The “Invisible Enemy” SARS-CoV-2: Viral Spread and Drug Treatment
Abstract
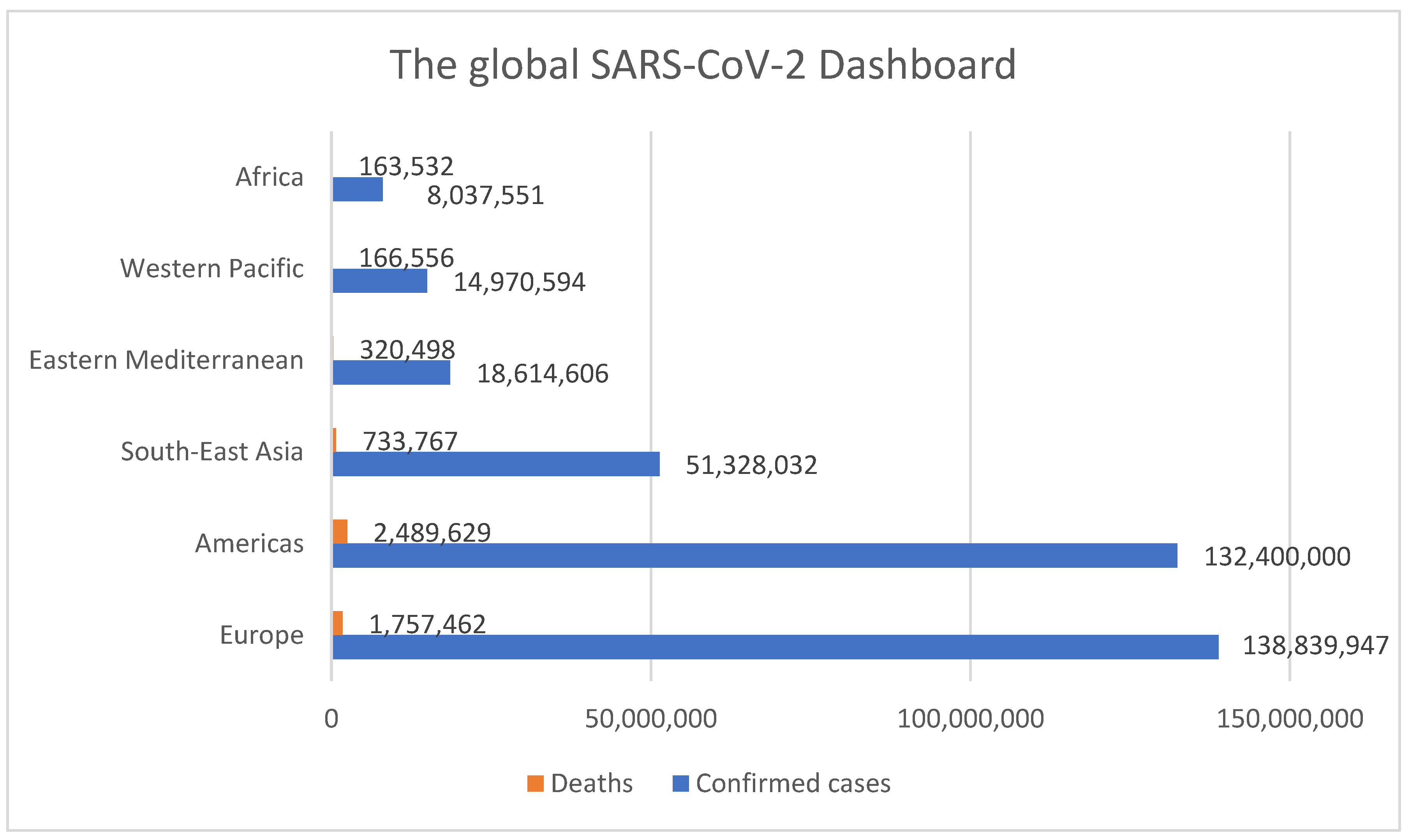
:1. Context of the COVID-19 Pandemic
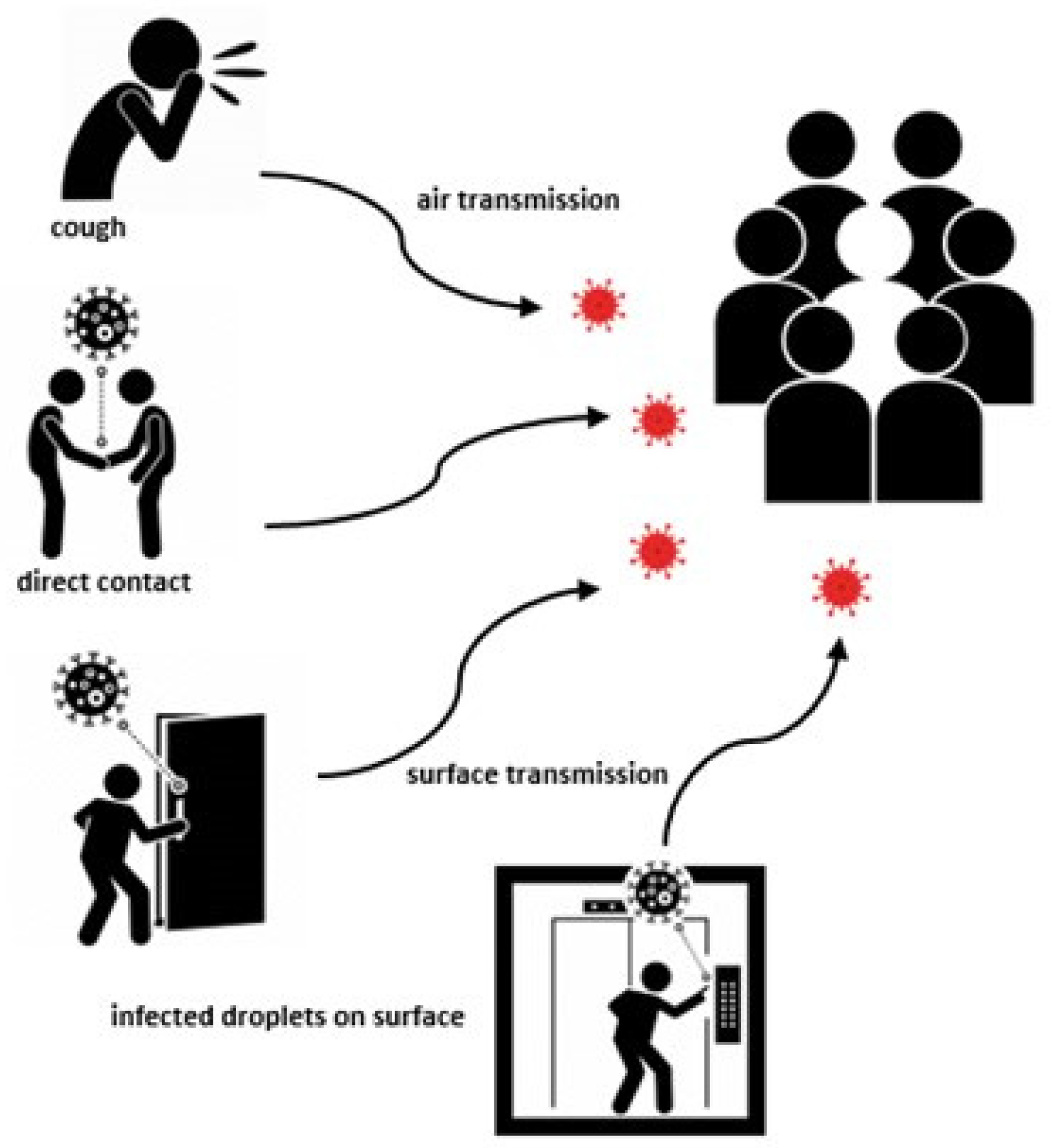
2. Coronavirus Transmission
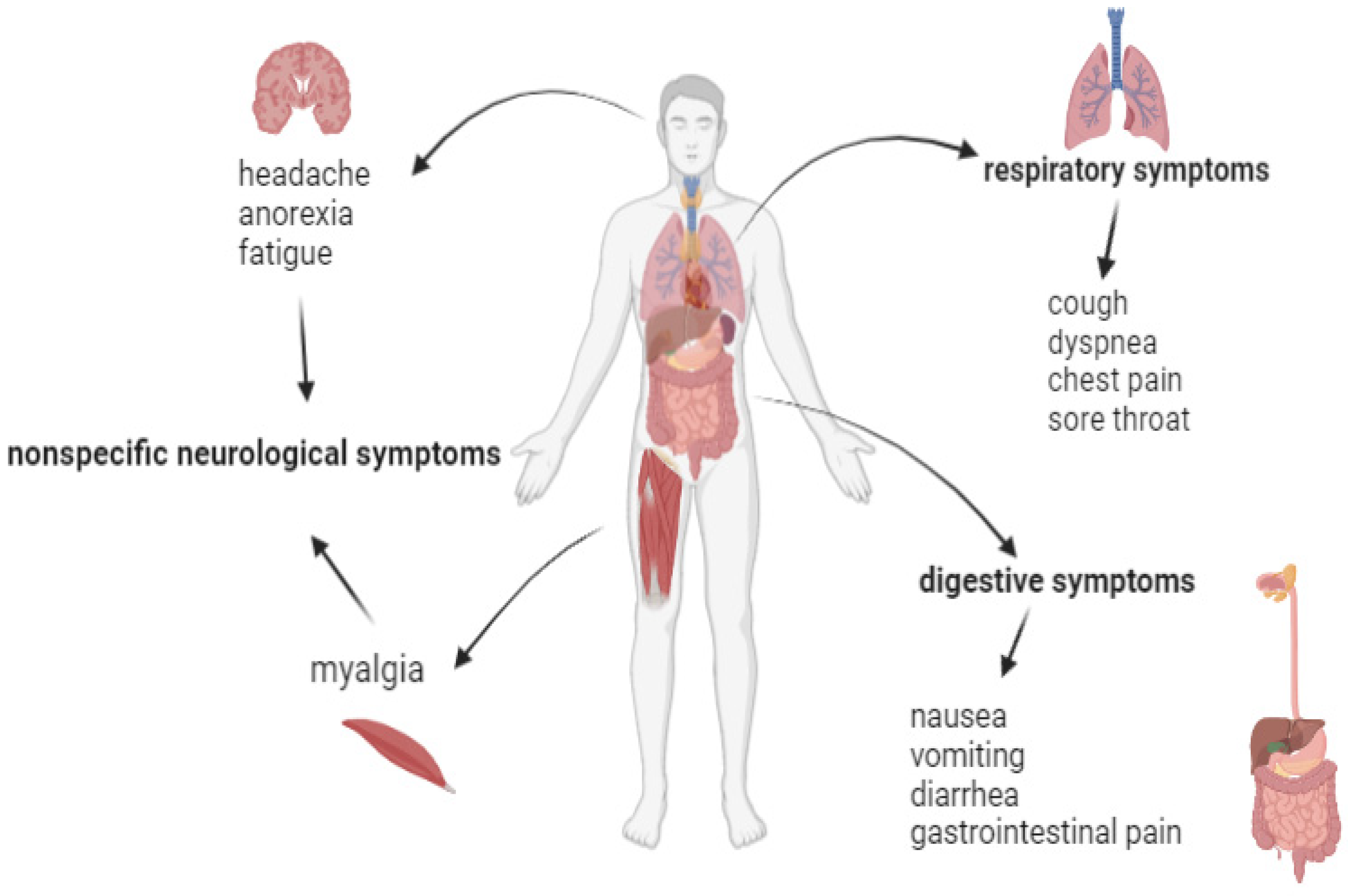
3. Clinical Symptoms
4. Complications
5. COVID-19 Treatment
5.1. Antiviral Medication
5.1.1. Remdesivir
5.1.2. Lopinavir/Ritonavir
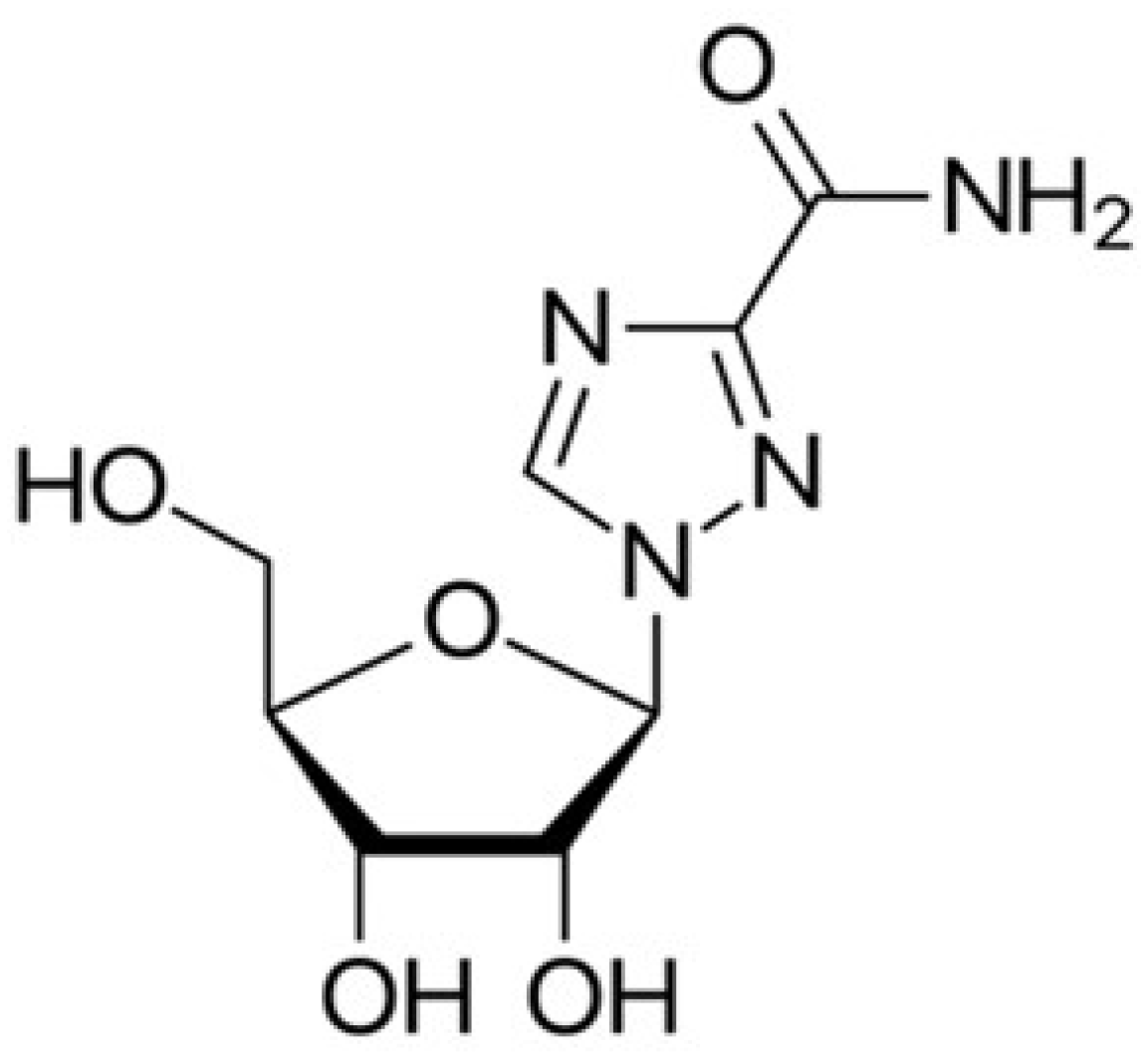
5.1.3. Ribavirin
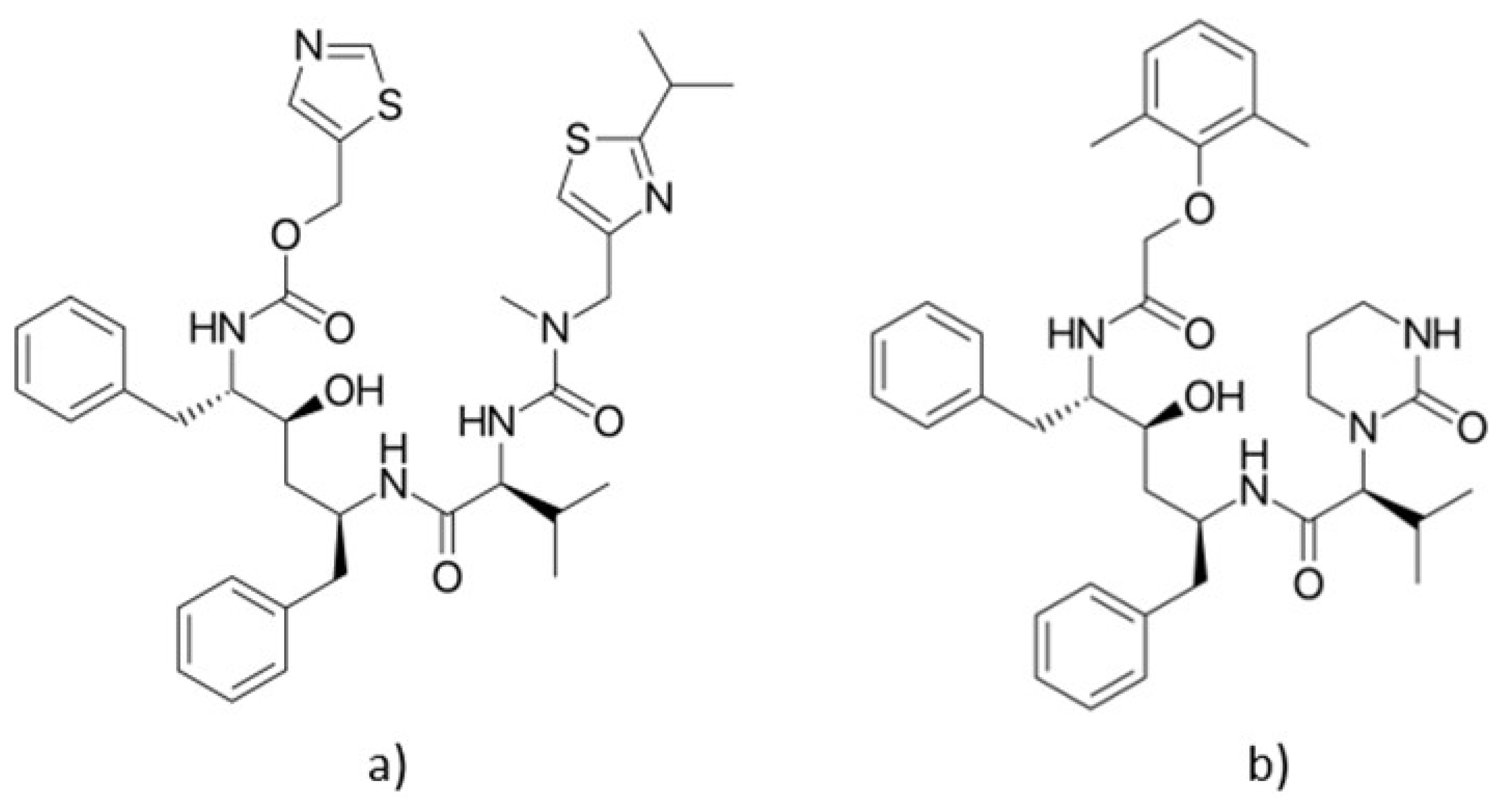
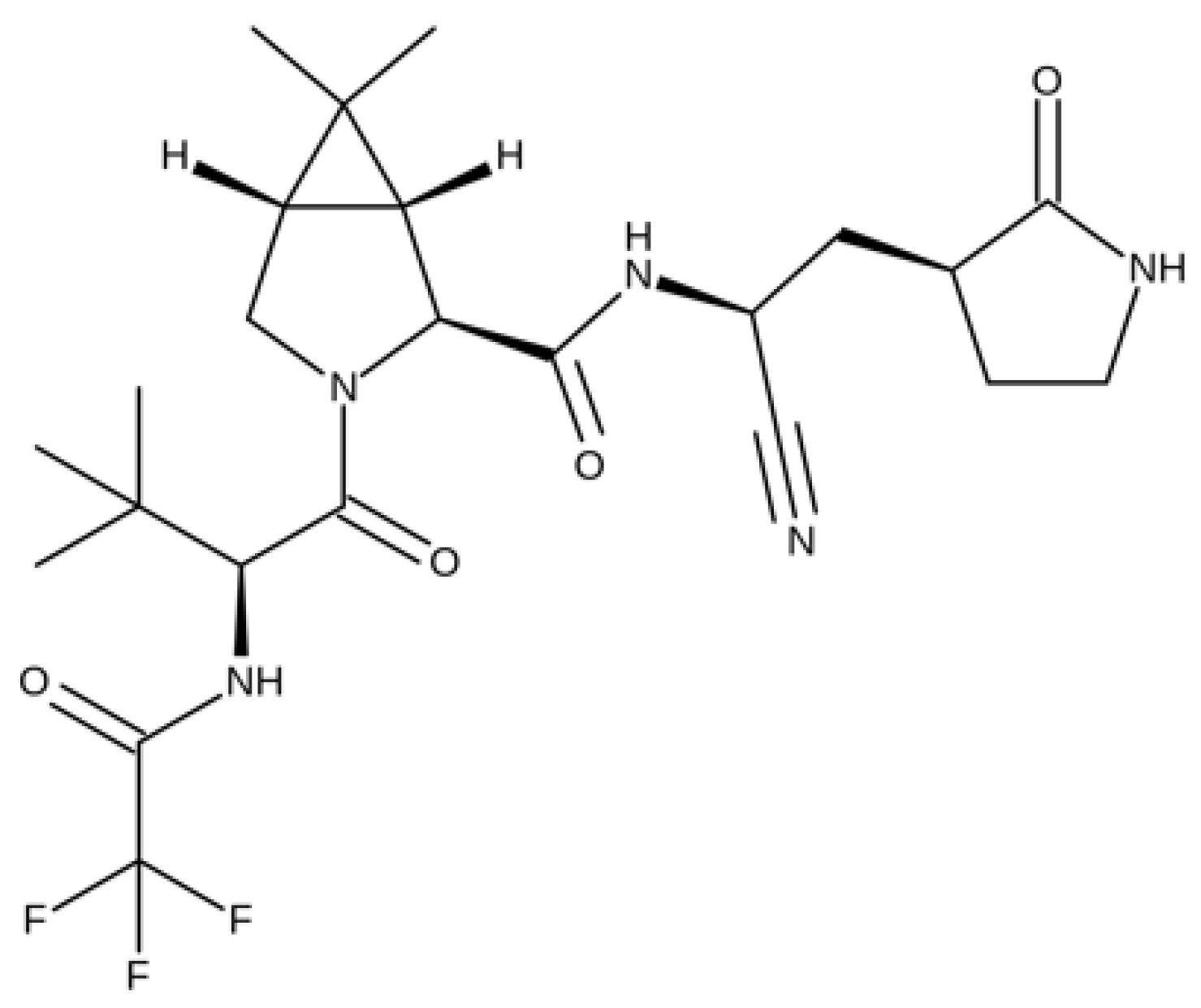
5.1.4. Nirmatrelvil/Ritonavir
5.2. Antimalarial Drugs
Chloroquine and Hydroxychloroquine
5.3. Corticosteroids
5.4. Immunomodulatory Drugs
Interleukin-6 Inhibitors
5.5. Antibodies
5.5.1. Convalescent Plasma Therapy
5.5.2. Special Monoclonal Therapy
6. Implications of Malpractice in the Context of the SARS-CoV-2 Crisis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Litvinova, M.; Wang, W.; Wang, Y.; Deng, X.; Chen, X.; Li, M.; Zheng, W.; Yi, L.; Chen, X.; et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: A descriptive and modelling study. Lancet Infect. Dis. 2020, 20, 793–802. [Google Scholar] [CrossRef]
