Quality of Life Predictors in a Group of Informal Caregivers during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measures
- Socio-demographic data: we designed a semi-structured questionnaire to collect the following information: gender, age, living area, marital status, children, education, profession, working status. It included COVID questions regarding infection and vaccination. There were questions relating to the patient: relationship to the caregiver, diagnosis, last Mini Mental State Examination (MMSE) score, the number of caregivers for the same patient [19].
- Open-answer questions: “What difficulties have you encountered while being an informal caregiver?” and “What type of assistance do you wish you had?”
- The Maslach Burnout Inventory—General Survey: considered a “gold standard” method for measuring burnout using a 16-item scale with a 7-point Likert-type scale that addresses the following components: exhaustion (Cronbach’s alpha coefficient rating: 0.90), cynicism (Cronbach’s alpha coefficient rating: 0.76), and professional efficacy (Cronbach’s alpha coefficient rating: 0.76) (e.g., “I feel emotionally drained from my work”). For test–retest reliability, time periods of a couple of weeks (scores of 20.60–0.82), 3 months, and 1 year (scores of 0.54–0.60) were used. Score interpretation for exhaustion was 0–18 for low burnout level, 19–26 for moderate, ≥27 for high; for cynicism, 1–5 was a low level of burnout, 6–9 was moderate, ≥10 was high; for professional efficacy, ≥40 was a low level of burnout, 34–39 was moderate, 0–33 was high [20,21].
- The Hamilton Anxiety Rating Scale (HARS): designed for measuring the severity of anxiety through 14 items for both psychic and somatic symptoms (e.g., “Anxious mood: Worries, anticipation of the worst, fearful anticipation, irritability”); the scale is clinician-administered, and a score of ≤17 indicates mild anxiety, 18–24 moderate anxiety, and 25–30 moderate to severe anxiety. The predictive validity using Cronbach’s alpha coefficient was 0.921 [22].
- Hamilton Depression Rating Scale (HDRS): designed for measuring the severity of depression, with 17 items describing both psychic and somatic symptoms (e.g., “Depressed mood: Gloomy attitude, pessimism about the future, feeling of sadness, tendency to weep”); the scale is clinician-administered, and a score of 0–7 is considered normal, 8–16 is mild depression, 17–23 is moderate depression, and ≥24 is severe depression. This scale has adequate internal consistency (α = 0.77), a high degree of inter-rater reliability (ICC = 0.82), and a Cronbach’s alpha coefficient <0.01 [23].
- World Health Organization Quality of Life (WHOQOL) brief version: used for measuring occupational burnout through 26 items assessing four areas: firstly, physical health (seven questions regarding daily activities, mobility, energy, functional capacity, pain, and sleep); secondly, mental health (six questions related to self-image, ability to learn, positive attitudes, negative thoughts, mentality, religion, focus, and mental status); thirdly, social relationships (three questions about personal relationships, sex life, social support); and lastly, environmental health (eight questions about safety, financial resources, health and social services, the environment in which they live, recreation, opportunities to acquire new knowledge, the environment, and their means of transport), e.g., “To what extent do you have the opportunity for leisure activities?” The World Health Organization developed this widely used scale for cross-cultural comparison. The reliability is assessed using the Cronbach’s alpha coefficient (0.91) and, according to the convergent validity, the correlation coefficient values are strongly associated at 0.01 [24,25].
- Pittsburg Sleep Quality Index (PSQI): evaluates the overall sleep quality in the prior month (sleep quality, latency, duration, efficiency, sleep disturbances, the use of sleep medication, and dysfunction during the daytime), e.g., “During the past month, how would you rate your sleep quality overall?” The validity of this test using Cronbach’s alpha coefficient is between 0.70 and 0.85 [26].
- Fear of COVID-19 scale: was developed by a group of researchers in Iran for measuring fear of COVID using 7 questions with 5 possible answers (e.g., “I cannot sleep because I am worried about getting COVID-19”). Total scores range between 7 and 35. This scale has been shown to be a valid and reliable way to assess the fear of COVID in the general population, with robust psychometric properties (test–retest reliability ICC = 0.72, internal consistency of α = 0.82) [27,28].
- Caregiver burden scale: used for measuring the burden of the caregivers using 15 items describing the assistance provided to the patient: transportation, housekeeping, cooking, shopping, decision making, financial record keeping, walking, making house repairs, farming/yardwork, administering medication, dressing, bathing, eating, toileting, and leaving patient unattended (e.g., “Does the patient need assistance with transportation?”), with a total score range between 0 and 15.
