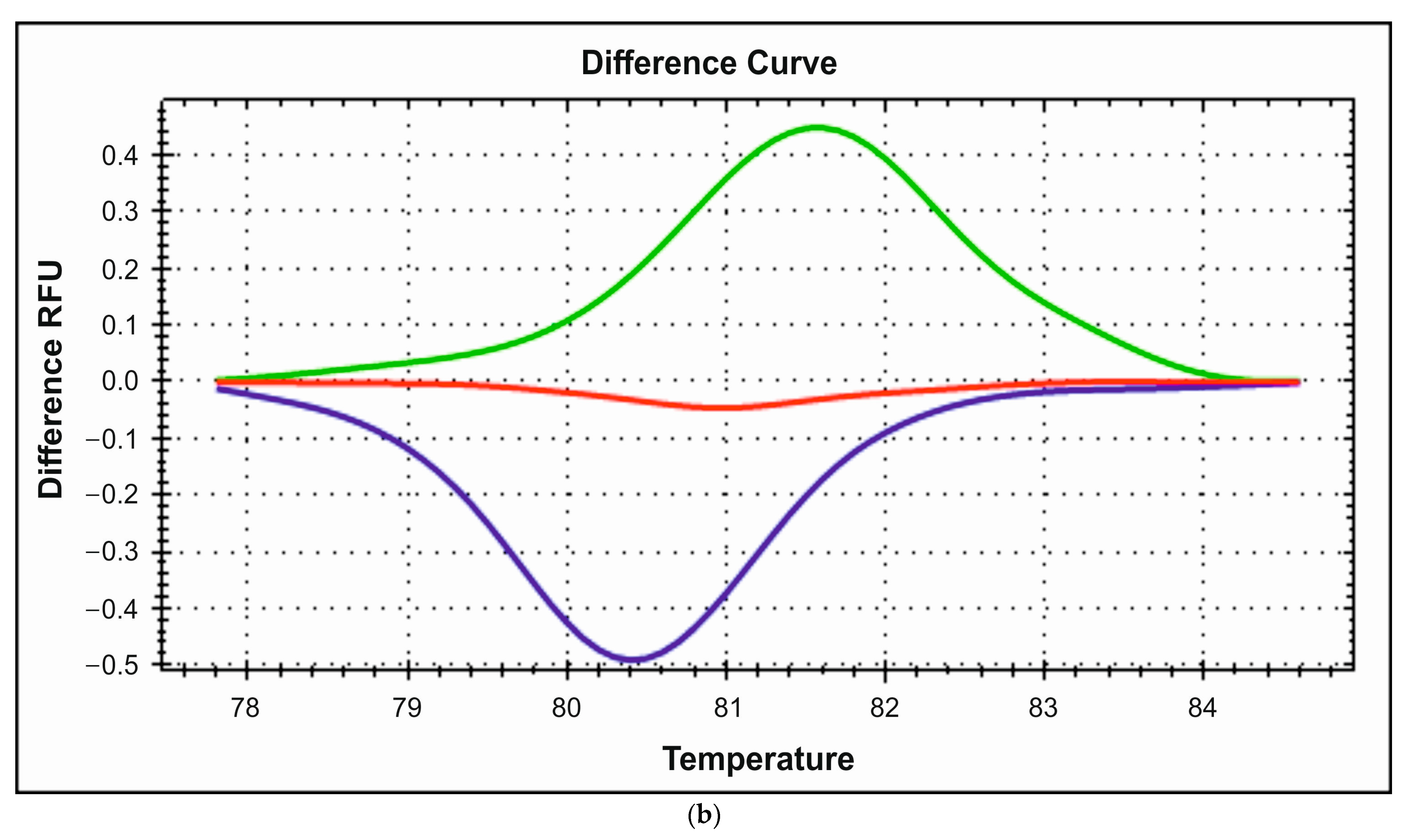
3.2. HRM Analysis
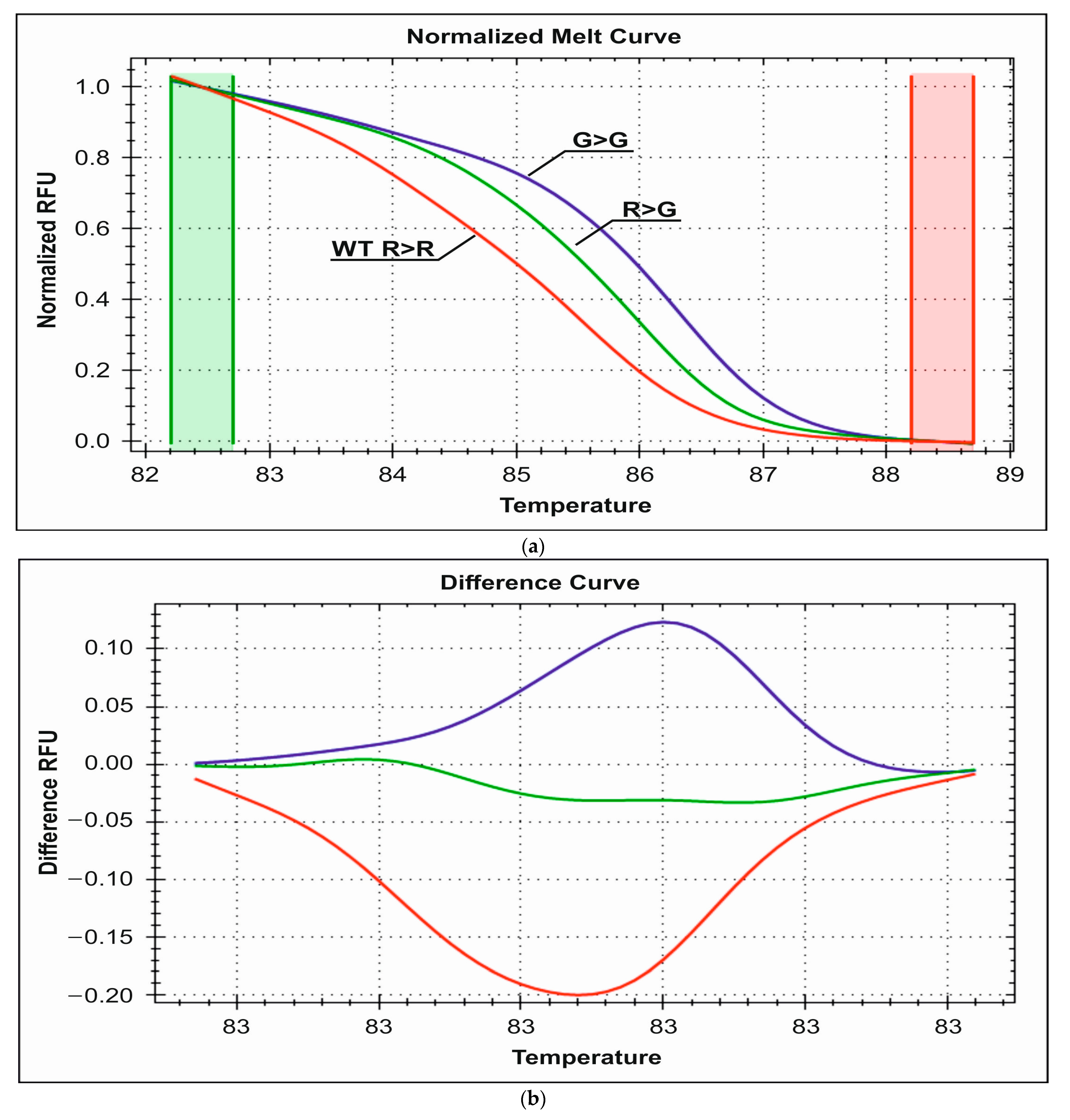
3.2.1. Detection of R389G Polymorphism
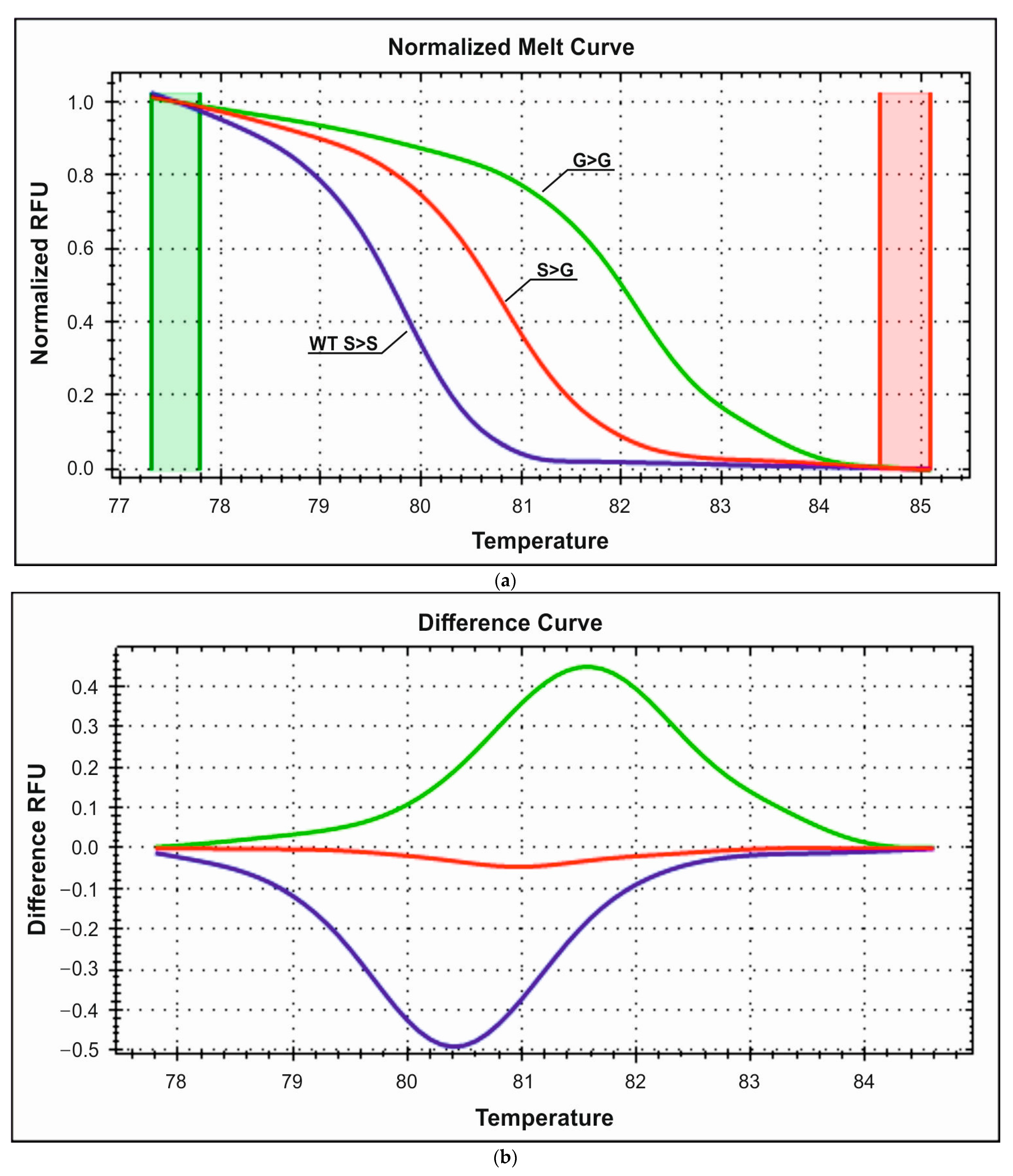
3.2.2. Detection of S49G Polymorphism
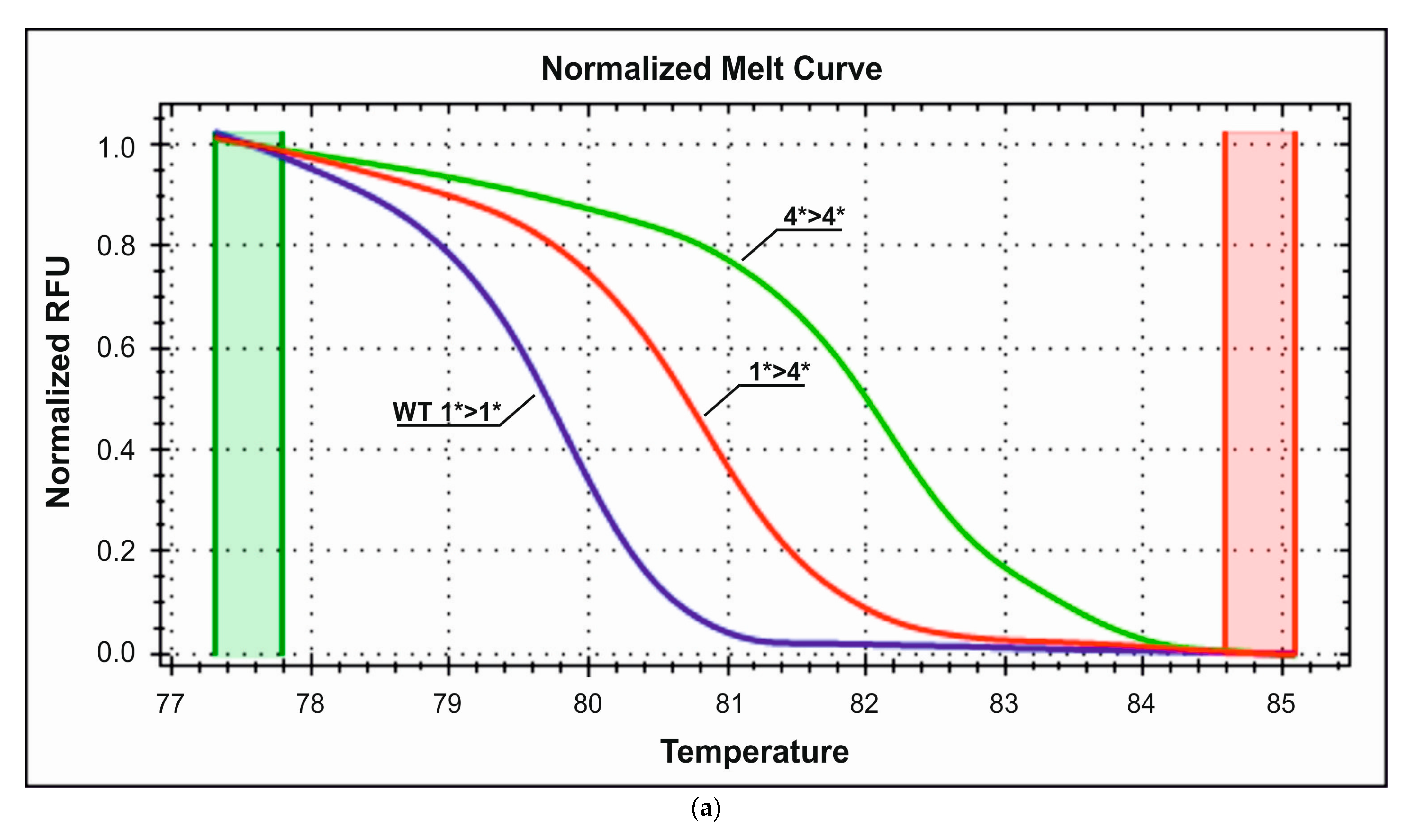
3.2.3. Detection of CYP2D6*4 Polymorphism
4. Discussion
5. Conclusions
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Liu, Z.; Yu, B.; Xu, F.; Mo, W.; Zhou, G.; Liu, Y.; Li, Q.; Zhou, H. Β1-Adrenergic Receptor Polymorphisms Influence the Response to Metoprolol Monotherapy in Patients with Essential Hypertension. Clin. Pharmacol. Ther. 2006, 80, 23–32. [Google Scholar] [CrossRef]
- Si, D.; Wang, J.; Xu, Y.; Chen, X.; Zhang, M.; Zhou, H. Association of Common Polymorphisms in Β1-Adrenergic Receptor With Antihypertensive Response to Carvedilol. J. Cardiovasc. Pharmacol. 2014, 64, 306–309. [Google Scholar] [CrossRef]
- Biolo, A.; Clausell, N.; Santos, K.G.; Salvaro, R.; Ashton-Prolla, P.; Borges, A.; Rohde, L.E. Impact of Beta1-Adrenergic Receptor Polymorphisms on Susceptibility to Heart Failure, Arrhythmogenesis, Prognosis, and Response to Beta-Blocker Therapy. Am. J. Cardiol. 2008, 102, 726–732. [Google Scholar] [CrossRef]
- Kao, D.P.; Davis, G.; Aleong, R.; O’Connor, C.M.; Fiuzat, M.; Carson, P.E.; Anand, I.S.; Plehn, J.F.; Gottlieb, S.S.; Silver, M.A.; et al. Effect of Bucindolol on Heart Failure Outcomes and Heart Rate Response in Patients with Reduced Ejection Fraction Heart Failure and Atrial Fibrillation. Eur. J. Heart Fail. 2013, 15, 324–333. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; McCrink, K.A.; Brill, A. Impact of CYP2D6 Genetic Variation on the Response of the Cardiovascular Patient to Carvedilol and Metoprolol. Curr. Drug Metab. 2015, 17, 30–36. [Google Scholar] [CrossRef]
- Jiang, Q.; Yuan, H.; Xing, X.; Liu, J.; Huang, Z.; Du, X. Methylation of Adrenergic Β1 Receptor Is a Potential Epigenetic Mechanism Controlling Antihypertensive Response to Metoprolol. Indian J. Biochem. Biophys. 2011, 48, 301–307. [Google Scholar]
- Cacabelos, R. Pharmacoepigenetics; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-813939-4. [Google Scholar]
- Voora, D.; Ginsburg, G.S. Clinical Application of Cardiovascular Pharmacogenetics. J. Am. Coll. Cardiol. 2012, 60, 9–20. [Google Scholar] [CrossRef]
- Roden, D.M. Cardiovascular Pharmacogenomics: Current Status and Future Directions. J. Hum. Genet. 2016, 61, 79–85. [Google Scholar] [CrossRef]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.-G.; Schiöth, H.B. The G-Protein-Coupled Receptors in the Human Genome Form Five Main Families. Phylogenetic Analysis, Paralogon Groups, and Fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Bylund, D.B.; Eikenberg, D.C.; Hieble, J.P.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Molinoff, P.B.; Ruffolo, R.R.; Trendelenburg, U. International Union of Pharmacology Nomenclature of Adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar]
