Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
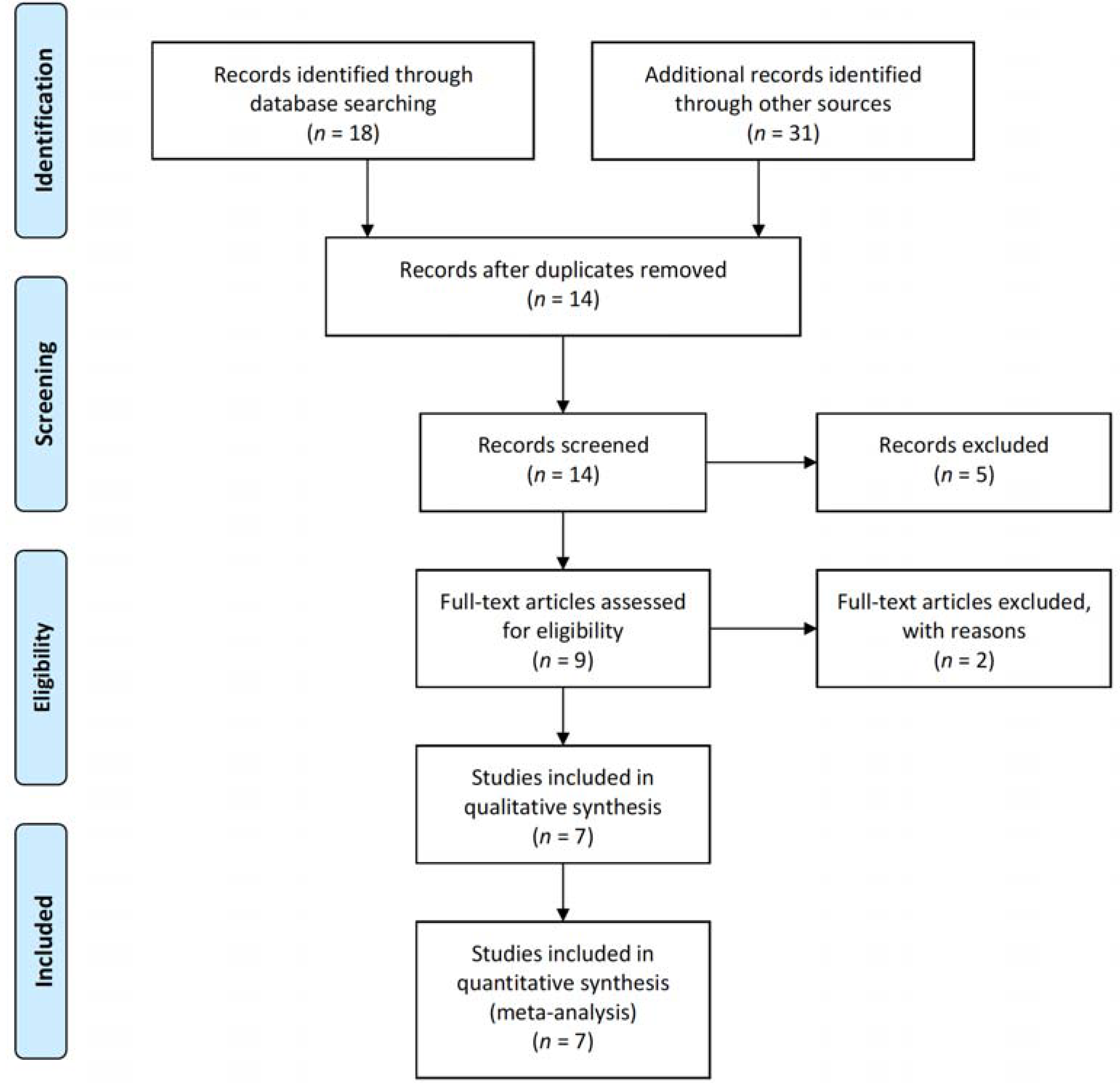
2.2. Search Strategy and Selection of Studies
2.2.1. Inclusion Criteria
- ParticipantsParticipants were not classified according to specific diseases or conditions.