- Lasalvia, A.; Bonetto, C.; Porru, S.; Carta, A.; Tardivo, S.; Bovo, C.; Ruggeri, M.; Amaddeo, F. Psychological impact of COVID-19 pandemic on healthcare workers in a highly burdened area of north-east Italy. Epidemiol. Psychiatr. Sci. 2020, 30, e1. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Linka, K.; Peirlinck, M.; Costabal, F.S.; Kuhl, E. Outbreak dynamics of COVID-19 in Europe and the effect of travel restrictions. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 710–717. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus Disease 2019 (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 28 January 2022).
- Callaway, E.; Ledford, H. How bad is Omicron? What scientists know so far. Nature 2021, 600, 197–199. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021, 375, n2943. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. arXiv 2021, arXiv:2112.01318v1. [Google Scholar] [CrossRef]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [CrossRef]
- Salian, V.S.; Wright, J.A.; Vedell, P.T.; Nair, S.; Li, C.; Kandimalla, M.; Tang, X.; Porquera, E.M.C.; Kalari, K.R.; Kandimalla, K.K. COVID-19 Transmission, Current Treatment, and Future Therapeutic Strategies. Mol. Pharm. 2021, 18, 754–771. [Google Scholar] [CrossRef]
- Tazerji, S.S.; Duarte, P.M.; Rahimi, P.; Shahabinejad, F.; Dhakal, S.; Malik, Y.S.; Shehata, A.A.; Lama, J.; Klein, J.; Safdar, M.; et al. Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: An updated review. J. Transl. Med. 2020, 18, 358. [Google Scholar] [CrossRef]
- Munnink, B.B.O.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2020, 371, 172–177. [Google Scholar] [CrossRef]
- Joonaki, E.; Hassanpouryouzband, A.; Heldt, C.L.; Areo, O. Surface Chemistry Can Unlock Drivers of Surface Stability of SARS-CoV-2 in a Variety of Environmental Conditions. Chem 2020, 6, 2135–2146. [Google Scholar] [CrossRef]
