Extruded Nucleoli of Human Dental Pulp Cells
Abstract
1. Introduction
2. Materials and Methods
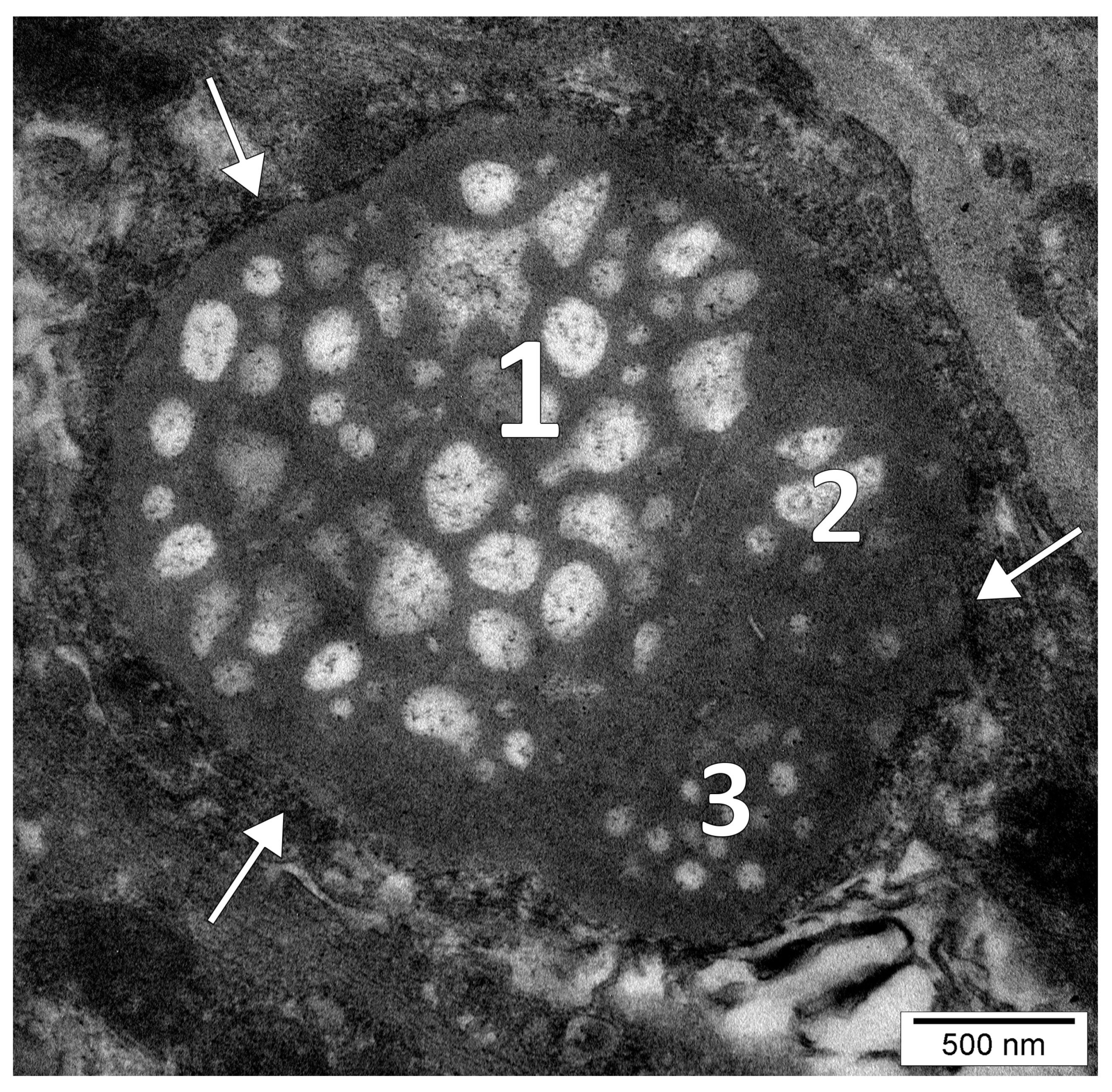
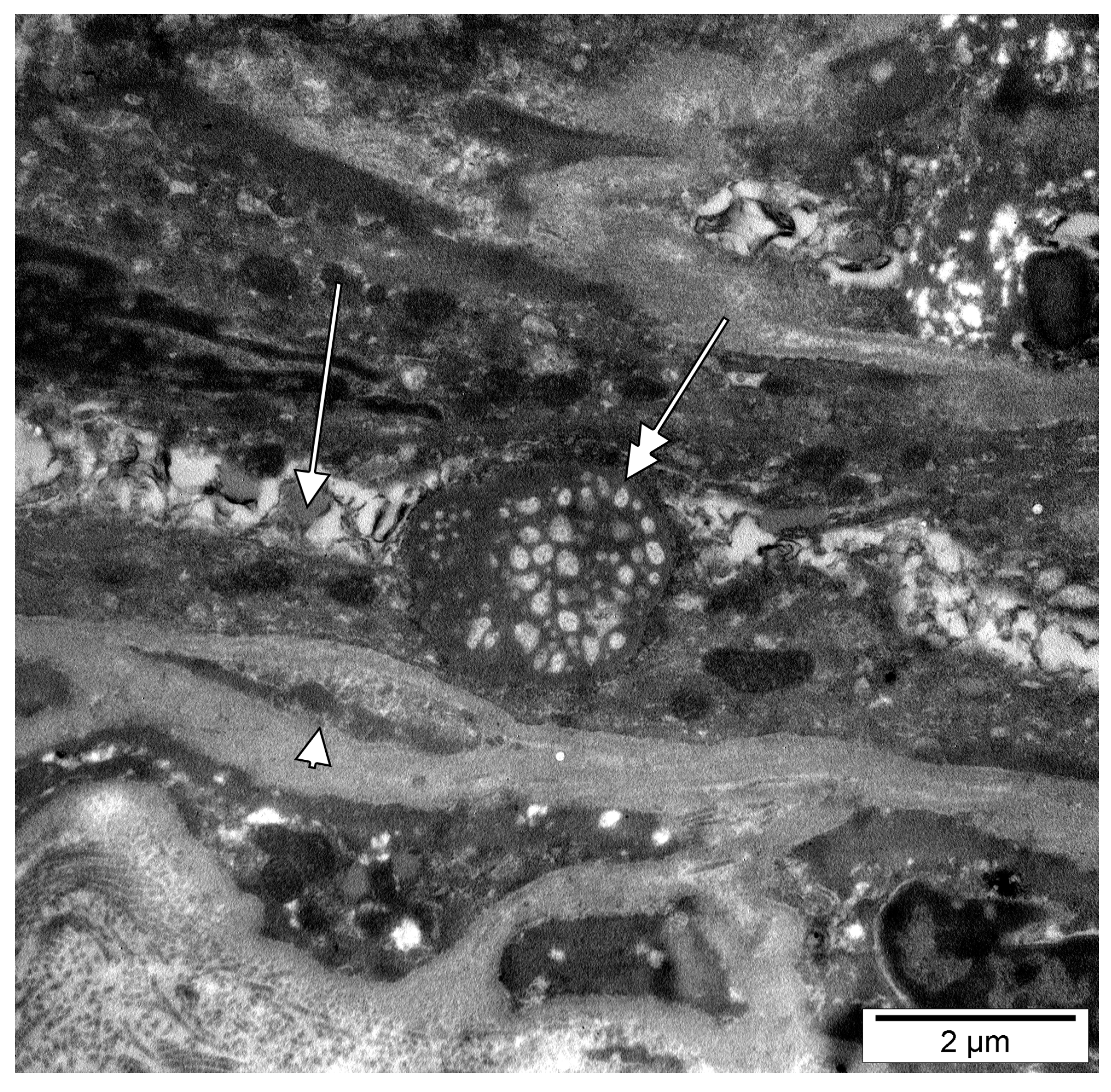
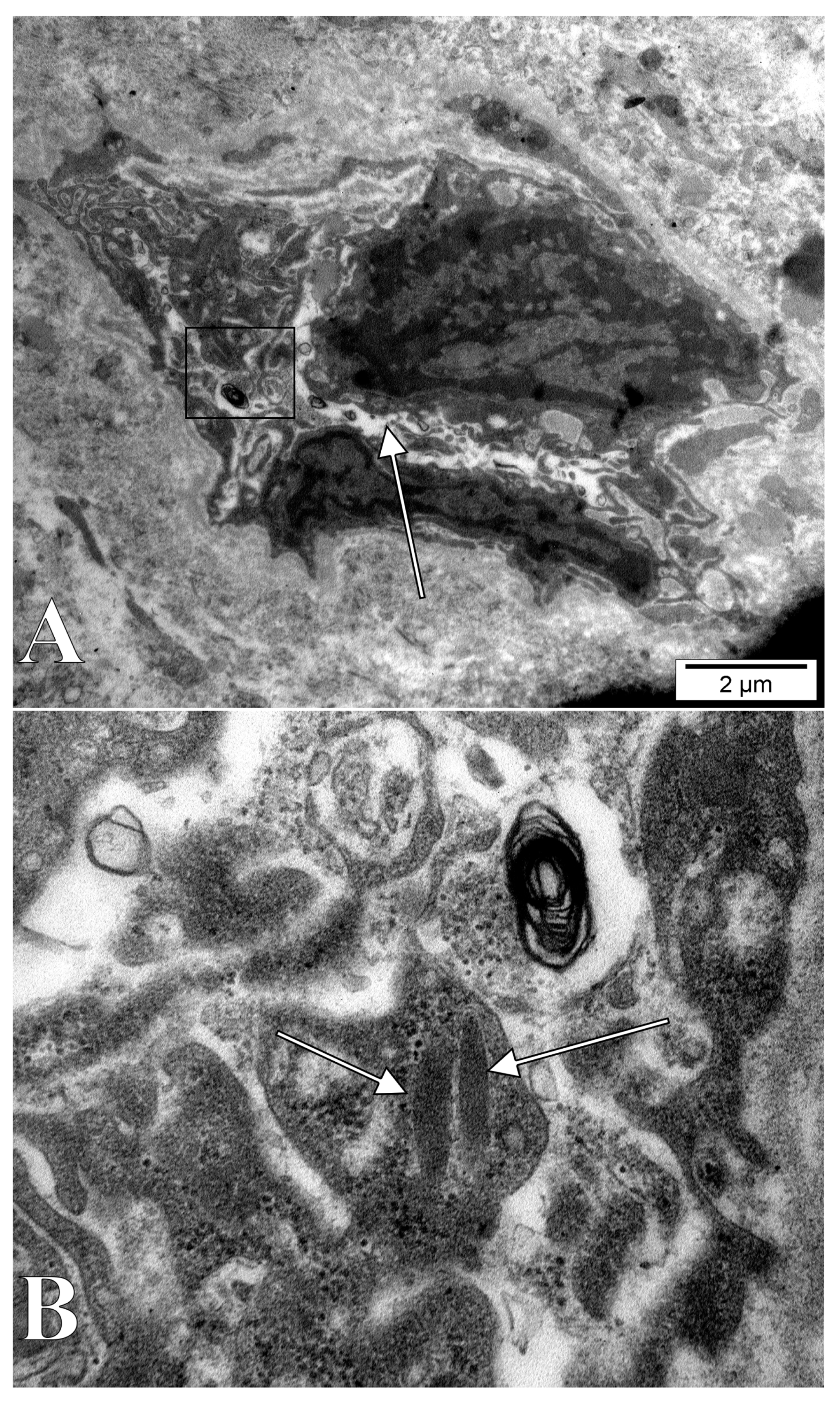
3. Results
4. Discussion
4.1. The Nucleolar Channel System
4.2. Intracytoplasmic Extruded Nuclear Chromatin and Nucleoli
4.3. Nucleolar Extrusion in Cancer Stem Cells
4.4. Which Is the Role of the Extruded Nucleoli in the Dental Pulp?
5. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atari, M.; Gil-Recio, C.; Fabregat, M.; Garcia-Fernandez, D.; Barajas, M.; Carrasco, M.A.; Jung, H.S.; Alfaro, F.H.; Casals, N.; Prosper, F.; et al. Dental pulp of the third molar: A new source of pluripotent-like stem cells. J. Cell Sci. 2012, 125, 3343–3356. [Google Scholar] [PubMed]
- d’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohshima, H. Distribution and organization of peripheral capillaries in dental pulp and their relationship to odontoblasts. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 1996, 245, 313–326. [Google Scholar] [CrossRef]
- Marchionni, C.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Di Tullio, A.; Costa, R.; Montanari, M.; Tazzari, P.L.; Ricci, F.; Pasquinelli, G.; et al. Angiogenic potential of human dental pulp stromal (stem) cells. Int. J. Immunopathol. Pharmcol. 2009, 22, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Viana, B.; Vieira, I.; Tavares, J.; Lobo, R.; Portela, A.; Vasconcelos, M. Dental stem cells characterization and bone regenerative potential in oral medicine. Int. J. Stem. Cell Res. Ther. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Zhang, W.; Walboomers, X.F.; Shi, S.; Fan, M.; Jansen, J.A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006, 12, 2813–2823. [Google Scholar] [CrossRef]
- Rombouts, C.; Giraud, T.; Jeanneau, C.; About, I. Pulp Vascularization during Tooth Development, Regeneration, and Therapy. J. Dent. Res. 2017, 96, 137–144. [Google Scholar] [CrossRef]
- Suchanek, J.; Soukup, T.; Visek, B.; Ivancakova, R.; Kucerova, L.; Mokry, J. Dental pulp stem cells and their characterization. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2009, 153, 31–35. [Google Scholar] [CrossRef]