- InterventionIn the experimental group, interventions in which exercise was the main focus were selected.
- ComparisonsInactivity or usual care was selected for comparison with interventions that included exercise.
- OutcomesIn our review, only the effect on telomere length was investigated.
- Types of studiesOnly RCT retrieved through international electronic databases were included.
2.2.2. Exclusion Criteria
2.2.3. Literature-Search Strategy
2.2.4. Study Selection and Data Extraction
2.2.5. Quality Assessment
2.3. Strategy for Data Synthesis
3. Results
3.1. Literature Search and Characteristics of the Included Randomized Clinical Trials
3.2. Methodological Quality Assessment
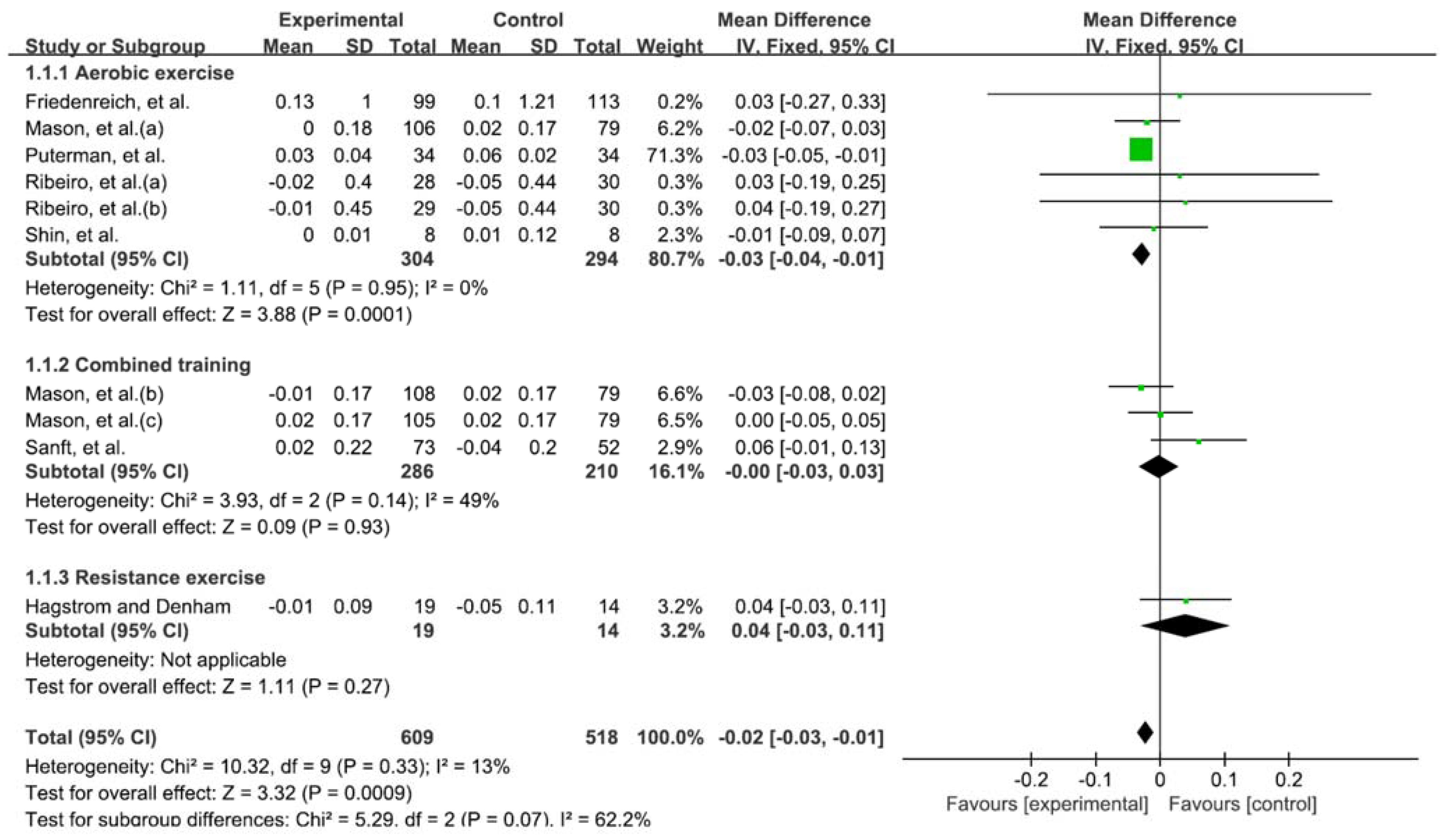
3.3. Exercise and Telomere Length
3.4. Types of Exercise That Affect Telomere Length
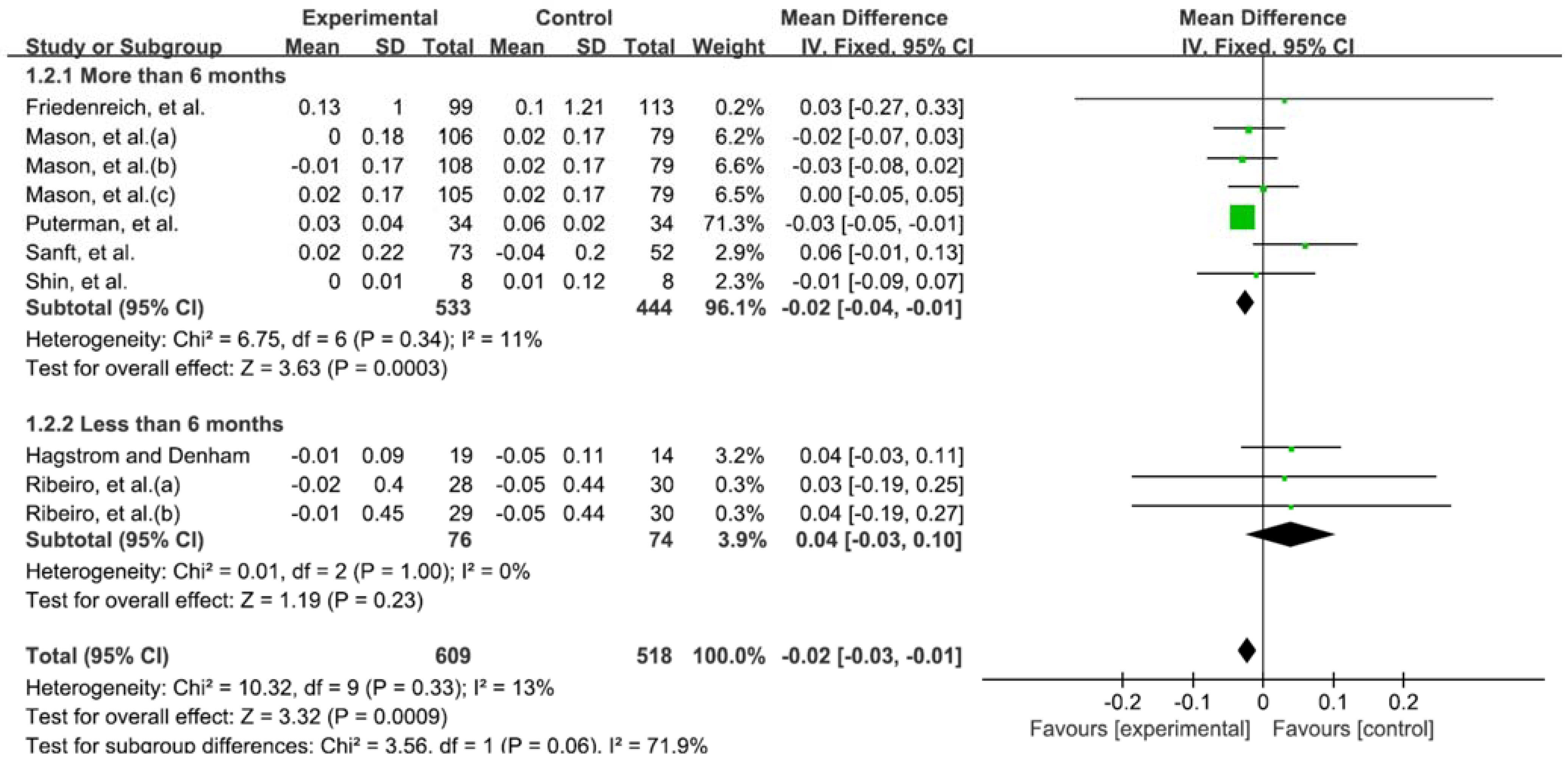
3.5. Telomere Length According to Duration of Exercise
3.6. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, E.H. Telomeres and telomerase: The means to the end (Nobel lecture). Angew. Chem. Int. Ed. 2010, 49, 