Successful Resection of Retrobulbar Carcinosarcoma without Recurrence: A Case Report
Abstract
1. Introduction
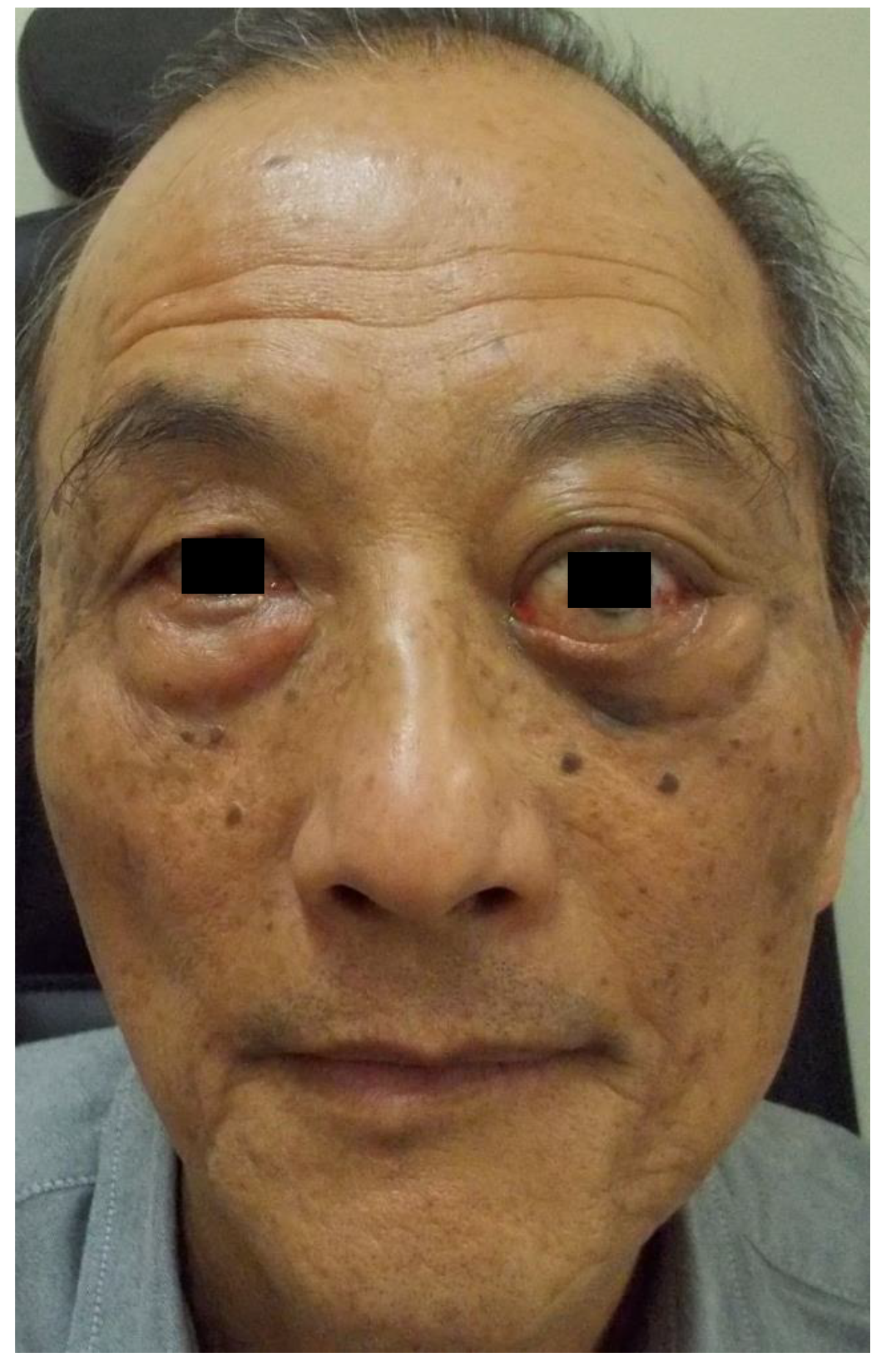
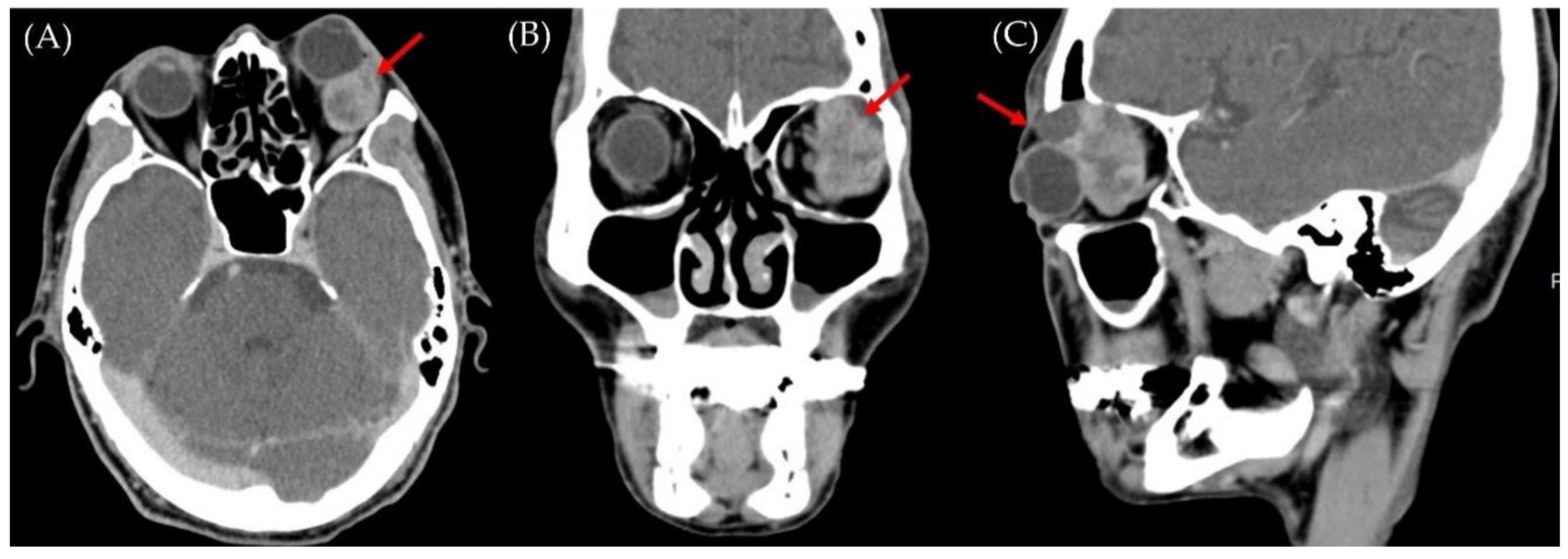
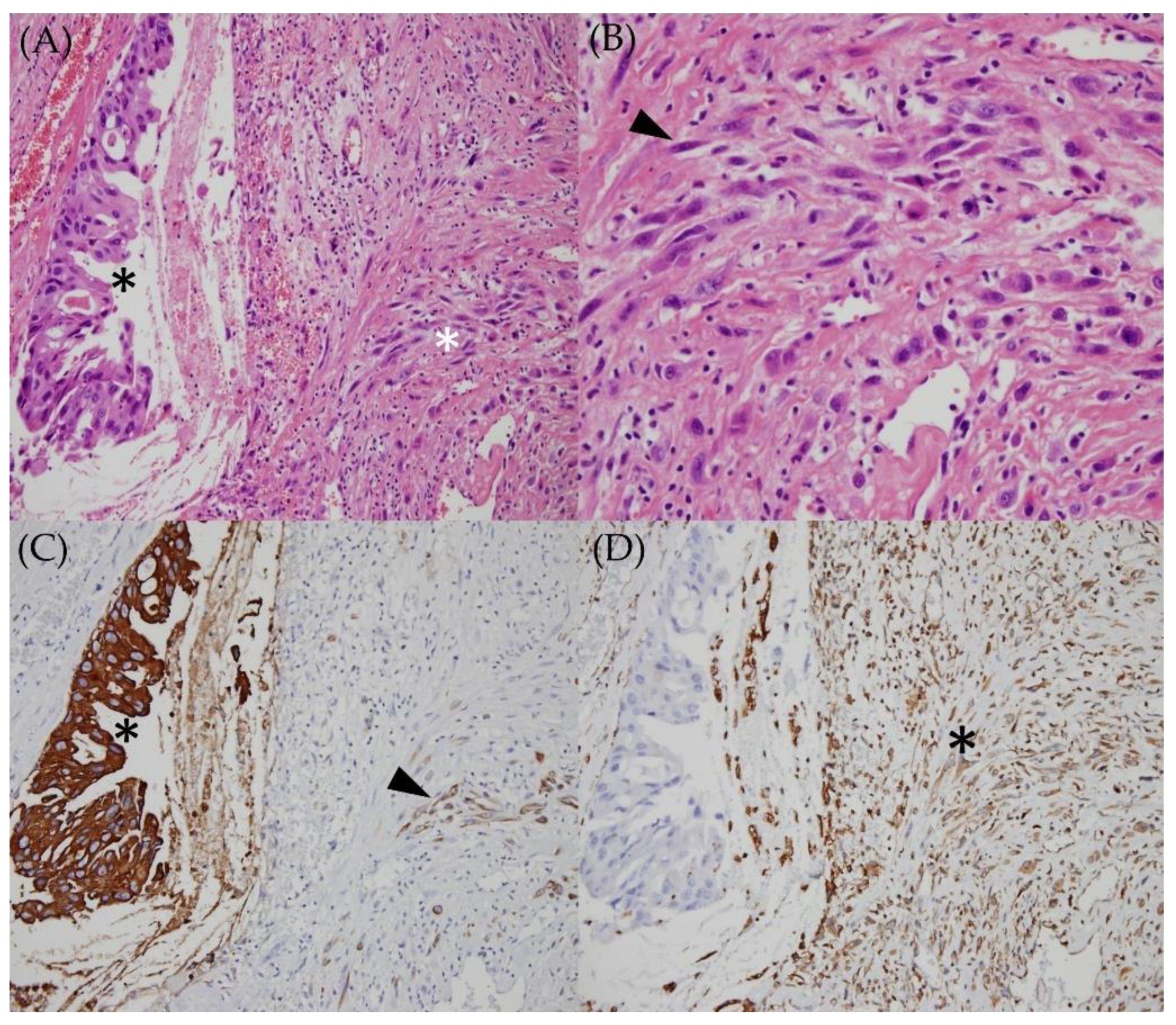
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Götte, K.; Riedel, F.; Coy, J.F.; Spahn, V.; Hörmann, K. Salivary gland carcinosarcoma: Immunohistochemical, molecular genetic and electron microscopic findings. Oral Oncol. 2000, 36, 360–364. [Google Scholar] [CrossRef]
- Cantrell, L.A.; Blank, S.V.; Duska, L.R. Uterine carcinosarcoma: A review of the literature. Gynecol Oncol. 2015, 137, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Berton-Rigaud, D.; Devouassoux-Shisheboran, M.; Ledermann, J.A.; Leitao, M.M.; Powell, M.A.; Poveda, A.; Beale, P.; Glasspool, R.M.; Creutzberg, C.L.; Harter, P.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int. J. Gynecol. Cancer 2014, 24, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Basibuyuk, I.; Topaktaş, R.; Elbir, F. Bladder carcinosarcoma: A case report with review of the literature. Arch. Ital. Urol. Androl. 2017, 89, 240–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biernat, W.; Kordek, R.; Liberski, P.P.; Woźniak, L. Carcinosarcoma of the skin. Case report and literature review. Am. J. Dermatopathol. 1996, 18, 614–619. [Google Scholar] [CrossRef]
- Madan, A.K.; Long, A.E.; Weldon, C.B.; Jaffe, B.M. Esophageal carcinosarcoma. J. Gastrointest. Surg. 2001, 5, 414–417. [Google Scholar] [CrossRef]