- Carlile, M.J.; Sturrock, M.G.; Chisholm, D.M.; Ogden, G.R.; Schor, A.M. The presence of pericytes and transitional cells in the vasculature of the human dental pulp: An ultrastructural study. Histochem. J. 2000, 32, 239–245. [Google Scholar] [CrossRef]
- Gwinnett, A.J.; Tay, F. Early and intermediate time response of the dental pulp to an acid etch technique in vivo. Am. J. Dent. 1998, 11, S35–S44. [Google Scholar]
- Rivera, E.M.; Vargas, M.; Ricks-Williamson, L. Considerations for the aesthetic restoration of endodontically treated anterior teeth following intracoronal bleaching. Pract. Periodontics Aesthet. Dent. 1997, 9, 117–128. [Google Scholar] [PubMed]
- Trushkowsky, R.D. Esthetic and functional consideration in restoring endodontically treated teeth. Dent. Clin. N. Am. 2011, 55, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Mankoo, T. Discussion: The ideal restoration of endodontically treated teeth: Structural and esthetic considerations. Eur. J. Esthet. Dent. 2013, 8, 269–277. [Google Scholar] [PubMed]
- Rusu, M.C.; Nicolescu, M.I.; Jianu, A.M.; Manoiu, V.S.; Ilie, A.C.; Dinca, D. The ultrastructural anatomy of the nuclear envelope in the masseter muscle indicates its role in the metabolism of the intracellular Ca(+). Ann. Anat. 2019, 224, 117–123. [Google Scholar] [CrossRef]
- Rybak, E.A.; Szmyga, M.J.; Zapantis, G.; Rausch, M.; Beshay, V.E.; Polotsky, A.J.; Coutifaris, C.; Carr, B.R.; Santoro, N.; Meier, U.T. The nucleolar channel system reliably marks the midluteal endometrium regardless of fertility status: A fresh look at an old organelle. Fertil. Steril. 2011, 95, 1385–1389.e1381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Busch, H. The Cell Nucleus; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kittur, N.; Zapantis, G.; Aubuchon, M.; Santoro, N.; Bazett-Jones, D.P.; Meier, U.T. The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings. Mol. Biol. Cell 2007, 18, 2296–2304. [Google Scholar] [CrossRef]