7405–7421. [Google Scholar] [CrossRef] [PubMed]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalson, N.S.; Brock, T.M.; Mangino, M.; Fabiane, S.M.; Mann, D.A.; Borthwick, L.A.; Deehan, D.J.; Williams, F.M. Reduced telomere length is associated with fibrotic joint disease suggesting that impaired telomere repair contributes to joint fibrosis. PLoS ONE 2018, 13, e0190120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goglin, S.E.; Farzaneh-Far, R.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Whooley, M.A. Change in leukocyte telomere length predicts mortality in patients with stable coronary heart disease from the heart and soul study. PLoS ONE 2016, 11, e0160748. [Google Scholar]
- Werner, C.; Fürster, T.; Widmann, T.; Pöss, J.; Roggia, C.; Hanhoun, M.; Scharhag, J.; Büchner, N.; Meyer, T.; Kindermann, W. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 2009, 120, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, A.T.; Ludlow, L.W.; Roth, S.M. Do telomeres adapt to physiological stress? Exploring the effect of exercise on telomere length and telomere-related proteins. Biomed. Res. Int. 2013, 2013, 601368. [Google Scholar] [CrossRef] [PubMed]
- Mundstock, E.; Zatti, H.; Louzada, F.M.; Oliveira, S.G.; Guma, F.T.; Paris, M.M.; Rueda, A.B.; Machado, D.G.; Stein, R.T.; Jones, M.H. Effects of physical activity in telomere length: Systematic review and meta-analysis. Ageing Res. Rev. 2015, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2019, 40, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, V.B.; Pedroso, D.C.C.; Kogure, G.S.; Lopes, I.P.; Santana, B.A.; Dutra de Souza, H.C.; Ferriani, R.A.; Calado, R.T.; Furtado, C.L.M.; Reis, R.M.D. Short-Term Aerobic Exercise Did Not Change Telomere Length While It Reduced Testosterone Levels and Obesity Indexes in PCOS: A Randomized Controlled Clinical Trial Study. Int. J. Environ. Res. 2021, 18, 11274. [Google Scholar] [CrossRef] [PubMed]
- Schutte, N.S.; Malouff, J.M.; Keng, S.-L. Meditation and telomere length: A meta-analysis. Psychol. Health 2020, 35, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5; The Cochrane Collaboration: Oxford, UK, 2011.
