Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect?
Abstract
1. Introduction
2. Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect?
2.1. Impact of Capacitance Vessels and Autonomic Regulation on OR
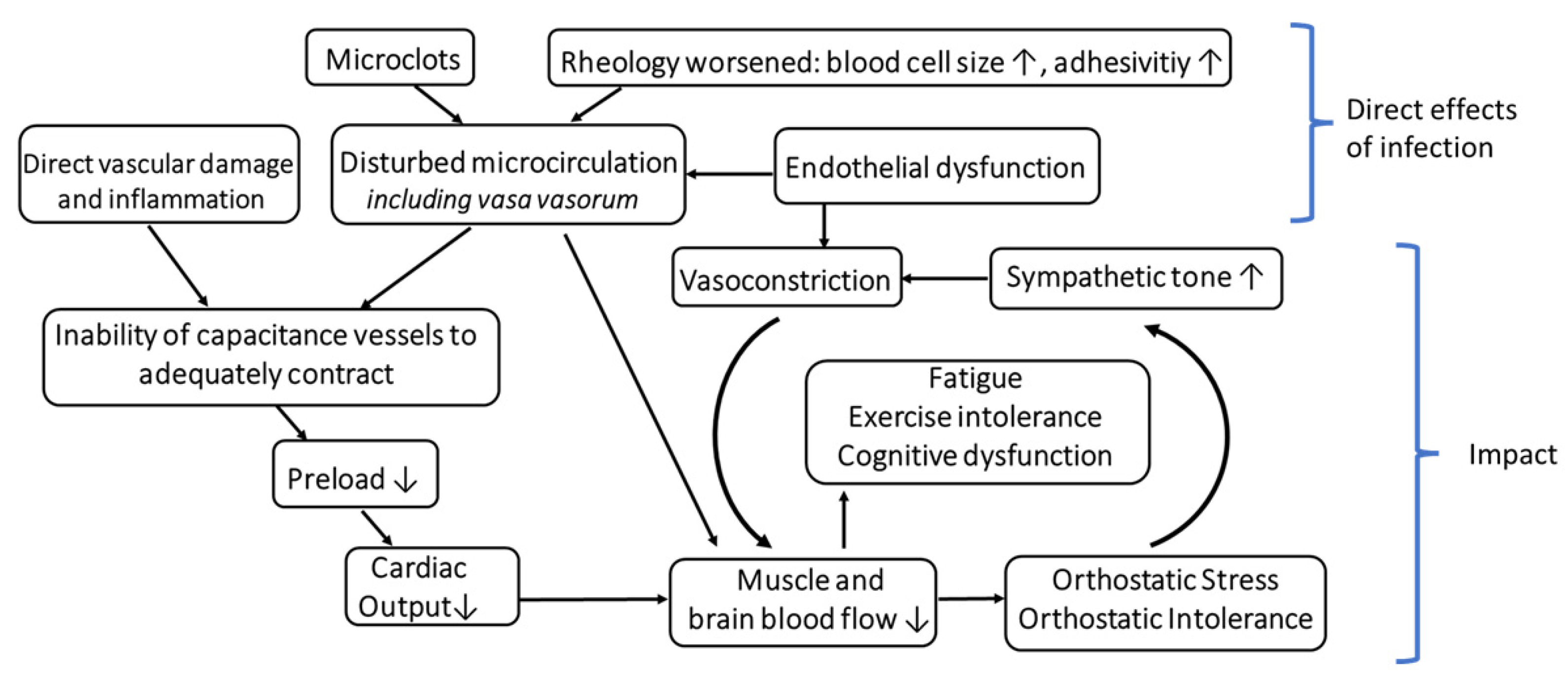
2.2. Disturbed Microcirculation as an Alternative Trigger for a Dysfunctional OR?
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, T.L.; Weitzer, D.J. Long Covid and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)—A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Petracek, L.S.; Suskauer, S.J.; Vickers, R.F.; Patel, N.R.; Violand, R.L.; Swope, R.L.; Rowe, P.C. Adolescent and Young Adult ME/CFS After Confirmed or Probable COVID-19. Front. Med. 2021, 8, 668944. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Islam, M.F.; Conroy, K.; Cotler, J.; Torres, C.; Johnson, M.; Mabie, B. COVID-19 symptoms over time: Comparing long-haulers to ME/CFS. Fatigue Biomed. Health Behav. 2021, 9, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review. Ther. Adv. Infect. Dis. 2021, 8, 20499361211009385. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Mantovani, E.A.O.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic fatigue syndrome: An emerging sequela in COVID-19 survivors? J. Neurovirol. 2021, 27, 631–637. [Google Scholar] [CrossRef]
- Freitag, H.; Szklarski, M.; Lorenz, S.; Sotzny, F.; Bauer, S.; Philippe, A.; Kedor, C.; Grabowski, P.; Lange, T.; Riemekasten, G.; et al. Autoantibodies to Vasoregulative G-Protein-Coupled Receptors Correlate with Symptom Severity, Autonomic Dysfunction and Disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Clin. Med. 2021, 10, 3675. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; Volk, H.D.; et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany—A first analysis of a prospective observational study. medRxiv 2021. [Google Scholar] [CrossRef]
- van Campen, C.M.C.; Rowe, P.C.; Visser, F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2021, 58, 28. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Eldokla, A.M.; Ali, S.T. Autonomic function testing in long-COVID syndrome patients with orthostatic intolerance. Auton. Neurosci. 2022, 241, 102997. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; Jamal, S.; Kichloo, A.; Grubb, B.P. New-onset Postural Orthostatic Tachycardia Syndrome following Coronavirus Disease 2019 Infection. J. Innov. Card. Rhythm. Manag. 2020, 11, 4302–4304. [Google Scholar] [CrossRef] [PubMed]
- Novak, P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: A case report. eNeurologicalSci 2020, 21, 100276. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2011, 11, 7–115. [Google Scholar] [CrossRef]
- Roma, M.; Marden, C.L.; Flaherty, M.A.K.; Jasion, S.E.; Cranston, E.M.; Rowe, P.C. Impaired Health-Related Quality of Life in Adolescent Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Impact of Core Symptoms. Front. Pediatr. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.M.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Mukerji, S.S.; Alabsi, H.S.; Systrom, D.; Marciano, S.P.; Felsenstein, D.; Mullally, W.J.; Pilgrim, D.M. Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. Ann. Neurol. 2021, 91, 367–379. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and Cytokines in Myalgic Encephalomyeli-tis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front Neurol. 2018, 9, 1033. [Google Scholar] [CrossRef]