2.3. Participants
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic Data
3.2. Questions Regarding the Dementia Patient
3.3. COVID-Related Questions
3.4. Caregivers’ Burden and Needs
3.5. Questionnaires
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Alzheimer’s Association Report. Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Onetiu, V.; Sorina Maria Aurelian, A.; Capisizu, F.; Ileana Codruta Zus, K. Cost of Dementia in Romania: A Cross-Sectional Cost-of-Illness Study Undertaken in Bucharest. Zdr. Pu-Bliczne I Zarządzanie 2016, 14, 204–226. [Google Scholar]
- Cheng, S.-T. Dementia Caregiver Burden: A Research Update and Critical Analysis. Curr. Psychiatry Rep. 2017, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Archbold, P.G. Impact of Parent Caring on Women. In Proceedings of the XII International Congress of Gerontology, Hamburg, Germany, 12–14 July 1981; National Council on Family Relations: Saint Paul, MN, USA, 1983; Volume 32, pp. 39–45. [Google Scholar]
- Rapp, S.R.; Chao, D. Appraisals of Strain and of Gain: Effects on Psychological Wellbeing of Caregivers of Dementia Patients. Aging Ment. Health 2000, 4, 142–147. [Google Scholar] [CrossRef]
- Jeste, D.V.; Mausbach, B.; Lee, E.E. Caring for Caregivers/Care Partners of Persons with Dementia. Int. Psychogeriatry 2021, 33, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Lowery, K.; Mynt, P.; Aisbett, J.; Dixon, T.; Obrien, J.; Ballard, C. Depression in the Carers of Dementia Sufferers: A Com-Parison of the Carers of Patients Suffering from Dementia with Lewy Bodies and the Carers of Patients with Alzheimer’s Disease. J. Affect. Disord. 2000, 59, 61–65. [Google Scholar] [CrossRef]
- Brodaty, H.; Hadzi-Pavlovic, D. Psychosocial Effects on Carers of Living with Persons with Dementia. Aust. N. Z. J. Psychiatry 1990, 24, 351–361. [Google Scholar] [CrossRef]
- Cohen, G.; Russo, M.J.; Campos, J.A.; Allegri, R.F. Living with Dementia: Increased Level of Caregiver Stress in Times of COVID-19. Int. Psychogeriatry 2020, 32, 1377–1381. [Google Scholar] [CrossRef]
- Aledeh, M.; Habib Adam, P. Caring for Dementia Caregivers in Times of the COVID-19 Crisis: A Systematic Review. Am. J. Nurs. Res. 2020, 8, 552–561. [Google Scholar] [CrossRef]
- Bailey, C.; Guo, P.; MacArtney, J.; Finucane, A.; Swan, S.; Meade, R.; Wagstaff, E. The Experiences of Informal Carers during the COVID-19 Pandemic: A Qualitative Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 13455. [Google Scholar] [CrossRef]
- Eggert, S.; Teubner, C.; Budnick, A.; Gellert, P.; Kuhlmey, A. Pflegende Angehörige in Der COVID-19-Krise; Stiftung ZQP: Berlin, Germany, 2020. [Google Scholar]
- Wolf-Ostermann, K.; Schmidt, A.; Preuß, B.; Heinze, F.; Seibert, K.; Friedrich, A.-C.; Domhoff, D.; Stolle, C.; Rothgang, H. Pflege in Zeiten von Corona: Ergebnisse Einer Deutschlandweiten Querschnittbefragung von Ambulanten Pflegediensten Und Teilstationären Einrichtungen. Pflege 2020, 33, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Leiblfinger, M.; Prieler, V.; Schwiter, K.; Steiner, J.; Benazha, A.; Lutz, H. Impact of COVID-19 Policy Responses on Live-in Care Workers in Austria, Germany, and Switzerland. J. Long Term Care 2020, 144–150. [Google Scholar] [CrossRef]