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Wang, Q.; Ting, N.S.; Brenner, D.R.; Conroy, S.M.; McIntyre, J.B.; Mickle, A.; Courneya, K.S.; Beattie, T. Effect of a 12-month exercise intervention on leukocyte telomere length: Results from the ALPHA Trial. Cancer Epidemiol. 2018, 56, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Denham, J. The effect of resistance training on telomere length in women recovering from breast cancer. J. Funct. Morphol. Kinesiol. 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.; Risques, R.A.; Xiao, L.; Duggan, C.R.; Imayama, I.; Campbell, K.L.; Kong, A.; Foster-Schubert, K.E.; Wang, C.; Alfano, C.M. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity 2013, 21, E549–E554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanft, T.; Usiskin, I.; Harrigan, M.; Cartmel, B.; Lu, L.; Li, F.-Y.; Zhou, Y.; Chagpar, A.; Ferrucci, L.M.; Pusztai, L. Randomized controlled trial of weight loss versus usual care on telomere length in women with breast cancer: The lifestyle, exercise, and nutrition (LEAN) study. Breast Cancer Res. Treat 2018, 172, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-A.; Lee, J.-H.; Song, W.; Jun, T.-W. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech. Ageing Dev. 2008, 129, 254–260. [Google Scholar] [CrossRef]
- Puterman, E.; Weiss, J.; Lin, J.; Schilf, S.; Slusher, A.L.; Johansen, K.L.; Epel, E.S. Aerobic exercise lengthens telomeres and reduces stress in family caregivers: A randomized controlled trial-Curt Richter Award Paper 2018. Psychoneuroendocrinology 2018, 98, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhou, J.; Dong, B. Effect of different levels of exercise on telomere length: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Ornish, D.; Lin, J.; Chan, J.M.; Epel, E.; Kemp, C.; Weidner, G.; Marlin, R.; Frenda, S.J.; Magbanua, M.J.M.; Daubenmier, J. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013, 14, 1112–1120. [Google Scholar] [CrossRef]
- Harrigan, M.; Cartmel, B.; Loftfield, E.; Sanft, T.; Chagpar, A.B.; Zhou, Y.; Playdon, M.; Li, F.; Irwin, M.L. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The lifestyle, exercise, and nutrition (LEAN) study. Am. J. Clin. Oncol. 2016, 34, 669. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Wing, R.R.; Koeske, R.; Valoski, A. A comparison of lifestyle exercise, aerobic exercise, and calisthenics on weight loss in obese children. Behav. Ther. 1985, 16, 345–356. [Google Scholar] [CrossRef]
- Rönn, T.; Volkov, P.; Tornberg, Å.; Elgzyri, T.; Hansson, O.; Eriksson, K.F.; Groop, L.; Ling, C.J.A.P. Extensive changes in the transcriptional profile of human adipose tissue including genes involved in oxidative phosphorylation after a 6-month exercise intervention. Acta Physiol. 2014, 211, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, A.; De Lange, T. Removal of shelterin reveals the telomere end-protection problem. Science 2012, 336, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denham, J.; Nelson, C.P.; O’Brien, B.J.; Nankervis, S.A.; Denniff, M.; Harvey, J.T.; Marques, F.Z.; Codd, V.; Zukowska-Szczechowska, E.; Samani, N.J. Longer leukocyte telomeres are associated with ultra-endurance exercise independent of cardiovascular risk factors. PLoS ONE 2013, 8, e69377. [Google Scholar] [CrossRef] [PubMed]
- Østhus, I.B.Ø.; Sgura, A.; Berardinelli, F.; Alsnes, I.V.; Brønstad, E.; Rehn, T.; Støbakk, P.K.; Hatle, H.; Wisløff, U.; Nauman, J. Telomere length and long-term endurance exercise: Does exercise training affect biological age? A pilot study. PLoS ONE 2012, 7, e52769. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Seals, D.R.; Pierce, G.L. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech. Ageing Dev. 2010, 131, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Gomes, E.C.; Silva, A.N.; Oliveira, M.R.D. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid. Med. Cell. Longev. 2012, 2012, 756132. [Google Scholar] [CrossRef]
- Samjoo, I.; Safdar, A.; Hamadeh, M.; Raha, S.; Tarnopolsky, M. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr. Diabetes 2013, 3, e88. [Google Scholar] [CrossRef] [Green Version]