- Jamal, S.M.; Landers, D.B.; Hollenberg, S.M.; Turi, Z.G.; Glotzer, T.V.; Tancredi, J.; Parrillo, J.E. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2325–2330. [Google Scholar] [CrossRef]
- Acanfora, D.; Nolano, M.; Acanfora, C.; Colella, C.; Provitera, V.; Caporaso, G.; Rodolico, G.R.; Bortone, A.S.; Galasso, G.; Casucci, G. Impaired Vagal Activity in Long-COVID-19 Patients. Viruses 2022, 14, 1035. [Google Scholar] [CrossRef] [PubMed]
- Soliński, M.; Pawlak, A.; Petelczyc, M.; Buchner, T.; Aftyka, J.; Gil, R.; Król, Z.J.; Żebrowski, J.J. Heart rate variability comparison between young males after 4–6 weeks from the end of SARS-CoV-2 infection and controls. Sci. Rep. 2022, 12, 8832. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.C.; Costa Silva, C.; da Silva Trindade, S.; de Souza Santos, M.C.; Barbosa Rocha, R.S.; da Costa Vasconcelos, P.F.; Simões Quaresma, J.A.; Magno Falcão, L.F. Reduction of Cardiac Autonomic Modulation and Increased Sympathetic Activity by Heart Rate Varia-bility in Patients with Long Covid. Front. Cardiovasc. Med. 2022, 9, 862001. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, A.; Martínez-Cava, A.; Courel-Ibáñez, J. Chronotropic Incompetence in Non-Hospitalized Patients with Post-COVID-19 Syndrome. J. Clin. Med. 2021, 10, 5434. [Google Scholar] [CrossRef]
- Barizien, N.; Le Guen, M.; Russel, S.; Touche, P.; Huang, F.; Vallée, A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 2021, 11, 14042. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K. Down-regulation of renin-aldosterone and antidiuretic hormone systems in patients with myalgic encephalomy-elitis/chronic fatigue syndrome. J. Cardiol. 2017, 69, 684–688. [Google Scholar] [CrossRef]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef]
- Savastano, M.C.; Santoro, L.; Crincoli, E.; Fossataro, C.; Gambini, G.; Savastano, A.; De Vico, U.; Santoliquido, A.; Nesci, A.; Landi, F.; et al. Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection. Vision 2022, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long Covid 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Travieso, A.; Sagir, A.; Martínez-Gómez, E.; Carrascosa-Granada, A.; Toya, T.; Núñez-Gil, I.J.; Estrada, V.; Lerman, A.; Escaned, J. In-vivo evidence of systemic endothelial vascular dysfunction in COVID-19. Int. J. Cardiol. 2021, 345, 153–155. [Google Scholar] [CrossRef]
- Sang, C.J.; Burkett, A.; Heindl, B.; Litovsky, S.H.; Prabhu, S.D.; Benson, P.V.; Rajapreyar, I. Cardiac pathology in COVID-19: A single center autopsy experience. Cardiovasc. Pathol. 2021, 54, 107370. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long Covid/Post-Acute Sequelae of COVID-19 (PASC) is accom-panied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Saha, A.K.; Schmidt, B.R.; Wilhelmy, J.; Nguyen, V.; Abugherir, A.; Do, J.K.; Nemat-Gorgani, M.; Davis, R.W.; Ramasubramanian, A.K. Red blood cell deformability is diminished in patients with Chronic Fatigue Syndrome. Clin. Hemorheol. Microcirc. 2019, 71, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Liu, X. NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. 2022, 13, 838011. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; Ciavarella, C.; Collura, S.; Mascoli, C.; Valente, S.; Degiovanni, A.; Gargiulo, M.; Capri, M.; Pasquinelli, G. Adventitial Microcirculation Is a Major Target of SARS-CoV-2-Mediated Vascular Inflammation. Biomolecules 2021, 11, 1063. [Google Scholar] [CrossRef]