- Weidner, N.; Zekan, P. Carcinosarcoma of the colon. Report of a unique case with light and immunohistochemical studies. Cancer 1986, 58, 1126–1130. [Google Scholar] [CrossRef]
- Koss, M.N.; Hochholzer, L.; Frommelt, R.A. Carcinosarcomas of the lung: A clinicopathologic study of 66 patients. Am. J. Surg. Pathol. 1999, 23, 1514–1526. [Google Scholar] [CrossRef]
- Takahira, M.; Nakamura, Y.; Shimizu, S.; Minato, H.; Katori, N.; Kobayashi, H.; Nakatani, Y.; Sugiyama, K. Carcinosarcoma of the lacrimal gland arising from a pleomorphic adenoma. Am. J. Ophthalmol. 2005, 140, 337–340. [Google Scholar] [CrossRef]
- Prakalapakorn, S.G.; Bernardino, C.R.; Auclair, P.L.; Grossniklaus, H.E. Carcinosarcoma of the orbit: Report of two cases and review of the literature. Ophthalmology 2008, 115, 2065–2070. [Google Scholar] [CrossRef]
- Ni, C.; Kuo, P.K.; Dryja, T.P. Histopathological classification of 272 primary epithelial tumors of the lacrimal gland. Chin. Med. J. (Engl.) 1992, 105, 481–485. [Google Scholar] [PubMed]
- Font, R.L.; Smith, S.L.; Bryan, R.G. Malignant epithelial tumors of the lacrimal gland: A clinicopathologic study of 21 cases. Arch. Ophthalmol. 1998, 116, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.; Chang, B.; Barsky, S.H. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am. J. Surg. Pathol. 1996, 20, 277–285. [Google Scholar] [CrossRef]