- Rothgang, H.; Wolf-Ostermann, K.; Domhoff, D.; Friedrich, A.-C.; Heinze, F.; Heß, M. Zur Situation Der Häuslichen Pflege in Deutschland Während Der Corona-Pandemie-Ergebnisse Einer Online—Befragung von Informellen Pflegepersonen Im Erwerbsfähigen Alter. Bremen University: Bremen, Germany, 2020. [Google Scholar]
- Bergmann, M.; Wagner, M. The Impact of COVID-19 on Informal Caregiving and Care Receiving across Europe during the First Phase of the Pandemic. Front. Public Health 2021, 9, 673874. [Google Scholar] [CrossRef]
- Ciobanu, A.M.; Damian, A.C.; Neagu, C. Association between Burnout and Immunological and Endocrine Alterations. Rom. J. Morphol. Embryol. 2021, 62, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Enciu, B.G.; Tănase, A.A.; Drăgănescu, A.C.; Aramă, V.; Pițigoi, D.; Crăciun, M.-D. The COVID-19 Pandemic in Romania: A Comparative Description with Its Border Countries. Healthcare 2022, 10, 1223. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State. J. Psychiatry Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Portoghese, I.; Leiter, M.P.; Maslach, C.; Galletta, M.; Porru, F.; D’Aloja, E.; Finco, G.; Campagna, M. Measuring Burnout among University Students: Factorial Validity, Invariance, and Latent Profiles of the Italian Version of the Maslach Burnout Inventory Student Survey (MBI-SS). Front. Psychol. 2018, 9, 2105. [Google Scholar] [CrossRef]
- Hallit, S.; Haddad, C.; Hallit, R.; Akel, M.; Obeid, S.; Haddad, G.; Soufia, M.; Khansa, W.; Khoury, R.; Kheir, N.P. Validation of the Hamilton anxiety rating scale and state trait anxiety inventory A and B in Arabic among the Lebanese population. Clin. Epidemiol. Glob. Health 2020, 8, 1104–1109. [Google Scholar] [CrossRef]
- Wickramasinghe, N.D.; Dissanayake, D.S.; Abeywardena, G.S. Validity and Reliability of the Maslach Burnout Inventory-Student Survey in Sri Lanka. BMC Psychol. 2018, 6, 52. [Google Scholar] [CrossRef]
- Olden, M.; Rosenfeld, B.; Pessin, H.; Breitbart, W. Measuring Depression at the End of Life: Is the Hamilton Depression Rating Scale a Valid Instrument? Assessment 2009, 16, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Ilić, I.; Šipetić-Grujičić, S.; Grujičić, J.; Živanović Mačužić, I.; Kocić, S.; Ilić, M. Psychometric Properties of the World Health Organization’s Quality of Life (WHOQOL-BREF) Questionnaire in Medical Students. Medicina 2019, 55, 772. [Google Scholar] [CrossRef]
- Almarabheh, A.; Ghamdi, M.A.; Elbarbary, A.; Alqashar, A.; Alserdieh, F.; Alahmed, F.; Alhaddar, H.; AlSada, L.; Yosri, M.; Omran, M.; et al. Validity and Reliability of the WHOQOL-BREF in the Measurement of the Quality of Life of Sickle Disease Patients in Bahrain. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Midorikawa, H.; Aiba, M.; Lebowitz, A.; Taguchi, T.; Shiratori, Y.; Ogawa, T.; Takahashi, A.; Takahashi, S.; Nemoto, K.; Arai, T.; et al. Confirming Validity of The Fear of COVID-19 Scale in Japanese with a Nationwide Large-Scale Sample. PLoS ONE 2021, 16, e0246840. [Google Scholar] [CrossRef]
- Ahorsu, D.K.; Lin, C.-Y.; Imani, V.; Saffari, M.; Griffiths, M.D.; Pakpour, A.H. The Fear of COVID-19 Scale: Development and Initial Validation. Int. J. Ment. Health Addict. 2022, 20, 1537–1545. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.-X.; Lin, Y.-Q.; Wang, L.; Zeng, Z.-N.; Xie, X.-L.; Chen, Q.-Y.; Wei, S.-C. Reliability and Validity of the Pittsburgh Sleep Quality Index among Frontline COVID-19 Health Care Workers Using Classical Test Theory and Item Response Theory. J. Clin. Sleep Med. 2022, 18, 541–551. [Google Scholar] [CrossRef]