- Boyle, E.C.; Haverich, A. Microvasculature dysfunction as the common thread between atherosclerosis, Kawasaki disease, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated multi-system inflammatory syndrome in children. Eur. J. Cardiothorac. Surg. 2020, 58, 1109–1110. [Google Scholar] [CrossRef]
- Daisley, H., Jr.; Rampersad, A.; Daisley, M.; Ramdin, A.; Acco, O.; Narinesingh, F.; Humphrey, O.; James, E. The vasa vasorum of the large pulmonary vessels are involved in COVID-19. Autops. Case Rep. 2021, 11, e2021304. [Google Scholar] [CrossRef]
- Faa, G.; Gerosa, C.; Fanni, D.; Barcellona, D.; Cerrone, G.; Orrù, G.; Scano, A.; Marongiu, F.; Suri, J.S.; Demontis, R.; et al. Aortic vulnerability to COVID-19: Is the microvasculature of vasa vasorum a key factor? A case report and a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6439–6442. [Google Scholar]
- Sollini, M.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Gelardi, F.; Chiti, A. Vasculitis changes in COVID-19 survivors with persistent symptoms: An [18F]FDG-PET/CT study. Eur. J. Nucl. Med. 2020, 48, 1460–1466. [Google Scholar] [CrossRef]
- Heistad, D.D.; Armstrong, M.L.; Amundsen, S. Blood flow through vasa vasorum in arteries and veins: Effects of luminal PO2. Am. J. Physiol. Circ. Physiol. 1986, 250, H434–H442. [Google Scholar] [CrossRef]
- Brook, W. Vasa Vasorum of Veins in Dog and Man. Angiology 1977, 28, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Terentes-Printzios, D.; Katsaounou, P.; Solomou, E.; Gardikioti, V.; Exarchos, D.; Economou, D.; Christopoulou, G.; Kalkinis, A.-D.; Kafouris, P.; et al. Time-related aortic inflammatory response, as assessed with 18F-FDG PET/CT, in patients hospitalized with severely or critical COVID-19: The COVAIR study. J. Nucl. Cardiol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C. Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2021, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C. Dyspnea in Post-COVID Syndrome following Mild Acute COVID-19 Infections: Potential Causes and Consequences for a Therapeutic Approach. Medicina 2022, 58, 419. [Google Scholar] [CrossRef] [PubMed]
- Hejbøl, E.K.; Harbo, T.; Agergaard, J.; Madsen, L.B.; Pedersen, T.H.; Østergaard, L.J.; Andersen, H.; Schrøder, H.D.; Tankisi, H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle his-topathology. Eur. J. Neurol. 2022, 29, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.; Rowe, P.; Visser, F. Reductions in Cerebral Blood Flow Can Be Provoked by Sitting in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare 2020, 8, 394. [Google Scholar] [CrossRef]
- Van Campen, C.L.M.C.; Rowe, P.C.; Visser, F.C. Cerebral Blood Flow Is Reduced in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients During Mild Orthostatic Stress Testing: An Exploratory Study at 20 Degrees of Head-Up Tilt Testing. Healthcare 2020, 8, 169. [Google Scholar] [CrossRef]
- Wirth, K.J.; Scheibenbogen, C.; Paul, F. An attempt to explain the neurological symptoms of Myalgic Encephalomyeli-tis/Chronic Fatigue Syndrome. J. Transl. Med. 2021, 19, 471. [Google Scholar] [CrossRef]