- Brueckner, F.; Piscitelli, C.L.; Tsai, C.-J.; Standfuss, J.; Deupi, X.; Schertler, G.F.X. Structure of β-Adrenergic Receptors. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 520, pp. 117–151. ISBN 978-0-12-391861-1. [Google Scholar]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional Beta3-Adrenoceptor in the Human Heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef]
- Zhu, W.; Woo, A.Y.-H.; Zhang, Y.; Cao, C.-M.; Xiao, R.-P. β-Adrenergic Receptor Subtype Signaling in the Heart: From Bench to the Bedside. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2011; Volume 67, pp. 191–204. ISBN 978-0-12-384921-2. [Google Scholar]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S. Beta 1- and Beta 2-Adrenergic-Receptor Subpopulations in Nonfailing and Failing Human Ventricular Myocardium: Coupling of Both Receptor Subtypes to Muscle Contraction and Selective Beta 1-Receptor down-Regulation in Heart Failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef]
- Lohse, M.J.; Engelhardt, S.; Eschenhagen, T. What Is the Role of β-Adrenergic Signaling in Heart Failure? Circ. Res. 2003, 93, 896–906. [Google Scholar] [CrossRef]
- Moniotte, S.; Kobzik, L.; Feron, O.; Trochu, J.-N.; Gauthier, C.; Balligand, J.-L. Upregulation of β3-Adrenoceptors and Altered Contractile Response to Inotropic Amines in Human Failing Myocardium. Circulation 2001, 103, 1649–1655. [Google Scholar] [CrossRef]
- Myagmar, B.-E.; Flynn, J.M.; Cowley, P.M.; Swigart, P.M.; Montgomery, M.D.; Thai, K.; Nair, D.; Gupta, R.; Deng, D.X.; Hosoda, C.; et al. Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent. Circ. Res. 2017, 120, 1103–1115. [Google Scholar] [CrossRef]
- Rockman, H.A.; Koch, W.J.; Lefkowitz, R.J. Seven-Transmembrane-Spanning Receptors and Heart Function. Nature 2002, 415, 206–212. [Google Scholar] [CrossRef]
- Katsarou, M.-S.; Karathanasopoulou, A.; Andrianopoulou, A.; Desiniotis, V.; Tzinis, E.; Dimitrakis, E.; Lagiou, M.; Charmandari, E.; Aschner, M.; Tsatsakis, A.M.; et al. Beta 1, Beta 2 and Beta 3 Adrenergic Receptor Gene Polymorphisms in a Southeastern European Population. Front. Genet. 2018, 9, 560. [Google Scholar] [CrossRef]
- Ahles, A.; Engelhardt, S. Polymorphic Variants of Adrenoceptors: Pharmacology, Physiology, and Role in Disease. Pharmacol. Rev. 2014, 66, 598–637. [Google Scholar] [CrossRef]