- Brodaty, H.; Donkin, M. Family Caregivers of People with Dementia. Dialogues Clin. Neurosci. 2009, 11, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Wister, A.; Li, L.; Mitchell, B.; Wolfson, C.; McMillan, J.; Griffith, L.E.; Kirkland, S.; Raina, P.; Costa, A.; Anderson, L.; et al. Levels of Depression and Anxiety among Informal Caregivers during the COVID-19 Pandemic: A Study Based on the Canadian Longitudinal Study on Aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77, 1740–1757. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chang, M.-C.; Wang, W.-F.; Jhang, K.-M. A Comparison of Caregiver Burden for Different Types of Dementia: An 18-Month Retrospective Cohort Study. Front. Psychol. 2022, 12, 798315. [Google Scholar] [CrossRef]
- Bruno, F.; Malvaso, A.; Chiesi, F.; Laganà, V.; Servidio, R.; Isella, V.; Ferrarese, C.; Gottardi, F.; Stella, E.; Agosta, F.; et al. COVID-19 Vaccine Uptake among Family Caregivers of People with Dementia: The Role of Attitudes toward Vaccination, Perceived Social Support and Personality Traits. Front. Psychol. 2022, 13, 923316. [Google Scholar] [CrossRef]
- Oortwijn, R.; Van Leeuwen, F.; Ren, D. How Openness to Experience Relates to Conspiracy Mentality and Vaccine Hesitancy. Available online: http://arno.uvt.nl/show.cgi?fid=151204 (accessed on 10 August 2023).
- Le Couteur, D.G.; Anderson, R.M.; Newman, A.B. COVID-19 through the Lens of Gerontology. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, A. COVID-19: GPs Can Stop Health Checks for over 75s and Routine Medicine Reviews. BMJ 2020, 368, m1157. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Hoben, M.; Amuah, J.E.; Hogan, D.B.; Baumbusch, J.; Gruneir, A.; Chamberlain, S.A.; Griffith, L.E.; McGrail, K.M.; Corbett, K.; et al. Prevalence and Correlates of Anxiety and Depression in Caregivers to Assisted Living Residents during COVID-19: A Cross-Sectional Study. BMC Geriatr. 2022, 22, 662. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.C.N.; Belfort, T.; Monteiro, A.; de Lucena, A.T.; Lacerda, I.B.; Gaigher, J.; Baptista, M.A.T.; Brandt, M.; Kimura, N.R.; de Souza, N.; et al. COVID-19: Challenges for Dementia Care and Research. Dement. Neuropsychol. 2020, 14, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.; Reiboldt, W.; Claver, M.; Fay, J. Caregiving in Quarantine: Evaluating the Impact of the Covid-19 Pandemic on Adult Child Informal Caregivers of a Parent. Gerontol. Geriatr. Med. 2021, 7, 233372142199015. [Google Scholar] [CrossRef]
- Budnick, A.; Hering, C.; Eggert, S.; Teubner, C.; Suhr, R.; Kuhlmey, A.; Gellert, P. Informal Caregivers during the COVID-19 Pandemic Perceive Additional Burden: Findings from an Ad-Hoc Survey in Germany. BMC Health Serv. Res. 2021, 21, 353. [Google Scholar] [CrossRef]
- Cohen, S.A.; Kunicki, Z.J.; Drohan, M.M.; Greaney, M.L. Exploring Changes in Caregiver Burden and Caregiving Intensity Due to COVID-19. Gerontol. Geriatr. Med. 2021, 7, 233372142199927. [Google Scholar] [CrossRef]
- Zwaanswijk, M.; Peeters, J.M.; van Beek, A.P.A.; Meerveld, J.H.C.M.; Francke, A.L. Informal Caregivers of People with Dementia: Problems, Needs and Support in the Initial Stage and in Subsequent Stages of Dementia: A Questionnaire Survey. Open Nurs. J. 2013, 7, 6–13. [Google Scholar] [CrossRef]