- Sreenan, J.J.; Hart, W.R. Carcinosarcomas of the female genital tract. A pathologic study of 29 metastatic tumors: Further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am. J. Surg. Pathol. 1995, 19, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Enomoto, T.; Fujita, M.; Yoshino, K.; Nakashima, R.; Kurachi, H.; Haba, T.; Wakasa, K.; Shroyer, K.R.; Tsujimoto, M.; et al. Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors. Cancer Res. 1997, 57, 5379–5385. [Google Scholar] [PubMed]
- Amant, F.; Vloeberghs, V.; Woestenborghs, H.; Moerman, P.; Vergote, I. Transition of epithelial toward mesenchymal differentiation during ovarian carcinosarcoma tumorigenesis. Gynecol. Oncol. 2003, 90, 372–377. [Google Scholar] [CrossRef]
- Harada, H. Histomorphological investigation regarding to malignant transformation of pleomorphic adenoma (so-called malignant mixed tumor) of the salivary gland origin: Special reference to carcinosarcoma. Kurume Med. J. 2000, 47, 307–323. [Google Scholar] [CrossRef][Green Version]
- Fujii, H.; Yoshida, M.; Gong, Z.X.; Matsumoto, T.; Hamano, Y.; Fukunaga, M.; Hruban, R.H.; Gabrielson, E.; Shirai, T. Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res. 2000, 60, 114–120. [Google Scholar]
- Stephen, J.; Batsakis, J.G.; Luna, M.A.; von der Heyden, U.; Byers, R.M. True malignant mixed tumors (carcinosarcoma) of salivary glands. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 597–602. [Google Scholar] [CrossRef]
- Wargotz, E.S.; Norris, H.J. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer 1989, 64, 1490–1499. [Google Scholar] [CrossRef]
- Nagao, K.; Matsuzaki, O.; Saiga, H.; Sugano, I.; Shigematsu, H.; Kaneko, T.; Katoh, T.; Kitamura, T. Histopathologic studies on carcinoma in pleomorphic adenoma of the parotid gland. Cancer 1981, 48, 113–121. [Google Scholar] [CrossRef]