- Terzakis, J.A. The nucleolar channel system of human endometrium. J. Cell Biol. 1965, 27, 293–304. [Google Scholar] [CrossRef]
- Shimizu, N.; Ishii, S. Electron-microscopic observations on nucleolar extrusion in nerve cells of the rat hypothalamus. Z. Zellforsch. Mikrosk. Anat. 1965, 67, 367–372. [Google Scholar] [CrossRef]
- Biggiogera, M.; Bottone, M.G.; Scovassi, A.I.; Soldani, C.; Vecchio, L.; Pellicciari, C. Rearrangement of nuclear ribonucleoprotein (RNP)-containing structures during apoptosis and transcriptional arrest. Biol. Cell 2004, 96, 603–615. [Google Scholar] [CrossRef]
- Caspersson, T.; Schultz, J. Ribonucleic Acids in Both Nucleus and Cytoplasm, and the Function of the Nucleolus. Proc. Natl. Acad. Sci. USA 1940, 26, 507–515. [Google Scholar] [CrossRef]
- Holmgren, E. Zur Kenntnis der Spinalganglienzellen des Kaninchens und des Frosches. Anat. Anz. 1899, 16, 161–171. [Google Scholar]
- Santolaya, R.C. Nucleolus-like bodies in the neuronal cytoplasm of the mouse arcuate nucleus. Z. Zellforsch. Mikrosk. Anat. 1973, 146, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Körner, F. Beobachtungen fiber den Austritt geformter Substanzen aus dem Kernk6rperchen bei menschliehen Nervenzellen. Z. Mikr.-Anat. Forsch. 1937, 42, 362–378. [Google Scholar]
- Bargmann, W. Die Epiphysis Cerebri, Handbuch der Mikroskopischen Anatomic des Menschen; Springer: Berlin, Germany, 1943; Volume III, pp. 308–502. [Google Scholar]
- Tewari, H.B.; Bourne, G.H. The histochemistry of the nucleus and nucleolus with reference to nucleo-cytoplasmic relations in the spinal ganglion neuron of the rat. Acta Histochem. 1962, 13, 323–350. [Google Scholar]
- Ehret, C.F.; Powers, E.L. Macronuclear and nucleolar development in Paramecium bursaria. Exp. Cell Res. 1955, 9, 241–257. [Google Scholar] [CrossRef]
- Weibel, E.R.; Palade, G.E. New Cytoplasmic Components in Arterial Endothelia. J. Cell Biol. 1964, 23, 101–112. [Google Scholar] [CrossRef]
- Kawabata, I. Electron microscopy of the rat hypothalamic neuro-secretory system. II. Nucleolus-like inclusion bodies in the cytoplasm of neurosecretory cells. Arch. Histol. Jpn. 1965, 26, 101–113. [Google Scholar] [CrossRef][Green Version]