- Shin, J.; Johnson, J.A. β-Blocker Pharmacogenetics in Heart Failure. Heart Fail. Rev. 2010, 15, 187–196. [Google Scholar] [CrossRef]
- Fayed, M.S.; Saleh, M.A.; Sabri, N.A.; Elkholy, A.A. Β1-Adrenergic Receptor Polymorphisms: A Possible Genetic Predictor of Bisoprolol Response in Acute Coronary Syndrome. Future Sci. OA 2023, 9, FSO895. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.-P.; Zhang, X.-Z.; Huang, S.-Q.; Bartlam, M.; Zhou, S.-F. New Insights into the Structural Characteristics and Functional Relevance of the Human Cytochrome P450 2D6 Enzyme. Drug Metab. Rev. 2009, 41, 573–643. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Dong, G.; Yue, J. The Endogenous Substrates of Brain CYP2D. Eur. J. Pharmacol. 2014, 724, 211–218. [Google Scholar] [CrossRef]
- Teh, L.K.; Bertilsson, L. Pharmacogenomics of CYP2D6: Molecular Genetics, Interethnic Differences and Clinical Importance. Drug Metab. Pharmacokinet. 2012, 27, 55–67. [Google Scholar] [CrossRef]
- Walko, C.M.; McLeod, H. Use of CYP2D6 Genotyping in Practice: Tamoxifen Dose Adjustment. Pharmacogenomics 2012, 13, 691–697. [Google Scholar] [CrossRef]
- Leitão, L.P.C.; Souza, T.P.; Rodrigues, J.C.G.; Fernandes, M.R.; Santos, S.; Santos, N.P.C. The Metabolization Profile of the CYP2D6 Gene in Amerindian Populations: A Review. Genes 2020, 11, 262. [Google Scholar] [CrossRef]
- Saravia-Bartra, M.; Losno-García, R.; Valderrama-Wong, M.; Muñoz-Jáuregui, A.M.; Bendezú-Acevedo, M.; García-Ceccarelli, J.; Surco-Laos, F.; Basurto-Ayala, P.; Pineda-Pérez, M.; Alvarado-Yarasca, A.T. Interacciones farmacocinéticas de la azitromicina e implicación clínica. Rev. Cuba. Med. Mil. 2021, 50, e02101284. [Google Scholar]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Ray, B.; Ozcagli, E.; Sadee, W.; Wang, D. CYP2D6 Haplotypes with Enhancer Single-Nucleotide Polymorphism Rs5758550 and Rs16947 (*2 Allele): Implications for CYP2D6 Genotyping Panels. Pharmacogenetics Genom. 2019, 29, 39–47. [Google Scholar] [CrossRef]
- Jessurun, N.T.; Wijnen, P.A.; Bast, A.; van Puijenbroek, E.P.; Bekers, O.; Drent, M. Tamsulosin Associated with Interstitial Lung Damage in CYP2D6 Variant Alleles Carriers. Int. J. Mol. Sci. 2020, 21, 2770. [Google Scholar] [CrossRef]
- Ur Rasheed, M.S.; Mishra, A.K.; Singh, M.P. Cytochrome P450 2D6 and Parkinson’s Disease: Polymorphism, Metabolic Role, Risk and Protection. Neurochem. Res. 2017, 42, 3353–3361. [Google Scholar] [CrossRef]
- Zanger, U.M.; Raimundo, S.; Eichelbaum, M. Cytochrome P450 2D6: Overview and Update on Pharmacology, Genetics, Biochemistry. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 23–37. [Google Scholar] [CrossRef]