- Latkovich, P.; Johnson, R.L. Carcinosarcoma of the parotid gland: Report of a case with cytohistologic and immunohistochemical findings. Arch. Pathol. Lab. Med. 1998, 122, 743–746. [Google Scholar]
- Sigal, S.H.; Tomaszewski, J.E.; Brooks, J.J.; Wein, A.; LiVolsi, V.A. Carcinosarcoma of bladder following long-term cyclophosphamide therapy. Arch. Pathol. Lab. Med. 1991, 115, 1049–1051. [Google Scholar]
- Kiratli, H.; Soysal, H.G.; Demir, S. Orbital metastasis and intraocular invasion of malignant mixed tumor (carcinosarcoma) of the parotid gland in a child. Arch. Ophthalmol. 2004, 122, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Ferry, A.P.; Font, R.L. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch. Ophthalmol. 1974, 92, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Patel, N.A.; Patel, N.; Ghassibi, M.P.; Tse, D.T.; Dubovy, S.R. Metastatic Uterine Carcinosarcoma to the Orbit. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e198–e202. [Google Scholar] [CrossRef]
- Fernandez, C.; Forrest, P.; Sivalingam, M.; Milman, T.; Ramesh, S.; Shi, W. Exenteration and Adjuvant Radiotherapy for Primary Carcinosarcoma of the Anterior Orbit: A Case Report. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e136–e139. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.S.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Seshan, V.E.; Shah, M.; Schiff, P.B.; Burke, W.M.; Cohen, C.J.; Herzog, T.J. The role of radiation in improving survival for early-stage carcinosarcoma and leiomyosarcoma. Am. J. Obstet. Gynecol. 2008, 199, 536.e1–536.e8. [Google Scholar] [CrossRef]