- Giebel, C.; Lord, K.; Cooper, C.; Shenton, J.; Cannon, J.; Pulford, D.; Shaw, L.; Gaughan, A.; Tetlow, H.; Butchard, S.; et al. A UK Survey of COVID-19 Related Social Support Closures and Their Effects on Older People, People with Dementia, and Carers. Int. J. Geriatr. Psychiatry 2021, 36, 393–402. [Google Scholar] [CrossRef]
- Park, S.S. Caregivers’ Mental Health and Somatic Symptoms during COVID-19. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, e235–e240. [Google Scholar] [CrossRef]
- Savla, J.; Roberto, K.A.; Blieszner, R.; McCann, B.R.; Hoyt, E.; Knight, A.L. Dementia Caregiving during the “Stay-at-Home” Phase of COVID-19 Pandemic. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, e241–e245. [Google Scholar] [CrossRef]
- Sheth, K.; Lorig, K.; Stewart, A.; Parodi, J.F.; Ritter, P.L. Effects of COVID-19 on Informal Caregivers and the Development and Validation of a Scale in English and Spanish to Measure the Impact of COVID-19 on Caregivers. J. Appl. Gerontol. 2021, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bussè, C.; Barnini, T.; Zucca, M.; Rainero, I.; Mozzetta, S.; Zangrossi, A.; Cagnin, A. Depression, Anxiety and Sleep Alterations in Caregivers of Persons with Dementia after 1-Year of COVID-19 Pandemic. Front. Psychiatry 2022, 13, 826371. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.; Farina, N.; Hughes, L.; Armsby, E.; Akarsu, N.; Pooley, J.; Towson, G.; Feeney, Y.; Tabet, N.; Fine, B.; et al. COVID-19 and the Quality of Life of People with Dementia and Their Carers—The TFD-C19 Study. PLoS ONE 2022, 17, e0262475. [Google Scholar] [CrossRef]
- Leggett, A.N.; Carmichael, A.; Leonard, N.; Jackson, J.; Kirch, M.; Solway, E.; Kullgren, J.T.; Singer, D.; Malani, P.N.; Gonzalez, R. Care Challenges Due to COVID-19 and Mental Health among Caregivers of U.S. Adults with a Chronic or Disabling Condition. Innov. Aging 2021, 5, igab031. [Google Scholar] [CrossRef] [PubMed]
- Ślusarska, B.; Bartoszek, A.; Kocka, K.; Deluga, A.; Chrzan-Rodak, A.; Nowicki, G. Quality of Life Predictors in Informal Caregivers of Seniors with a Functional Performance Defici—An Example of Home Care in Poland. Clin. Interv. Aging 2019, 14, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Ichimiya, A.; Igata, R.; Ogomori, K. Care-Givers IT. QOL and the Burden of Care-Giving for De-Mented Elderly at Home. Ronen Seishin Igaku Zasshi 2001, 12, 1159–1167. [Google Scholar]
- Glozman, J.M. Quality of Life of Caregivers. Neuropsychol. Rev. 2004, 14, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Takahashi, M.; Iwamitsu, Y.; Oishi, S.; Miyaoka, H. Subjective Experiences of Family Caregivers of Patients with Dementia as Predictive Factors of Quality of Life: Predictive Factors for QOL in Caregivers. Psychogeriatrics 2011, 11, 98–104. [Google Scholar] [CrossRef]
- Hajjar, L.; Kragen, B. Timely Communication through Telehealth: Added Value for a Caregiver during COVID-19. Front. Public Health 2021, 9, 755391. [Google Scholar] [CrossRef]
- Roach, P.; Zwiers, A.; Cox, E.; Fischer, K.; Charlton, A.; Josephson, C.B.; Patten, S.B.; Seitz, D.; Ismail, Z.; Smith, E.E. Understanding the Impact of the COVID-19 Pandemic on Well-Being and Virtual Care for People Living with Dementia and Care Partners Living in the Community. Dementia 2021, 20, 2007–2023. [Google Scholar] [CrossRef]