- Dorado, P.; González, I.; Naranjo, M.E.G.; de Andrés, F.; Peñas-Lledó, E.M.; Calzadilla, L.R.; LLerena, A. Lessons from Cuba for Global Precision Medicine: CYP2D6 Genotype Is Not a Robust Predictor of CYP2D6 Ultrarapid Metabolism. OMICS J. Integr. Biol. 2017, 21, 17–26. [Google Scholar] [CrossRef]
- Salyakina, D.; Roy, S.; Wang, W.; Oliva, M.; Akhouri, R.; Sotto, I.; Mulas, N.; Solano, R.; Fernández, J.R.; Sanchez, S.; et al. Results and Challenges of Cytochrome P450 2D6 (CYP2D6) Testing in an Ethnically Diverse South Florida Population. Mol. Genet. Genom. Med. 2019, 7, e922. [Google Scholar] [CrossRef]
- Lu, Y.; Mo, C.; Zeng, Z.; Chen, S.; Xie, Y.; Peng, Q.; He, Y.; Deng, Y.; Wang, J.; Xie, L.; et al. CYP2D6*4 Allele Polymorphism Increases the Risk of Parkinson’s Disease: Evidence from Meta-Analysis. PLoS ONE 2013, 8, e84413. [Google Scholar] [CrossRef]
- De Andrés, F.; Sosa-Macías, M.; Ramos, B.P.L.; Naranjo, M.-E.G.; LLerena, A. CYP450 Genotype/Phenotype Concordance in Mexican Amerindian Indigenous Populations–Where to from Here for Global Precision Medicine? OMICS J. Integr. Biol. 2017, 21, 509–519. [Google Scholar] [CrossRef]
- Quiñones, L.; Roco, Á.; Cayún, J.P.; Escalante, P.; Miranda, C.; Varela, N.; Meneses, F.; Gallegos, B.; Zaruma-Torres, F.; Lares-Asseff, I. Farmacogenómica Como Herramienta Fundamental Para La Medicina Personalizada: Aplicaciones En La Práctica Clínica. Rev. Méd. Chile 2017, 145, 483–500. [Google Scholar] [CrossRef]
- Alvarado, A.T.; Ybañez-Julca, R.; Muñoz, A.M.; Tejada-Bechi, C.; Cerro, R.; Quiñones, L.A.; Varela, N.; Alvarado, C.A.; Alvarado, E.; Bendezú, M.R.; et al. Frequency of CYP2D6*3 and *4 and Metabolizer Phenotypes in Three Mestizo Peruvian Populations. Pharmacia 2021, 68, 891–898. [Google Scholar] [CrossRef]
- Tamburro, M.; Ripabelli, G. High Resolution Melting as a Rapid, Reliable, Accurate and Cost-Effective Emerging Tool for Genotyping Pathogenic Bacteria and Enhancing Molecular Epidemiological Surveillance: A Comprehensive Review of the Literature. Ann. Ig. 2017, 29, 293–316. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Dybska, E.; Barciszewska, A.-M. DNA Methylation Analysis with Methylation-Sensitive High-Resolution Melting (MS-HRM) Reveals Gene Panel for Glioma Characteristics. CNS Neurosci. Ther. 2020, 26, 1303–1314. [Google Scholar] [CrossRef]
- Molenaar, P.; Rabnott, G.; Yang, I.; Fong, K.M.; Savarimuthu, S.M.; Li, L.; West, M.J.; Russell, F.D. Conservation of the Cardiostimulant Effects of (−)-Norepinephrine across Ser49Gly and Gly389Arg Beta1-Adrenergic Receptor Polymorphisms in Human Right Atrium in Vitro. J. Am. Coll. Cardiol. 2002, 40, 1275–1282. [Google Scholar] [CrossRef]
- Shin, J.; Johnson, J.A. Pharmacogenetics of β-Blockers. Pharmacotherapy 2007, 27, 874–887. [Google Scholar] [CrossRef]