- Biggiogera, M.; Bottone, M.G.; Martin, T.E.; Uchiumi, T.; Pellicciari. Still immunodetectable nuclear RNPs are extruded from the cytoplasm of spontaneously apoptotic thymocytes. Exp. Cell Res. 1997, 234, 512–520. [Google Scholar] [CrossRef]
- Biggiogera, M.; Bottone, M.G.; Pellicciari, C. Nuclear RNA is extruded from apoptotic cells. J. Histochem. Cytochem. 1998, 46, 999–1005. [Google Scholar] [CrossRef]
- Sparrow, A.; Hammond, M.R. Cytological evidence for the transfer of desoxyribose nucleic acid from nucleus to cytoplasm in certain plant cells. Am. J. Bot. 1947, 34, 439–445. [Google Scholar] [CrossRef]
- Li, M.; Qin, R.; Jiang, W.; Liu, D. Cytogenetical effects of aluminum on root meristem cells of Helianthus annuus L. Bot. Sci. 2015, 93, 15–22. [Google Scholar] [CrossRef][Green Version]
- Shi, Q.; Wang, J.; Jiang, Z.; Wu, H.; Wang, J.; Jiang, W.; Liu, D.; Zou, J. Effects of cadmium on nucleolus in root tips of Hordeum vulgare. Bot. Sci. 2017, 95, 93–102. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhang, S.; Meng, Q.; Zou, J.; Jiang, W.; Liu, D. Effects of aluminum on nucleoli in root tip cells, root growth and the antioxidant defense system in Vicia faba L. Acta Biol. Crac. Ser. Bot. 2009, 51, 99–106. [Google Scholar]
- De la Pompa, J.L.; Wakeham, A.; Correia, K.M.; Samper, E.; Brown, S.; Aguilera, R.J.; Nakano, T.; Honjo, T.; Mak, T.W.; Rossant, J.; et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 1997, 124, 1139–1148. [Google Scholar] [CrossRef]
- Berger, C.M.; Gaume, X.; Bouvet, P. The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78–85. [Google Scholar] [CrossRef]
- Borer, R.A.; Lehner, C.F.; Eppenberger, H.M.; Nigg, E.A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989, 56, 379–390. [Google Scholar] [CrossRef]
- Ginisty, H.; Sicard, H.; Roger, B.; Bouvet, P. Structure and functions of nucleolin. J. Cell Sci. 1999, 112 Pt 6, 761–772. [Google Scholar] [CrossRef]
- The critical role of the nucleolus in cell differentiation and stem cell development—The concept as it applies to the malignant cell. Med. Hypotheses 1980, 6, 1289–1298. [CrossRef]