- Szewczykowski, C.; Mardin, C.; Lucio, M.; Wallukat, G.; Hoffmanns, J.; Schröder, T.; Raith, F.; Rogge, L.; Heltmann, F.; Moritz, M.; et al. Long COVID: Association of Functional Autoantibodies against G-Protein-Coupled Receptors with an Impaired Retinal Microcirculation. Int. J. Mol. Sci. 2022, 23, 7209. [Google Scholar] [CrossRef]
- Miwa, K.; Fujita, M. Cardiovascular Dysfunction with Low Cardiac Output Due to a Small Heart in Patients with Chronic Fatigue Syndrome. Intern. Med. 2009, 48, 1849–1854. [Google Scholar] [CrossRef][Green Version]
- Hollingsworth, K.G.; Hodgson, T.; MacGowan, G.A.; Blamire, A.M.; Newton, J.L. Impaired cardiac function in chronic fatigue syndrome measured using magnetic resonance cardiac tagging. J. Intern. Med. 2011, 271, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K. Cardiac dysfunction and orthostatic intolerance in patients with myalgic encephalomyelitis and a small left ventricle. Heart Vessel. 2015, 30, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.L.; Finkelmeyer, A.; Petrides, G.; Frith, J.; Hodgson, T.; Maclachlan, L.; MacGowan, G.; Blamire, A.M. Reduced cardiac volumes in chronic fatigue syndrome associate with plasma volume but not length of disease: A cohort study. Open Hear. 2016, 3, e000381. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, B.E.; Coryell, V.T.; Parker, M.; Martin, P.; Laperriere, A.; Klimas, N.G.; Sfakianakis, G.N.; Bilsker, M.S. Chronic fatigue syndrome: Illness severity, sedentary lifestyle, blood volume and evidence of di-minished cardiac function. Clin. Sci. 2009, 118, 125–135. [Google Scholar] [CrossRef]
- De Becker, P.; Dendale, P.; De Meirleir, K.; Campine, I.; Vandenborne, K.; Hagers, Y. Autonomic testing in patients with chronic fatigue syndrome. Am. J. Med. 1998, 105, 22S–26S. [Google Scholar] [CrossRef]
- Wyller, V.B.; Saul, J.P.; Walløe, L.; Thaulow, E. Sympathetic cardiovascular control during orthostatic stress and isometric exercise in adolescent chronic fatigue syndrome. Eur. J. Appl. Physiol. 2007, 102, 623–632. [Google Scholar] [CrossRef]
- Rimes, K.A.; Lievesley, K.; Chalder, T. Stress vulnerability in adolescents with chronic fatigue syndrome: Experimental study investigating heart rate variability and skin conductance responses. J. Child Psychol. Psychiatry 2017, 58, 851–858. [Google Scholar] [CrossRef]
- Burton, A.R.; Rahman, K.; Kadota, Y.; Lloyd, A.; Vollmer-Conna, U. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syn-drome. Exp. Brain Res. 2010, 204, 71–78. [Google Scholar] [CrossRef]
- Joseph, P.; Arevalo, C.; Oliveira, R.K.F.; Faria-Urbina, M.; Felsenstein, D.; Oaklander, A.L.; Systrom, D.M. Insights From Invasive Cardiopulmonary Exercise Testing of Patients with Myalgic Encephalomyeli-tis/Chronic Fatigue Syndrome. Chest 2021, 160, 642–651. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirth, K.J.; Löhn, M. Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect? Medicina 2022, 58, 1807. https://doi.org/10.3390/medicina58121807
Wirth KJ, Löhn M. Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect? Medicina. 2022; 58(12):1807. https://doi.org/10.3390/medicina58121807
Chicago/Turabian StyleWirth, Klaus J., and Matthias Löhn. 2022. "Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect?" Medicina 58, no. 12: 1807. https://doi.org/10.3390/medicina58121807
APA StyleWirth, K. J., & Löhn, M. (2022). Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect? Medicina, 58(12), 1807. https://doi.org/10.3390/medicina58121807