- Hakalahti, A.E.; Tapanainen, J.M.; Junttila, J.M.; Kaikkonen, K.S.; Huikuri, H.V.; Petaja-Repo, U.E. Association of the Beta-1 Adrenergic Receptor Carboxyl Terminal Variants with Left Ventricular Hypertrophy among Diabetic and Non-Diabetic Survivors of Acute Myocardial Infarction. Cardiovasc. Diabetol. 2010, 9, 42. [Google Scholar] [CrossRef]
- Liu, W.-N.; Fu, K.-L.; Gao, H.-Y.; Shang, Y.-Y.; Wang, Z.-H.; Jiang, G.-H.; Zhang, Y.; Zhang, W.; Zhong, M. Β1 Adrenergic Receptor Polymorphisms and Heart Failure: A Meta-Analysis on Susceptibility, Response to β-Blocker Therapy and Prognosis. PLoS ONE 2012, 7, e37659. [Google Scholar] [CrossRef]
- Mahesh Kumar, K.N.; Ramu, P.; Rajan, S.; Shewade, D.G.; Balachander, J.; Adithan, C. Genetic Polymorphisms of Β1 Adrenergic Receptor and Their Influence on the Cardiovascular Responses to Metoprolol in a South Indian Population. J. Cardiovasc. Pharmacol. 2008, 52, 459–466. [Google Scholar] [CrossRef]
- Sawczuk, M.; Maciejewska-Karlowska, A.; Cieszczyk, P.; Zarebska, A. Ser49Gly and Arg389Gly Polymorphisms of the ADRB1 Gene and Endurance Performance. Open Life Sci. 2012, 7, 794–800. [Google Scholar] [CrossRef]
- Albuquerque, F.N.d.; Brandão, A.A.; Silva, D.A.; Rocha, R.M.; Bittencourt, M.I.; Sales, A.L.F.; Spineti, P.P.d.M.; Duque, G.S.; Azevedo, L.R.d.S.; Pozzan, R.; et al. O Polimorfismo Genético Do Receptor Beta-Adrenérgico Tipo 1 Ser49Gly é Preditor de Morte Em Pacientes Brasileiros Com Insuficiência Cardíaca. Arq. Bras. Cardiol. 2020, 114, 616–624. [Google Scholar] [CrossRef]
- Santos, K.T.; De Freitas, R.G.A.; Manta, F.S.N.; De Carvalho, E.F.; Silva, D.A. Ser49Gly Polymorphism in the β-Adrenergic Receptor 1 Gene in a Population Sample from Rio de Janeiro State, Brazil, Stratified by Self-Identified Skin Color and Genetic Ancestry. Mol. Med. Rep. 2015, 12, 1591–1597. [Google Scholar] [CrossRef]
- Rau, T.; Düngen, H.-D.; Edelmann, F.; Waagstein, F.; Lainščak, M.; Dimković, S.; Apostolović, S.; Nešković, A.N.; Haverkamp, W.; Gelbrich, G.; et al. Impact of the Β1-Adrenoceptor Arg389Gly Polymorphism on Heart-Rate Responses to Bisoprolol and Carvedilol in Heart-Failure Patients. Clin. Pharmacol. Ther. 2012, 92, 21–28. [Google Scholar] [CrossRef]
- Gu, R.; Shen, Y.; Liu, J.; Qiao, S.; Wang, L.; Xu, B. Association of Arg389Gly Β1-Adrenergic Receptor Polymorphism with Effective Dose of β Blocker in Congestive Heart Failure among Chinese Han Population. Genetics. bioRxiv 2018. [Google Scholar] [CrossRef]
- Topolska, E.; Oszczygieł, A.; Mańkowska-Załuska, B.; Lubiński, A. Różnice w Występowaniu Poszczególnych Zaburzeń Rytmu Serca w Zależności Od Płci i Stężenia Hormonów Płciowych. Good Rythm. 2016, 1, 34–38. [Google Scholar] [CrossRef]