- Gupta, A.; Koochakzadeh, S.; Neskey, D.M.; Nguyen, S.A.; Lentsch, E.J. Salivary Carcinosarcoma: An Extremely Rare and Highly Aggressive Malignancy. Laryngoscope 2020, 130, E335–E339. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Lu, Z.; Wang, M.; Cong, L.; Yang, B.; Chen, X.; Cai, J.; Yang, X. Esophageal Carcinosarcoma: Analysis of Clinical Features and Prognosis of 24 Cases and a Literature Review. Cancer Control 2021, 28, 10732748211004886. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Lee, L.-C.; Gao, H.-W.; Chen, Y.-H.; Chien, K.-H. Successful Resection of Retrobulbar Carcinosarcoma without Recurrence: A Case Report. Medicina 2022, 58, 317. https://doi.org/10.3390/medicina58020317
Huang C-H, Lee L-C, Gao H-W, Chen Y-H, Chien K-H. Successful Resection of Retrobulbar Carcinosarcoma without Recurrence: A Case Report. Medicina. 2022; 58(2):317. https://doi.org/10.3390/medicina58020317
Chicago/Turabian StyleHuang, Chun-Hao, Lung-Chi Lee, Hong-Wei Gao, Yi-Hao Chen, and Ke-Hung Chien. 2022. "Successful Resection of Retrobulbar Carcinosarcoma without Recurrence: A Case Report" Medicina 58, no. 2: 317. https://doi.org/10.3390/medicina58020317
APA StyleHuang, C.-H., Lee, L.-C., Gao, H.-W., Chen, Y.-H., & Chien, K.-H. (2022). Successful Resection of Retrobulbar Carcinosarcoma without Recurrence: A Case Report. Medicina, 58(2), 317. https://doi.org/10.3390/medicina58020317