- Deng, G.; Ju, X.; Meng, Q.; Yu, Z.J.; Ma, L.B. Emodin inhibits the proliferation of PC3 prostate cancer cells in vitro via the Notch signaling pathway. Mol. Med. Rep. 2015, 12, 4427–4433. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Feki, A.; Papaccio, G.; Caton, J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 2011, 23, 275–279. [Google Scholar] [CrossRef]
- Zhu, T.S.; Costello, M.A.; Talsma, C.E.; Flack, C.G.; Crowley, J.G.; Hamm, L.L.; He, X.; Hervey-Jumper, S.L.; Heth, J.A.; Muraszko, K.M.; et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011, 71, 6061–6072. [Google Scholar] [CrossRef]
- Bhartiya, D. The need to revisit the definition of mesenchymal and adult stem cells based on their functional attributes. Stem Cell Res. Ther. 2018, 9, 78. [Google Scholar] [CrossRef]
- Caplan, A.I. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Montelatici, E.; Crisan, M.; Corselli, M.; Huard, J.; Lazzari, L.; Peault, B. Perivascular multi-lineage progenitor cells in human organs: Regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Okada, M.; Proto, J.D.; Gao, X.; Sekiya, N.; Beckman, S.A.; Corselli, M.; Crisan, M.; Saparov, A.; Tobita, K.; et al. Human pericytes for ischemic heart repair. Stem Cells 2013, 31, 305–316. [Google Scholar] [CrossRef]
- Chen, W.C.; Baily, J.E.; Corselli, M.; Diaz, M.E.; Sun, B.; Xiang, G.; Gray, G.A.; Huard, J.; Peault, B. Human myocardial pericytes: Multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 2015, 33, 557–573. [Google Scholar] [CrossRef]
- Corselli, M.; Chin, C.J.; Parekh, C.; Sahaghian, A.; Wang, W.; Ge, S.; Evseenko, D.; Wang, X.; Montelatici, E.; Lazzari, L.; et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood 2013, 121, 2891–2901. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- James, A.W.; Zara, J.N.; Corselli, M.; Chiang, M.; Yuan, W.; Nguyen, V.; Askarinam, A.; Goyal, R.; Siu, R.K.; Scott, V.; et al. Use of human perivascular stem cells for bone regeneration. J. Vis. Exp. 2012, 63, e2952. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Peault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytom. A 2010, 77, 22–30. [Google Scholar] [CrossRef]