- Machuki, J.O.; Zhang, H.Y.; Harding, S.E.; Sun, H. Molecular Pathways of Oestrogen Receptors and β-Adrenergic Receptors in Cardiac Cells: Recognition of Their Similarities, Interactions and Therapeutic Value. Acta Physiol. 2018, 222, e12978. [Google Scholar] [CrossRef]
- Villareal, R.P.; Woodruff, A.L.; Massumi, A. Gender and Cardiac Arrhythmias. Tex. Heart Inst. J. 2001, 28, 265–275. [Google Scholar]
- Zeitler, E.P.; Poole, J.E.; Albert, C.M.; Al-Khatib, S.M.; Ali-Ahmed, F.; Birgersdotter-Green, U.; Cha, Y.-M.; Chung, M.K.; Curtis, A.B.; Hurwitz, J.L.; et al. Arrhythmias in Female Patients: Incidence, Presentation and Management. Circ. Res. 2022, 130, 474–495. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic Polymorphisms of Cytochrome P450 2D6 (CYP2D6): Clinical Consequences, Evolutionary Aspects and Functional Diversity. Pharmacogenomics J. 2005, 5, 6–13. [Google Scholar] [CrossRef]
- Bradford, L.D. CYP2D6 Allele Frequency in European Caucasians, Asians, Africans and Their Descendants. Pharmacogenomics 2002, 3, 229–243. [Google Scholar] [CrossRef]
- Sachse, C.; Brockmöller, J.; Bauer, S.; Roots, I. Cytochrome P450 2D6 Variants in a Caucasian Population: Allele Frequencies and Phenotypic Consequences. Am. J. Hum. Genet. 1997, 60, 284–295. [Google Scholar]
- Luzum, J.A.; Sweet, K.M.; Binkley, P.F.; Schmidlen, T.J.; Jarvis, J.P.; Christman, M.F.; Sadee, W.; Kitzmiller, J.P. CYP2D6 Genetic Variation and Beta-Blocker Maintenance Dose in Patients with Heart Failure. Pharm. Res. 2017, 34, 1615–1625. [Google Scholar] [CrossRef]
- Wu, D.; Li, G.; Deng, M.; Song, W.; Huang, X.; Guo, X.; Wu, Z.; Wu, S.; Xu, J. Associations between ADRB1 and CYP2D6 Gene Polymorphisms and the Response to β-Blocker Therapy in Hypertension. J. Int. Med. Res. 2015, 43, 424–434. [Google Scholar] [CrossRef]
- Liu, J. Gly389Arg Polymorphism of Β1-Adrenergic Receptor Is Associated with the Cardiovascular Response to Metoprolol. Clin. Pharmacol. Ther. 2003, 74, 372–379. [Google Scholar] [CrossRef]
- Sethi, Y.; Patel, N.; Kaka, N.; Kaiwan, O.; Kar, J.; Moinuddin, A.; Goel, A.; Chopra, H.; Cavalu, S. Precision Medicine and the Future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. J. Clin. Med. 2023, 12, 1799. [Google Scholar] [CrossRef]

| General Characteristics | Study Group (N = 30) | Control Group (N = 20) | p-Value |
|---|---|---|---|
| Gender (F/M) | 16/14 | 12/8 | - |
| Mean age (years) | 15.5 ± 1.9 | 15.8 ± 1.2 | 0.59 |
| Height (cm) | 169.7 ± 9.7 | 167.4 ± 5.7 | 0.34 |
| Weight (kg) | 63.8 ± 18.3 | 63.5 ± 11.7 | 0.94 |
| BSA (m2) | 1.72 ± 0.24 | 1.71 ± 0.15 | 0.81 |