- Cuffaro, L.; Lorenzo, D.; Bonavita, F. Dementia Care and COVID-19 Pandemic: A Necessary Digital Revolu-Tion. Neurol. Sci 2020, 41, 1977–1979. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.K.; Nicholson, R.; Epstein, C.; Donley, T.; Salant, R.; Nguyen, A.H.; Shirk, S.; Stevenson, E.; Mittelman, M.S. Telehealth Support for Dementia Caregivers during the COVID-19 Pandemic: Lessons Learned from the NYU Family Support Program. Am. J. Geriatr. Psychiatry 2023, 31, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Quail, Z.; Bolton, L.; Massey, K. Digital Delivery of Non-Pharmacological Intervention Programmes for People Living with Dementia during the COVID-19 Pandemic. BMJ Case Rep. 2021, 14, e242550. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, L.; Kalankesh, L.R.; Doshmangir, L.; Farahbakhsh, M. Telehealth in Primary Health Care: A Scoping Review of the Literature. Perspect. Health Inf. Manag. 2022, 19, 1n. [Google Scholar]
- Rajasekaran, K. Access to Telemedicine-Are We Doing All That We Can during the COVID-19 Pandemic? Otolaryngol Head Neck Surg. 2020, 163, 104–106. [Google Scholar] [CrossRef]
- Dang, S.; Gomez-Orozco, C.A.; van Zuilen, M.H.; Levis, S. Providing Dementia Consultations to Veterans Using Clinical Video Telehealth: Results from a Clinical Demonstration Project. Telemed. J. E-Health 2018, 24, 203–209. [Google Scholar] [CrossRef]
- Gately, M.E.; Trudeau, S.A.; Moo, L.R. In-Home Video Telehealth for Dementia Management: Implications for Rehabilitation. Curr. Geriatr. Rep. 2019, 8, 239–249. [Google Scholar] [CrossRef]
- Langford, A.T.; Solid, C.A.; Scott, E.; Lad, M.; Maayan, E.; Williams, S.K.; Seixas, A.A. Mobile Phone Ownership, Health Apps, and Tablet Use in US Adults with a Self-Reported History of Hypertension: Cross-Sectional Study. JMIR MHealth UHealth 2019, 7, e12228. [Google Scholar] [CrossRef]
- Panerai, S.; Raggi, A.; Tasca, D.; Musso, S.; Gelardi, D.; Prestianni, G.; Catania, V.; Muratore, S.; Ferri, R. Telephone-Based Reality Orientation Therapy for Patients with Dementia: A Pilot Study during the COVID-19 Outbreak. Am. J. Occup. Ther. 2021, 75, 7502205130. [Google Scholar] [CrossRef]
- Lai, F.H.-Y.; Yan, E.W.-H.; Yu, K.K.-Y.; Tsui, W.-S.; Chan, D.T.-H.; Yee, B.K. The Protective Impact of Telemedicine on Persons with Dementia and Their Caregivers during the COVID-19 Pandemic. Am. J. Geriatr. Psychiatry 2020, 28, 1175–1184. [Google Scholar] [CrossRef]
- Capozzo, R.; Zoccolella, S.; Frisullo, M.; Barone, R.; Dellabate, M.; Barulli, M. Telemedicine for Delivery of Care in Fron-Totemporal Lobar Degeneration during COVID-19 Pandemic: Results from Southern Italy. J. Alzheimer’s Dis. 2020, 76, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Mansour, H.; Carter, C.; Rapaport, P.; Morgan-Trimmer, S.; Marchant, N.L.; Poppe, M.; Higgs, P.; Brierley, J.; Solomon, N.; et al. Social Connectedness and Dementia Prevention: Pilot of the APPLE-Tree Video-Call Intervention during the COVID-19 Pandemic. Dementia 2021, 20, 2779–2801. [Google Scholar] [CrossRef] [PubMed]
- Masoud, S.S.; Meyer, K.N.; Sweet, M.; Prado, L.; White, P.J. We Don’t Feel so Alone: A Qualitative Study of Virtual Memory Cafés to Support Social Connectedness among Individuals Living with Dementia and Care Partners during COVID-19. Front Public Health 2021, 9, 660144. [Google Scholar] [CrossRef]