- Grigoriu, F.; Hostiuc, S.; Vrapciu, A.D.; Rusu, M.C. Subsets of telocytes: The progenitor cells in the human endocardial niche. Rom. J. Morphol. Embryol. 2016, 57, 767–774. [Google Scholar]
- Colazzo, F.; Chester, A.H.; Taylor, P.M.; Yacoub, M.H. Induction of mesenchymal to endothelial transformation of adipose-derived stem cells. J. Heart Valve Dis. 2010, 19, 736–744. [Google Scholar]
- Kissa, K.; Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010, 464, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Abián, M.I.; Rufas, J.S.; de la Torre, C. Response of interphasic nucleoli to hypoxia in root meristems. Cell Biol. Int. Rep. 2010, 9, 699–708. [Google Scholar] [CrossRef]
- Qin, R.; Jiao, Y.; Zhang, S.; Jiang, W.; Liu, D. Effects of aluminum on nucleoli in root tip cells and selected physiological and biochemical characters in Allium cepa var. agrogarumL. BMC Plant Biol. 2010, 10, 225. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, M.C.; Vrapciu, A.D.; Nicolescu, M.I.; Stoenescu, M.D.; Jianu, A.M.; Lighezan, R.; Oancea, R.; Mănoiu, V.S.; Hostiuc, S. Extruded Nucleoli of Human Dental Pulp Cells. Medicina 2022, 58, 260. https://doi.org/10.3390/medicina58020260
Rusu MC, Vrapciu AD, Nicolescu MI, Stoenescu MD, Jianu AM, Lighezan R, Oancea R, Mănoiu VS, Hostiuc S. Extruded Nucleoli of Human Dental Pulp Cells. Medicina. 2022; 58(2):260. https://doi.org/10.3390/medicina58020260
Chicago/Turabian StyleRusu, Mugurel Constantin, Alexandra Diana Vrapciu, Mihnea Ioan Nicolescu, Mihai Dragomir Stoenescu, Adelina Maria Jianu, Rodica Lighezan, Roxana Oancea, Vasile Sorin Mănoiu, and Sorin Hostiuc. 2022. "Extruded Nucleoli of Human Dental Pulp Cells" Medicina 58, no. 2: 260. https://doi.org/10.3390/medicina58020260
APA StyleRusu, M. C., Vrapciu, A. D., Nicolescu, M. I., Stoenescu, M. D., Jianu, A. M., Lighezan, R., Oancea, R., Mănoiu, V. S., & Hostiuc, S. (2022). Extruded Nucleoli of Human Dental Pulp Cells. Medicina, 58(2), 260. https://doi.org/10.3390/medicina58020260