- Xue, X.; Ball, J.K.; Alexander, C.; Alexander, M.R. All Surfaces Are Not Equal in Contact Transmission of SARS-CoV-2. Matter 2020, 3, 1433–1441. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Schuit, M.; Ratnesar-Shumate, S.; Yolitz, J.; Williams, G.; Weaver, W.; Green, B.; Miller, D.; Krause, M.; Beck, K.; Wood, S.; et al. Airborne SARS-CoV-2 Is Rapidly Inactivated by Simulated Sunlight. J. Infect. Dis. 2020, 222, 564–571. [Google Scholar] [CrossRef]
- Ratnesar-Shumate, S.; Williams, G.; Green, B.; Krause, M.; Holland, B.; Wood, S.; Bohannon, J.; Boydston, J.; Freeburger, D.; Hooper, I.; et al. Simulated Sunlight Rapidly Inactivates SARS-CoV-2 on Surfaces. J. Infect. Dis. 2020, 222, 214–222. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Halley, J.M.; Vokou, D.; Pappas, G.; Sainis, I. SARS-CoV-2 mutational cascades and the risk of hyper-exponential growth. Microb. Pathog. 2021, 161, 105237. [Google Scholar] [CrossRef]
- Ren, S.-Y.; Wang, W.-B.; Gao, R.-D.; Zhou, A.-M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Rocklöv, J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Travel Med. 2021, 28, Taab124. [Google Scholar] [CrossRef]
- Elias, C.; Sekri, A.; Leblanc, P.; Cucherat, M.; Vanhems, P. The incubation period of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2021, 104, 708–710. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 (accessed on 6 December 2021).
- He, X.; Cheng, X.; Feng, X.; Wan, H.; Chen, S.; Xiong, M. Clinical Symptom Differences between Mild and Severe COVID-19 Patients in China: A Meta-Analysis. Front. Public Health 2021, 8, 561264. [Google Scholar] [CrossRef]
- Utku, A.Ç.; Budak, G.; Karabay, O.; Güçlü, E.; Okan, H.D.; Vatan, A. Main symptoms in patients presenting in the COVID-19 period. Scott. Med. J. 2020, 65, 127–132. [Google Scholar] [CrossRef]
- Alpha Variant vs. Delta Variant–How Are the Symptoms Different? Available online: https://health.fmolhs.org/body/covid-19/alpha-variant-vs-delta-variant-how-are-the-symptoms-different/ (accessed on 28 January 2022).