| BMI | 21.9 ± 5.2 | 22.4 ± 3.6 | 0.71 |
| BP syst (mmHg) | 117.5 ± 10.4 | 120.5 ± 14.5 | 0.40 |
| BP diast (mmHg) | 64.9 ± 8.0 | 62.6 ± 0.6 | 0.39 |
| Sat O2 (%) | 98.3 ± 0.7 | 98.4 ± 1.1 | 0.79 |
| Laboratory tests | |||
| CK-MB (U/L) | 14.1 ± 5.3 | 12.1 ± 5.8 | 0.35 |
| WBC (103 u/L) | 6.49 ± 1.83 | 6.88 ± 2.08 | 0.50 |
| RBC (106 u/L) | 4.88 ± 0.45 | 4.80 ± 0.47 | 0.53 |
| HGB (g/dL) | 13.08 ± 1.31 | 14.0 ± 1.25 | 0.61 |
| HCT (%) | 41.5 ± 2.7 | 41.1 ± 3.8 | 0.62 |
| TSH (mL U/L) | 2.43 ± 1.05 | 2.36 ± 1.27 | 0.85 |
| FT4 (ng/dL) | 1.3 ± 0.18 | 1.4 ± 0.39 | 0.14 |
| Echocardiography parameters | |||
| LVIDD (mm) | 48.7 ± 4.5 | 48.5 ± 3.1 | 0.86 |
| LVIDS (mm) | 31.7 ± 13.7 | 28.8 ± 3.3 | 0.38 |
| LV-EF (%) | 68.7 ± 7.9 | 69.9 ± 5.9 | 0.59 |
| LV-SF (%) | 40.7 ± 6.1 | 39.9 ± 4.9 | 0.63 |
| Exercise test | |||
| Stress test (gr) | 3.7 ± 0.6 | 3.8 ± 0.5 | 0.67 |
| METS | 11.2 ± 1.1 | 11.4 ± 0.73 | 0.56 |
| NYHA scale (I/II) | 22/8 | 18/2 | - |
| R389G Polymorphism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Group | Control Group | ||||||||||
| Genotype | Allele Frequency | Genotype | Allele Frequency | ||||||||
| Type of Arrhythmia | RR | RG | GG | R | G | RR | RG | GG | R | G | |
| VT+SVT (n = 30) | 13 | 10 | 7 | 36 (0.6) | 24 (0.4) | ||||||
| VT (n = 19) | 9 | 8 | 2 | 26 (0.69) | 12 (0.31) | (n = 20) | 5 | 12 | 3 | 22 (0.55) | 18 (0.45) |
| SVT (n = 11) | 4 | 2 | 5 | 10 (0.46) | 12 (0.54) | ||||||
| χ2 | 14.2 | p = 0.0002 | SVT vs. control | ||||||||
| 6.45 | p = 0.0002 | VT vs. control | |||||||||
| 12.5 | p = 0.0004 | SVT vs. VT | |||||||||
| gender | |||||||||||
| female (n = 16) | 7 | 4 | 5 | 18 (0.56) | 14 (0.44) | (n = 12) | 3 | 8 | 1 | 14 (0.58) | 10 (0.42) |
| SVT female (n = 7) | 3 | 0 | 4 | 6 (0.43) | 8 (0.57) | ||||||
| VT female (n = 9) | 4 | 4 | 1 | 12 (0.67) | 6 (0.33) | ||||||
| male (n = 14) | 6 | 6 | 2 | 18 (0.64) | 10 (0.36) | (n = 8) | 2 | 4 | 2 | 8 (0.5) | 8 (0.5) |
| χ2 | 3.12 | p = 0.007 | SVT girls vs. VT girls | ||||||||
| age | |||||||||||
| 11–14 (n = 10) | 4 | 5 | 1 | 13 (0.65) | 7 (0.35) | 11–14 (n = 3) | 0 | 2 | 1 | 2 (0.33) | 4 (0.67) |
| 15–16 (n = 6) | 2 | 1 | 3 | 5 (0.42) | 7 (0.58) | 15–16 (n = 9) | 2 | 6 | 1 | 10 (0.56) | 8 (0.44) |
| 16.5–18 (n = 14) | 7 | 4 | 3 | 18 (0.64) | 10 (0.36) | 16.5–18 (n = 8) | 3 | 4 | 1 | 10 (0.63) | 6 (0.37) |
| S49G Polymorphism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Group | Control Group | ||||||||||
| Genotype | Allele Frequency | Genotype | Allele Frequency | ||||||||
| Type of Arrhythmia | SS | SG | GG | S | G | SS | SG | GG | S | G | |
| VT + SVT (n = 30) | 24 | 5 | 1 | 53 (0.88) | 7 (0.12) | ||||||
| VT (n = 19) | 15 | 3 | 1 | 33 (0.87) | 5 (0.13) | (n = 20) | 16 | 3 | 1 | 35 (0.88) | 5 (0.12) |