- Gedde, M.H.; Husebo, B.S.; Erdal, A.; Puaschitz, N.G.; Vislapuu, M.; Angeles, R.C.; Berge, L.I. Access to and Interest in Assistive Technology for Home-Dwelling People with Dementia during the COVID-19 Pandemic (Pan.deM). Int. Rev. Psychiatry 2021, 33, 404–411. [Google Scholar] [CrossRef] [PubMed]



| Questionnaire | Measure | Scoring |
|---|---|---|
| The Maslach Burnout Inventory | Measuring burnout using exhaustion, cynicism, professional efficacy | Exhaustion:
|
| Hamilton Anxiety Rating Scale (HARS) (clinician administered) | Severity of anxiety using psychic and somatic symptoms |
|
| Hamilton Depression Rating Scale (HDRS) (clinician administered) | Severity of depression using psychic and somatic symptoms |
|
| World Health Organization Quality of Life (WHOQOL) |
|
|
| Pittsburg Sleep Quality Index (PSQI) | Overall sleep quality (sleep quality, latency, duration, efficiency, sleep disturbances, the use of sleep medication, and dysfunction during the daytime) | A seven-component score was derived from the items, each scored from 0 (no difficulty) to 3 (severe difficulty). The component scores were summed, ranging from 0 to 21. |
| Fear of COVID-19 scale | Fear of COVID-19 | Total score range of 7–35 |
| Caregiver burden scale | Burden of the caregiver (transportation, housekeeping, cooking, shopping, decision making, financial record keeping, walking, making house repairs, farming/yardwork, administering medication, dressing, bathing, eating, toileting, leaving patient unattended) | Total score range of 1–15 |
| Mean | Standard Deviation | |
|---|---|---|
| Age | 55.22 | 55.22 |
| Frequency | Percent | |
| Gender | ||
| Female | 78 | 70.9 |
| Male | 32 | 29.1 |
| Living area | ||
| Urban | 88 | 80.0 |
| Rural | 22 | 20.0 |
| Marital status | ||
| Single | 6 | 5.5 |
| Married | 76 | 69.1 |
| Divorced | 12 | 10.9 |
| Widow | 10 | 9.1 |
| In a relationship | 6 | 5.5 |
| Education | ||
| Middle school | 6 | 5.5 |
| High school | 58 | 52.7 |
| University | 46 | 41.8 |
| Occupation | ||
| Student | 2 | 1.8 |
| Working | 68 | 61.8 |
| Unemployed | 4 | 3.6 |
| Retired | 36 | 32.7 |
| Working status | ||
| Physical presence | 48 | 43.6 |
| Online presence | 6 | 5.5 |
| Hybrid presence | 18 | 16.4 |
| Relationship to the Patient | Frequency | Percent |
|---|---|---|
| Nephew/niece | 14 | 12.7 |
| Wife/husband | 20 | 18.2 |
| Daughter/son | 68 | 61.8 |
| Daughter-in-law | 8 | 7.3 |
| Type of dementia | ||
| Atypical or mixed Alzheimer’s dementia | 64 | 58.2 |
| Alzheimer’s dementia, late onset | 12 | 10.9 |
| Alzheimer’s dementia, unspecified | 28 | 25.5 |
| Alzheimer’s dementia, early onset | 6 | 5.5 |
| Mean | Standard deviation | |
| Last MMSE score | 13.32 | 6.789 |
| Total number of caregivers for one patient | 1.67 | 0.692 |
| Caregiver burden | 11.05 | 3.270 |
| Measure | Baseline | 3 Months | 6 Months | p | |||
|---|---|---|---|---|---|---|---|
| Mean | Std. Error | Mean | Std. Error | Mean | Std. Error | ||
| Maslach Burnout Inventory | |||||||
| Emotional exhaustion | 7.33 | 0.667 | 9.28 | 0.764 | 11.36 | 0.962 | 0.001 |
| Cynicism | 5.308 | 0.627 | 7.179 | 0.732 | 9.923 | 0.958 | 0.001 |
| Professional efficacy | 7.282 | 0.890 | 8.949 | 0.969 | 11.641 | 1.256 | 0.001 |
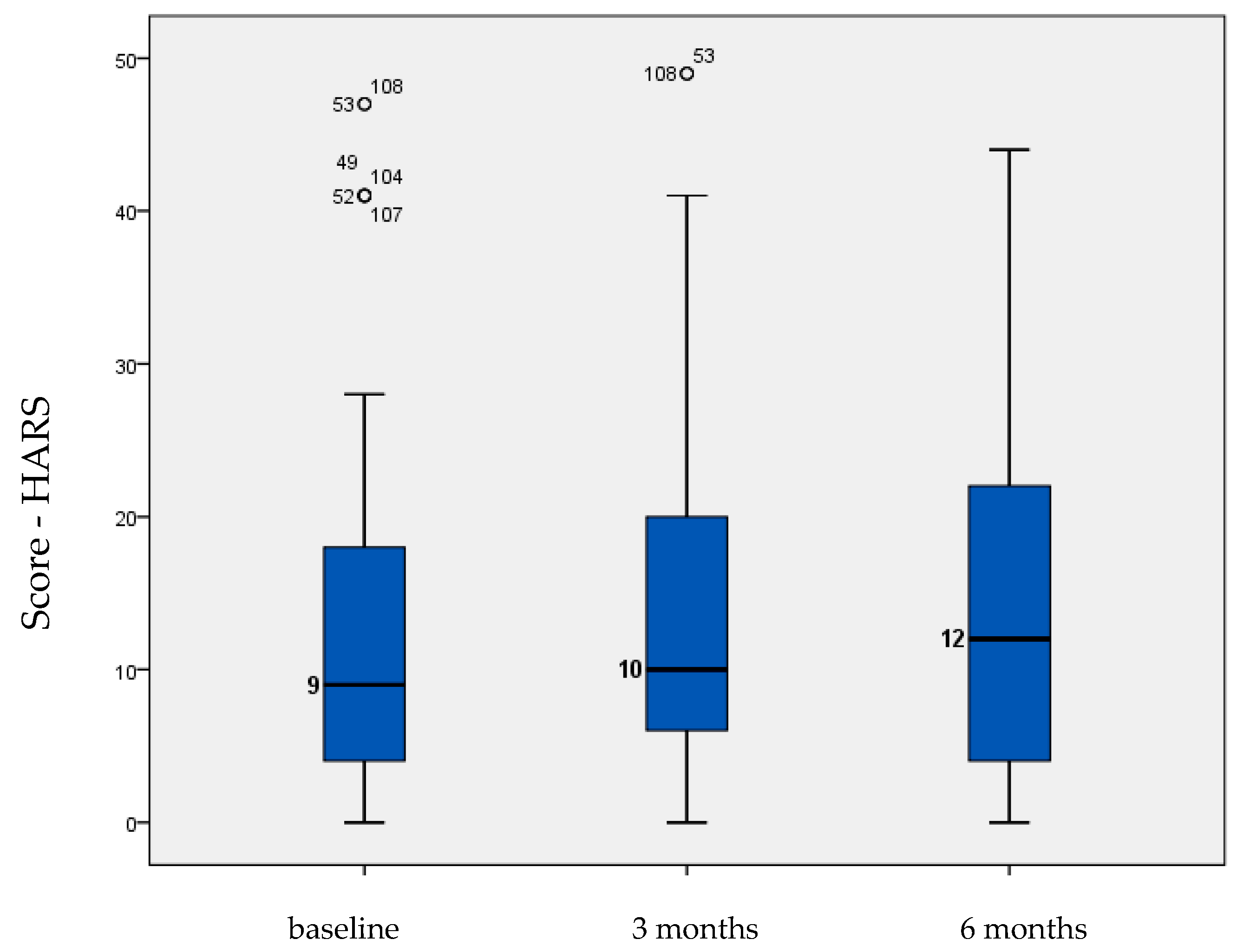
| HARS | 13.000 | 1.286 | 13.154 | 1.232 | 13.667 | 1.217 | 0.57 |