- Luna-Muschi, A.; Borges, I.C.; de Faria, E.; Barboza, A.S.; Maia, F.L.; Leme, M.D.; Guedes, A.R.; Mendes-Correa, M.C.; Kallas, E.G.; Segurado, A.C.; et al. Clinical features of COVID-19 by SARS-CoV-2 Gamma variant: A prospective cohort study of vaccinated and unvaccinated healthcare workers. J. Infect. 2021, in press. [Google Scholar] [CrossRef]
- Hoppe, L. Delta Variant Symptoms: What You Need to Know. Available online: https://www.bupa.co.uk/newsroom/ourviews/delta-variant-symptoms (accessed on 28 January 2022).
- Mukherjee, P.S. African Doctor Says Patients with Omicron Variant Have “Very Mild” Symptoms. Available online: https://www.reuters.com/world/africa/safrican-doctor-says-patients-with-omicron-variant-have-very-mild-symptoms-2021-11-28/ (accessed on 28 January 2022).
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID -19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.-L.; Li, J.; Liu, X.-H.; Zhu, C.-L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1116–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, E.S.; Kashani, N.R.; Nikzad, H.; Azadbakht, J.; Bafrani, H.H.; Kashani, H.H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McFee, R. COVID-19: Therapeutics and interventions currently under consideration. Dis. Mon. 2020, 66, 101058. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Liu, G.; Luo, W.; Xia, N. COVID-19: Progress in diagnostics, therapy and vaccination. Theranostics 2020, 10, 7821–7835. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 21 December 2021).
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- de Wit, E.; Rasmussen, A.; Falzarano, D.; Bushmaker, T.; Feldmann, F.; Brining, D.L.; Fischer, E.R.; Martellaro, C.; Okumura, A.; Chang, J.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc. Natl. Acad. Sci. USA 2013, 110, 16598–16603. [Google Scholar] [CrossRef] [Green Version]
- Aleissa, M.M.; Silverman, E.A.; Acosta, L.M.P.; Nutt, C.T.; Richterman, A.G.; Marty, F.M. New Perspectives on Antimicrobial Agents: Remdesivir Treatment for COVID-19. Antimicrob. Agents Chemother. 2020, 65, e01814-20. [Google Scholar] [CrossRef]
- Al-Tannak, N.F.; Novotny, L.; Alhunayan, A. Remdesivir—Bringing Hope for COVID-19 Treatment. Sci. Pharm. 2020, 88, 29. [Google Scholar] [CrossRef]
- Lahiry, S.; Chakraborty, D.S.; Choudhury, S.; Chatterjee, S. Past, Present, and Future of Remdesivir: An Overview of the Antiviral in Recent Times. Indian J. Crit. Care Med. 2020, 24, 570–574. [Google Scholar] [CrossRef]
- Malin, J.J.; Suárez, I.; Priesner, V.; Fätkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and Other Viral Diseases. Clin. Microbiol. Rev. 2020, 34, e00162-20. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Gilead. Gilead Announces Results from Phase 3 Trial of Investigational Antiviral Remdesivir in Patients with Severe COVID-19. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2020/4/gilead-announces-results-from-phase-3-trial-of-investigational-antiviral-remdesivir-in-patients-with-severe-covid-19 (accessed on 24 November 2021).
- Gillenwater, S.; Rahaghi, F.; Hadeh, A. Remdesivir for the Treatment of COVID-19—Preliminary Report. N. Engl. J. Med. 2020, 383, 992–994. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Chandwani, A.; Shuter, J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Ther. Clin. Risk Manag. 2008, 4, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Lin, W.; Cai, W.; Wen, C.; Guan, Y.; Mo, X.; Wang, J.; Wang, Y.; Peng, P.; et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med 2020, 1, 105–113.e4. [Google Scholar] [CrossRef]
- Pruijssers, A.J.; Denison, M. Nucleoside analogues for the treatment of coronavirus infections. Curr. Opin. Virol. 2019, 35, 57–62. [Google Scholar] [CrossRef]
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. 2007, 3, 218–225. [Google Scholar]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 (accessed on 28 January 2022).
- Wang, Z.; Yang, L. In the age of Omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J. Med. Virol. 2021. [Google Scholar] [CrossRef]
- Ma, C.; Sacco, M.D.; Hurst, B.; Townsend, J.A.; Hu, Y.; Szeto, T.; Zhang, X.; Tarbet, B.; Marty, M.; Chen, Y.; et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020, 30, 678–692. [Google Scholar] [CrossRef]
- Kneller, D.W.; Galanie, S.; Phillips, G.; O’Neill, H.M.; Coates, L.; Kovalevsky, A. Malleability of the SARS-CoV-2 3CL Mpro Active-Site Cavity Facilitates Binding of Clinical Antivirals. Structure 2020, 28, 1313–1320.e3. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Pfizer. Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate (accessed on 28 January 2022).
- Al-Bari, A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017, 5, e00293. [Google Scholar] [CrossRef] [PubMed]
- Coronado, L.M.; Nadovich, C.T.; Spadafora, C. Malarial hemozoin: From target to tool. Biochim. Biophys. Acta 2014, 1840, 2032–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plantone, D.; Koudriavtseva, T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef]
- Shippey, E.A.; Wagler, V.D.; Collamer, A.N. Hydroxychloroquine: An old drug with new relevance. Clevel. Clin. J. Med. 2018, 85, 459–467. [Google Scholar] [CrossRef]
- Dyavar, S.R.; Singh, R.; Emani, R.; Pawar, G.P.; Chaudhari, V.D.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V.; Salunke, D.B. Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV2 infection and potential therapeutic options. Biomed. Pharmacother. 2021, 141, 111794. [Google Scholar] [CrossRef]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2021, 94, 869–877. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 588, E6. [Google Scholar] [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020, 34, 101663. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.H.; Hardin, J.W.; Sutton, S.S.; Ambati, J. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Med 2020, 1, 114–127.e3. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19–Preliminary Report. MedRxiv 2020. [Google Scholar] [CrossRef]
- ClinialTrials.gov. Glucocorticoid Therapy for COVID-19 Critically III Patients with Severe Acute Respiratory Failure. Available online: https://clinicaltrials.gov/ct2/show/NCT04244591 (accessed on 25 November 2021).