| SVT (n = 11) | 9 | 2 | 0 | 20 (0.91) | 2 (0.09) | ||||||
| gender | |||||||||||
| female (n = 16) | 12 | 3 | 1 | 27 (0.84) | 5 (0.16) | (n = 12) | 10 | 1 | 1 | 21 (0.875) | 3 (0.125) |
| male (n = 14) | 12 | 2 | 0 | 26 (0.93) | 2 (0.07) | (n = 8) | 6 | 2 | 0 | 14 (0.875) | 2 (0.125) |
| age | |||||||||||
| 11–14 (n = 10) | 9 | 1 | 0 | 19 (0.95) | 1 (0.05) | 11–14 (n = 3) | 3 | 0 | 0 | 6 (1.00) | 0 (0) |
| 15–16 (n = 6) | 4 | 2 | 0 | 10 (0.83) | 2(0.17) | 15–16 (n = 9) | 7 | 2 | 0 | 16 (0.89) | 2 (0.11) |
| 16.5–18 (n = 14) | 11 | 2 | 1 | 24 (0.86) | 4 (0.14) | 16.5–18 (n = 8) | 6 | 1 | 1 | 13 (0.81) | 3 (0.19) |
| CYP2D6*4 Polymorphism | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Group | Control Group | ||||||||
| Genotype | Allele Frequency | Genotype | Allele Frequency | ||||||
| Type of Arrhythmia | WT/WT | WT/*4 | 4/4 | 4* | WT/WT | WT/*4 | 4/4 | 4* | |
| VT+SVT (n = 30) | 17 | 9 | 4 | 17 (0.28) | |||||
| VT (n = 19) | 11 | 4 | 4 | 12 (0.31) | (n = 20) | 15 | 3 | 2 | 7 (0.17) |
| SVT (n = 11) | 6 | 5 | 0 | 5 (0.23) | |||||
| gender | |||||||||
| female (n = 16) | 10 | 4 | 2 | 8 (0.25) | (n = 12) | 10 | 1 | 1 | 3 (0.125) |
| male (n = 14) | 7 | 5 | 2 | 9 (0.32) | (n = 8) | 5 | 2 | 1 | 4 (0.25) |
| age | |||||||||
| 11–14 (n = 10) | 7 | 3 | 0 | 3 (0.15) | 11–14 (n = 3) | 3 | 0 | 0 | 0 |
| 15–16 (n = 6) | 2 | 2 | 2 | 6 (0.5) | 15–16 (n = 9) | 5 | 3 | 1 | 5 (0.28) |
| 16.5–18 (n = 14) | 8 | 4 | 2 | 8 (0.28) | 16.5–18 (n = 8) | 7 | 0 | 1 | 2 (0.125) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moric-Janiszewska, E.; Smolik, S.; Szydłowski, L.; Kapral, M. Associations between Selected ADRB1 and CYP2D6 Gene Polymorphisms in Children with Ventricular and Supraventricular Arrhythmias. Medicina 2023, 59, 2057. https://doi.org/10.3390/medicina59122057
Moric-Janiszewska E, Smolik S, Szydłowski L, Kapral M. Associations between Selected ADRB1 and CYP2D6 Gene Polymorphisms in Children with Ventricular and Supraventricular Arrhythmias. Medicina. 2023; 59(12):2057. https://doi.org/10.3390/medicina59122057
Chicago/Turabian StyleMoric-Janiszewska, Ewa, Sławomir Smolik, Lesław Szydłowski, and Małgorzata Kapral. 2023. "Associations between Selected ADRB1 and CYP2D6 Gene Polymorphisms in Children with Ventricular and Supraventricular Arrhythmias" Medicina 59, no. 12: 2057. https://doi.org/10.3390/medicina59122057
APA StyleMoric-Janiszewska, E., Smolik, S., Szydłowski, L., & Kapral, M. (2023). Associations between Selected ADRB1 and CYP2D6 Gene Polymorphisms in Children with Ventricular and Supraventricular Arrhythmias. Medicina, 59(12), 2057. https://doi.org/10.3390/medicina59122057