- Nimmo, M.; Leggate, M.; Viana, J.; King, J. The effect of physical activity on mediators of inflammation. Obes. Metab. 2013, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]


| Study | Subject | Sample Size | Duration | Exercise Intensity | Control | Author’s Conclusion | Country, Setting |
|---|---|---|---|---|---|---|---|
| Friedenreich, et al., 2018 | 50–74 years old | AG = 99 CG = 113 | 12 months | Aerobic exercise five days/week (supervised three days/week), 45 min/session, achieving 70–80% heart rate reserve. | Usual inactivity | One year of aerobic exercise did not significantly change telomere attrition in healthy postmenopausal women. | Canada, Westside Recreation Centre in Calgary |
| Hagstrom and Denham, 2018 | Breast cancer | RG = 19 CG = 14 | 16 weeks | Supervised RT was performed three times per week for 60 min per session. The exercise load was set at an 8 repetition maximum. | Usual inactivity | Resistance training is a safe intervention that does not accelerate biological aging. | Australia, University of Western Sydney exercise science laboratories |
| Mason, et al., 2013 | Postmenopausal women | AG = 106 AWG = 108 WG = 105 CG = 79 | 12 months | - Combined training: total daily energy intake of 1200–2000 kcal/day based on baseline weight, <30% daily energy intake from fat, and a 10% reduction in body weight by 6 months with maintenance thereafter to 12 months. - Aerobic exercise: ≥45 min of moderate-to-vigorous intensity exercise, 5 days/week (225 min/week) for 12 months | Four group nutrition classes and 8 weeks of facility exercise training with individualized guidance from an exercise physiologist | Twelve months of dietary weight loss and aerobic exercise did not change telomere length in postmenopausal women. | USA, Fred Hutchinson Cancer Research Center |
| Puterman, et al., 2018 | Dementia caregivers | AG = 34 CG = 34 | 24 weeks | An exercise program starting with a self-selected activity 3 times of 20 min each week and increasing to 4 repetitions per week. | Received free gym memberships and a similar personalized fitness program | Aerobic exercise to improve health indicators and attenuate cellular aging in high-risk samples. | USA, Clinical & Translational Science Institute Clinical Research Services |
| Ribeiro, et al., 2021 | Polycystic ovary syndrome | CAI = 28 IAT = 29 CG = 30 | 4 months | It lasts evenly and gradually from 30 min in the first week to 50 min in the last week. Target strength training areas followed ACSM recommendations. | Maintaining daily physical activity | Booth exercises reduced obesity indices and hyperandrogenism on PCOS women without changes in telomere length or inflammatory biomarkers. | Brazil, Ribeirão Preto Medical School-University of São Paulo |
| Sanft, et al., 2018 | Breast cancer | WG = 73 CG = 52 | 6 months | Reducing calories to 1200–2000 kcal/day, adjusting to baseline body weight, and reducing dietary fat to less than 25% of total energy intake | Brochures on nutrition and physical activity are available | Suggests that weight loss interventions may prolong telomere length compared to shortening in usual care counterparts. | USA, Yale-New Haven Hospital |
| Shin, et al., 2008 | Obese middle-aged women | AG = 8 CG = 8 | 6 months | Three days a week for 6 months. Each session consisted of 10 min of warming up, 45 min of treadmill walking/running, and 5 min of cooling down. | Usual inactivity | The lengths of telomere in leukocytes were not influenced by both mid-intensity and high intensity of exercise stress. | Republic of Korea, Seoul national university |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Lee, E.; Kim, H. Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2022, 58, 242. https://doi.org/10.3390/medicina58020242
Song S, Lee E, Kim H. Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina. 2022; 58(2):242. https://doi.org/10.3390/medicina58020242
Chicago/Turabian StyleSong, Seonghyeok, Eunsang Lee, and Hyunjoong Kim. 2022. "Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Medicina 58, no. 2: 242. https://doi.org/10.3390/medicina58020242
APA StyleSong, S., Lee, E., & Kim, H. (2022). Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina, 58(2), 242. https://doi.org/10.3390/medicina58020242