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef]
- Preuss, C.V.; Anjum, F. Tocilizumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- van Rhee, F.; Wong, R.; Munshi, N.; Rossi, J.-F.; Ke, X.-Y.; Fosså, A.; Simpson, D.; Capra, M.; Liu, T.; Hsieh, R.K.; et al. Siltuximab for multicentric Castleman’s disease: A randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014, 15, 966–974. [Google Scholar] [CrossRef]
- Gritti, G.; Raimondi, F.; Ripamonti, D.; Riva, I.; Landi, F.; Alborghetti, L.; Frigeni, M.; Damiani, M.; Micò, C.; Fagiuoli, S.; et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: An observational cohort study. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically III Patients with COVID-19 with Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- van Griensven, J.; Edwards, T.; de Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; Horby, P.; Raoul, H.; Magassouba, N.; Antierens, A.; et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N. Engl. J. Med. 2016, 374, 33–42. [Google Scholar] [CrossRef]
- Zhou, B.; Zhong, N.; Guan, Y. Treatment with Convalescent Plasma for Influenza A (H5N1) Infection. N. Engl. J. Med. 2007, 357, 1450–1451. [Google Scholar] [CrossRef] [Green Version]
- Salazar, E.; Perez, K.K.; Ashraf, M.; Chen, J.; Castillo, B.; Christensen, P.A.; Eubank, T.; Bernard, D.W.; Eagar, T.N.; Long, S.W.; et al. Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. Am. J. Pathol. 2020, 190, 1680–1690. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [Green Version]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H.; et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly and Company. Lilly’s Neutralizing Antibody Bamlanivimab (LY-CoV555) Receives FDA Emergency Use Authorization for the Treatment of Recently Diagnosed COVID-19. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-receives-fda (accessed on 25 November 2021).
- Kumar, R.N.; Wu, E.-L.; Stosor, V.; Moore, W.J.; Achenbach, C.; Ison, M.G.; Angarone, M.P. Real-World Experience of Bamlanivimab for Coronavirus Disease 2019 (COVID-19): A Case-Control Study. Clin. Infect. Dis. 2021, 74, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab on Viral Load in Patients with Mild to Moderate COVID-19. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Regeneron Pharmaceuticals. Regeneron’s COVID-19 Outpatient Trial Prospectively Demonstrates that REGN-COV2 Antibody Cocktail Significantly Reduced Virus Levels and Need for Further Medical Attention. Available online: https://investor.regeneron.com/news-releases/news-release-details/regenerons-covid-19-outpatient-trial-prospectively-demonstrates (accessed on 25 November 2021).
- Regeneron Pharmaceuticals. Regeneron’s REGN-COV2 Antibody Cocktail Reduced Viral Levels and Improved Symptoms in Non-Hospitalized COVID-19 Patients. Available online: https://investor.regeneron.com/news-releases/news-release-details/regenerons-regn-cov2-antibody-cocktail-reduced-viral-levels-and (accessed on 25 November 2021).
- Ganesh, R.; Philpot, L.M.; Bierle, D.M.; Anderson, R.J.; Arndt, L.L.; Arndt, R.F.; Culbertson, T.L.; Borgen, M.J.D.; Hanson, S.N.; Kennedy, B.D.; et al. Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab Among High-Risk Patients with Mild to Moderate Coronavirus Disease 2019. J. Infect. Dis. 2021, 224, 1278–1286. [Google Scholar] [CrossRef]
- Liu, E.C.; Lee, J.H.; Loo, A.; Mazur, S.; Sultan, S.; Aull, M.; Lee, J.B.; Muthukumar, T.; Hartono, C. Casirivimab-Imdevimab (REGN-COV2) for Mild to Moderate SARS-CoV-2 Infection in Kidney Transplant Recipients. Kidney Int. Rep. 2021, 6, 2900–2902. [Google Scholar] [CrossRef]
- Mayer, C.; VanHise, K.; Caskey, R.; Naqvi, M.; Burwick, R.M. Monoclonal Antibodies Casirivimab and Imdevimab in Pregnancy for Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2021, 138, 937–939. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Radenkovic, D.; Chawla, S.; Pirro, M.; Sahebkar, A.; Banach, M. Cholesterol in Relation to COVID-19: Should We Care about It? J. Clin. Med. 2020, 9, 1909. [Google Scholar] [CrossRef]
- Palacios-Rápalo, S.N.; de Jesús-González, L.A.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Martínez-Mier, G.; Quistián-Galván, J.; Muñoz-Pérez, A.; Bernal-Dolores, V.; del Ángel, R.M.; et al. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front. Immunol. 2021, 12, 796855. [Google Scholar] [CrossRef]
- Kočar, E.; Režen, T.; Rozman, D. Cholesterol, lipoproteins, and COVID-19: Basic concepts and clinical applications. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158849. [Google Scholar] [CrossRef]
- Talha, B.; Dhamoon, A.S. Ritonavir. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Croxtall, J.D.; Perry, C.M. Lopinavir/Ritonavir: A review of its use in the management of HIV-1 infection. Drugs 2010, 70, 1885–1915. [Google Scholar] [CrossRef]
- Klibanov, O.M.; Gale, S.E.; Santevecchi, B. Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir Tablets for Hepatitis C Virus Genotype 1 Infection. Ann. Pharmacother. 2015, 49, 566–581. [Google Scholar] [CrossRef]