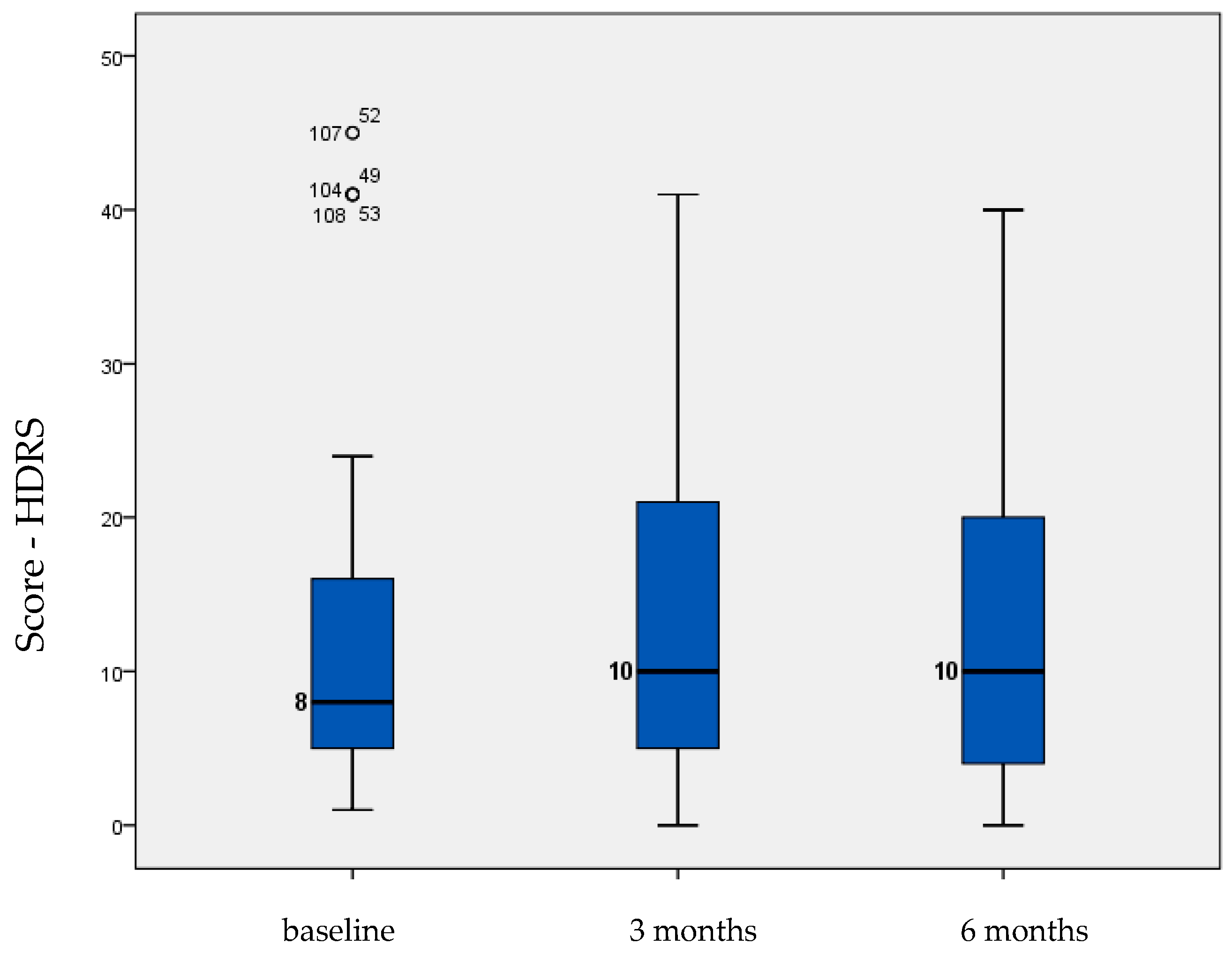
| HDRS | 12.282 | 1.235 | 13.282 | 1.184 | 13.846 | 1.203 | 0.068 |
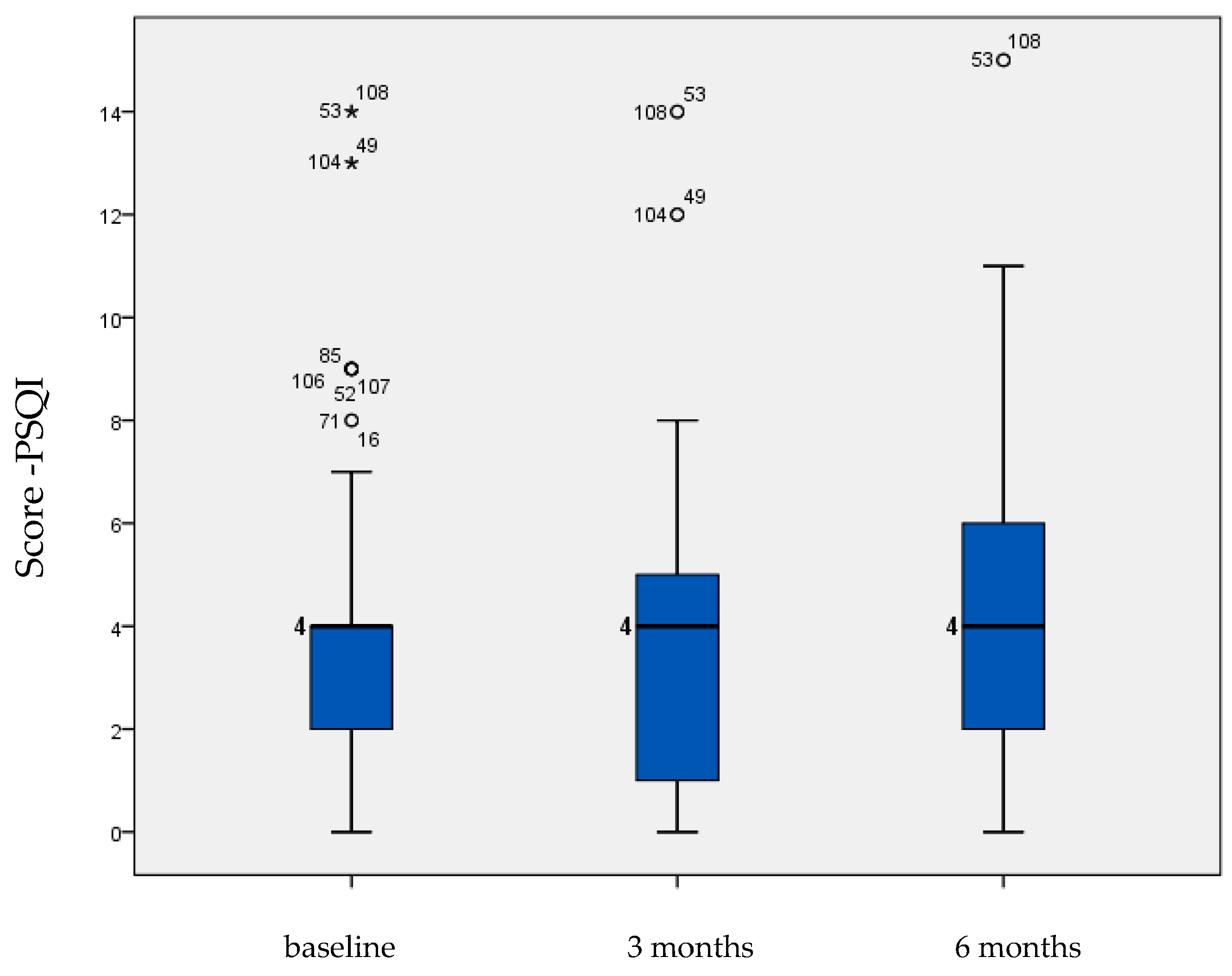
| Pittsburg Sleep Quality Index | 4.205 | 0.356 | 3.769 | 0.351 | 4.513 | 0.365 | 0.002 |
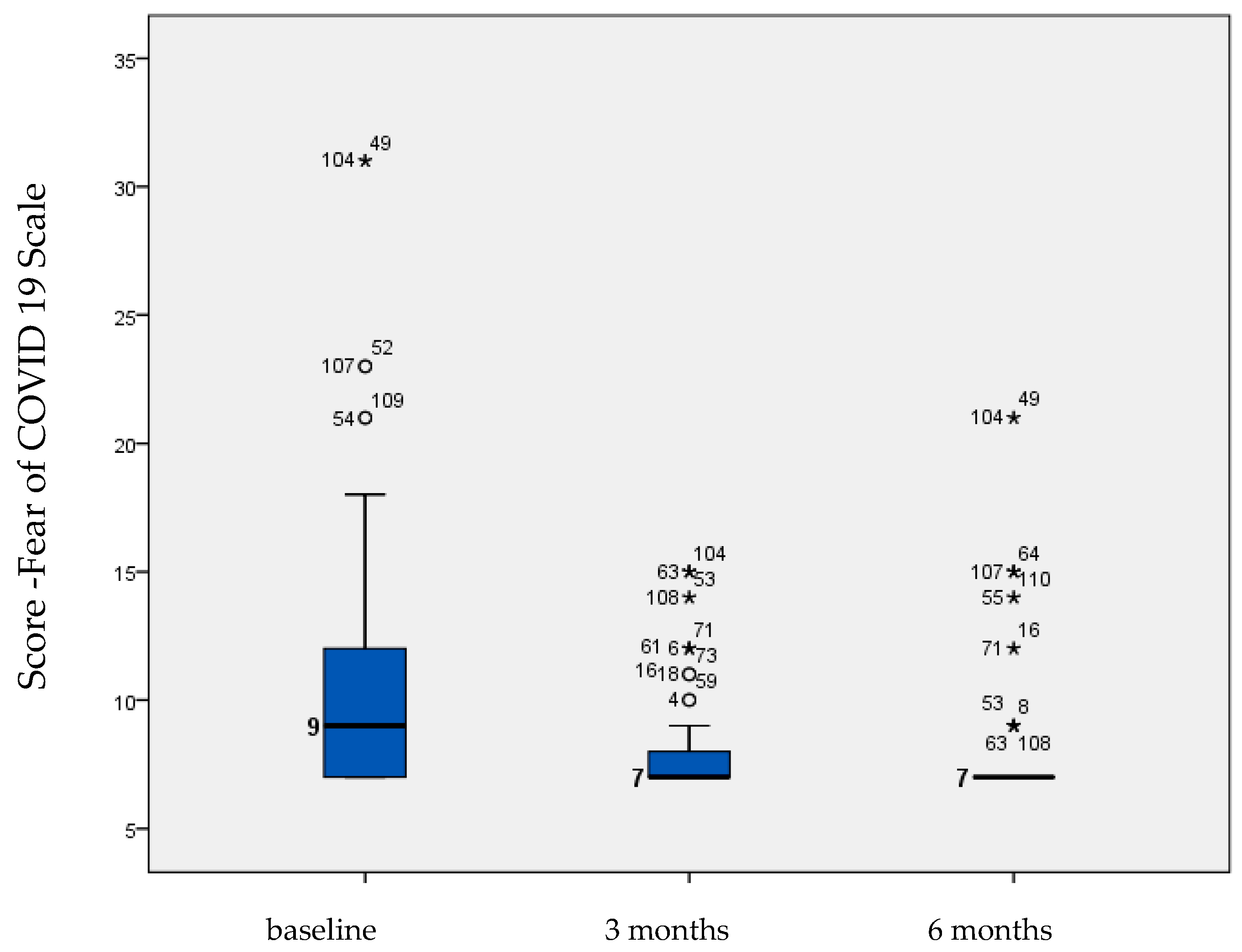
| Fear of COVID-19 | 10.795 | 0.586 | 8.179 | 0.263 | 8.179 | 0.342 | 0.001 |
| WHOQOL-BREF 26 | 58.667 | 1.354 | 57.359 | 1.316 | 56.821 | 1.196 | 0.03 |
| Measure | Baseline | 3 Months | 6 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | Correlation | r | p | Correlation | r | p | Correlation | |
| Maslach Burnout Inventory | |||||||||
| Emotional exhaustion | −0.66 | 0.001 | Inverse and moderate | - | - | - | - | - | - |
| Cynicism | −0.64 | 0.001 | Inverse and moderate | −0.76 | 0.001 | Inverse and strong | −0.75 | 0.001 | Inverse and strong |
| Professional efficacy | −0.65 | 0.001 | Inverse and moderate | −0.75 | 0.001 | Inverse and strong | −0.80 | 0.001 | Inverse and strong |
| HARS | −0.83 | 0.001 | Inverse and strong | −0.88 | 0.001 | Inverse and strong | −0.89 | 0.001 | Inverse and strong |
| HDRS | −0.84 | 0.001 | Inverse and strong | −0.92 | 0.001 | Inverse and strong | −0.93 | 0.001 | Inverse and strong |
| Pittsburg Sleep Quality Index | −0.71 | 0.001 | Inverse and strong | −0.70 | 0.001 | Inverse and strong | −0.73 | 0.001 | Inverse and strong |
| Fear of COVID-19 | −0.36 | 0.001 | Inverse and low | −0.42 | 0.001 | Inverse and moderate | −0.63 | 0.001 | Inverse and moderate |
| Timepoint | Model | R | R Square | Adjusted R Square | Std. Error of the Estimate | Change Statistics | |
|---|---|---|---|---|---|---|---|
| R Square Change | F Change | ||||||
| Baseline | (Constant), professional efficacy, cynicism, exhaustion, anxiety, depression, caregiver burden, sleep quality | 0.867 | 0.751 | 0.734 | 5.614 | 0.000 | 0.003 |
| 3 months | (Constant), professional efficacy, cynicism, exhaustion, anxiety, depression | 0.933 | 0.871 | 0.862 | 4.300 | 0.003 | 2.264 |
| 6 months | (Constant), professional efficacy, cynicism, exhaustion, anxiety, depression, sleep quality | 0.942 | 0.887 | 0.878 | 3.691 | 0.010 | 6.466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian, A.C.; Mihăilescu, A.I.; Anghele, C.; Ciobanu, C.A.; Petrescu, C.; Riga, S.; Dionisie, V.; Ciobanu, A.M. Quality of Life Predictors in a Group of Informal Caregivers during the COVID-19 Pandemic. Medicina 2023, 59, 1486. https://doi.org/10.3390/medicina59081486
Damian AC, Mihăilescu AI, Anghele C, Ciobanu CA, Petrescu C, Riga S, Dionisie V, Ciobanu AM. Quality of Life Predictors in a Group of Informal Caregivers during the COVID-19 Pandemic. Medicina. 2023; 59(8):1486. https://doi.org/10.3390/medicina59081486
Chicago/Turabian StyleDamian, Ana Claudia, Alexandra Ioana Mihăilescu, Cristina Anghele, Constantin Alexandru Ciobanu, Cristian Petrescu, Sorin Riga, Vlad Dionisie, and Adela Magdalena Ciobanu. 2023. "Quality of Life Predictors in a Group of Informal Caregivers during the COVID-19 Pandemic" Medicina 59, no. 8: 1486. https://doi.org/10.3390/medicina59081486
APA StyleDamian, A. C., Mihăilescu, A. I., Anghele, C., Ciobanu, C. A., Petrescu, C., Riga, S., Dionisie, V., & Ciobanu, A. M. (2023). Quality of Life Predictors in a Group of Informal Caregivers during the COVID-19 Pandemic. Medicina, 59(8), 1486. https://doi.org/10.3390/medicina59081486