- Kemnic, T.R.; Gulick, P.G. HIV Antiretroviral Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Loelius, S.G.; Lannan, K.L.; Blumberg, N.; Phipps, R.P.; Spinelli, S.L. The HIV protease inhibitor, ritonavir, dysregulates human platelet function in vitro. Thromb. Res. 2018, 169, 96–104. [Google Scholar] [CrossRef]
- Loustaud-Ratti, V.; Debette-Gratien, M.; Jacques, J.; Alain, S.; Marquet, P.; Sautereau, D.; Rousseau, A.; Carrier, P. Ribavirin: Past, present and future. World J. Hepatol. 2016, 8, 123–130. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatitis C Virus Infection. J. Hepatol. 2011, 55, 245–264. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, R.; Baroudy, B.M.; Malcolm, B.A.; Reyes, G.R. The effect of ribavirin and IMPDH inhibitors on hepatitis C virus subgenomic replicon RNA. Virology 2003, 310, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Freedman, D.O. Malaria Prevention in Short-Term Travelers. N. Engl. J. Med. 2008, 359, 603–612. [Google Scholar] [CrossRef]
- Park, T.-Y.; Jang, Y.; Kim, W.; Shin, J.; Toh, H.T.; Kim, C.-H.; Yoon, H.S.; Leblanc, P.; Kim, K.-S. Chloroquine modulates inflammatory autoimmune responses through Nurr1 in autoimmune diseases. Sci. Rep. 2019, 9, 15559. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.J.; Gluzman, I.Y.; Russell, D.G.; Goldberg, D.E. On the molecular mechanism of chloroquine’s antimalarial action. Proc. Natl. Acad. Sci. USA 1996, 93, 11865–11870. [Google Scholar] [CrossRef] [Green Version]
- Corssmit, E.P.; Dekkers, O.M. Screening in adrenal tumors. Curr. Opin. Oncol. 2019, 31, 243–246. [Google Scholar] [CrossRef]
- Bano, G.; Mir, F.; Beharry, N.; Wilson, P.; Hodgson, S.; Schey, S. A Novel Medical Treatment of Cushing’s Due to Ectopic ACTH in a Patient with Neurofibromatosis Type 1. Int. J. Endocrinol. Metab. 2013, 11, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Brinks, J.; van Dijk, E.H.C.; Habeeb, M.; Nikolaou, A.; Tsonaka, R.; Peters, H.A.B.; Sips, H.C.M.; van de Merbel, A.F.; de Jong, E.K.; Notenboom, R.G.E.; et al. The Effect of Corticosteroids on Human Choroidal Endothelial Cells: A Model to Study Central Serous Chorioretinopathy. Investig. Opthalmology Vis. Sci. 2018, 59, 5682–5692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebba, A. Tocilizumab: The first interleukin-6-receptor inhibitor. Am. J. Health Pharm. 2008, 65, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.-M. Raspunde sau nu raspunde personalul medical? Aceasta este intrebarea. In Probleme Si Solutii Legale Privind Criza COVID-19; C.H. Beck: Bucuresti, Romania, 2020; p. 299. [Google Scholar]
- Calin, R.M. Raspunderea personalului medical si a furnizorului de servicii medicale. In Practica Judiciara, 2nd ed.; Hamangiu: Bucuresti, Romania, 2016; pp. 214–222. [Google Scholar]


| Drug | Disease | Mechanism of Action | Ref. |
|---|---|---|---|
| Lopinavir/Ritonavir | HIV chronic hepatitis C | HIV-1 protease inhibitor: disrupts the cleavage of protein precursors inhibition of the liver enzyme cytochrome P450 3A4 | [115,116,117,118,119] |
| Ribavirin | chronic hepatitis C | reduces hepatitis C virus replicon colony-forming efficiency | [120,121,122] |
| Chloroquine Hydroxychloroquine | malaria autoimmune diseases | prevents polymerization of heme into hemozoin binds to transcriptional factors on T helper 17 cells | [123,124,125] |
| Dexamethasone | inflammation, asthma, allergies, drug hypersensitivity reactions, Cushing syndrome | suppresses neutrophil migration, decreases lymphocyte colony proliferation | [126,127,128] |
| Tocilizumab | cytokine release syndrome, giant cell arteritis rheumatoid arthritis | prevents IL-6 from binding to its interleukin-6 receptor | [88,129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, A.; Manea, A.; Scurtu, A.D.; Bratu, L.M.; Chioran, D.; Dolghi, A.; Alexoi, I.; AAbed, H.; Lazureanu, V.; Dehelean, C.A. The “Invisible Enemy” SARS-CoV-2: Viral Spread and Drug Treatment. Medicina 2022, 58, 261. https://doi.org/10.3390/medicina58020261
Tanase A, Manea A, Scurtu AD, Bratu LM, Chioran D, Dolghi A, Alexoi I, AAbed H, Lazureanu V, Dehelean CA. The “Invisible Enemy” SARS-CoV-2: Viral Spread and Drug Treatment. Medicina. 2022; 58(2):261. https://doi.org/10.3390/medicina58020261
Chicago/Turabian StyleTanase, Alina, Aniko Manea, Alexandra Denisa Scurtu, Lavinia Melania Bratu, Doina Chioran, Alina Dolghi, Iren Alexoi, Hazzaa AAbed, Voichita Lazureanu, and Cristina Adriana Dehelean. 2022. "The “Invisible Enemy” SARS-CoV-2: Viral Spread and Drug Treatment" Medicina 58, no. 2: 261. https://doi.org/10.3390/medicina58020261
APA StyleTanase, A., Manea, A., Scurtu, A. D., Bratu, L. M., Chioran, D., Dolghi, A., Alexoi, I., AAbed, H., Lazureanu, V., & Dehelean, C. A. (2022). The “Invisible Enemy” SARS-CoV-2: Viral Spread and Drug Treatment. Medicina, 58(2), 261. https://doi.org/10.3390/medicina58020261







