Investigating the Influence of Anaesthesiology for Cancer Resection Surgery on Oncologic Outcomes: The Role of Experimental In Vivo Models
Abstract
1. Introduction
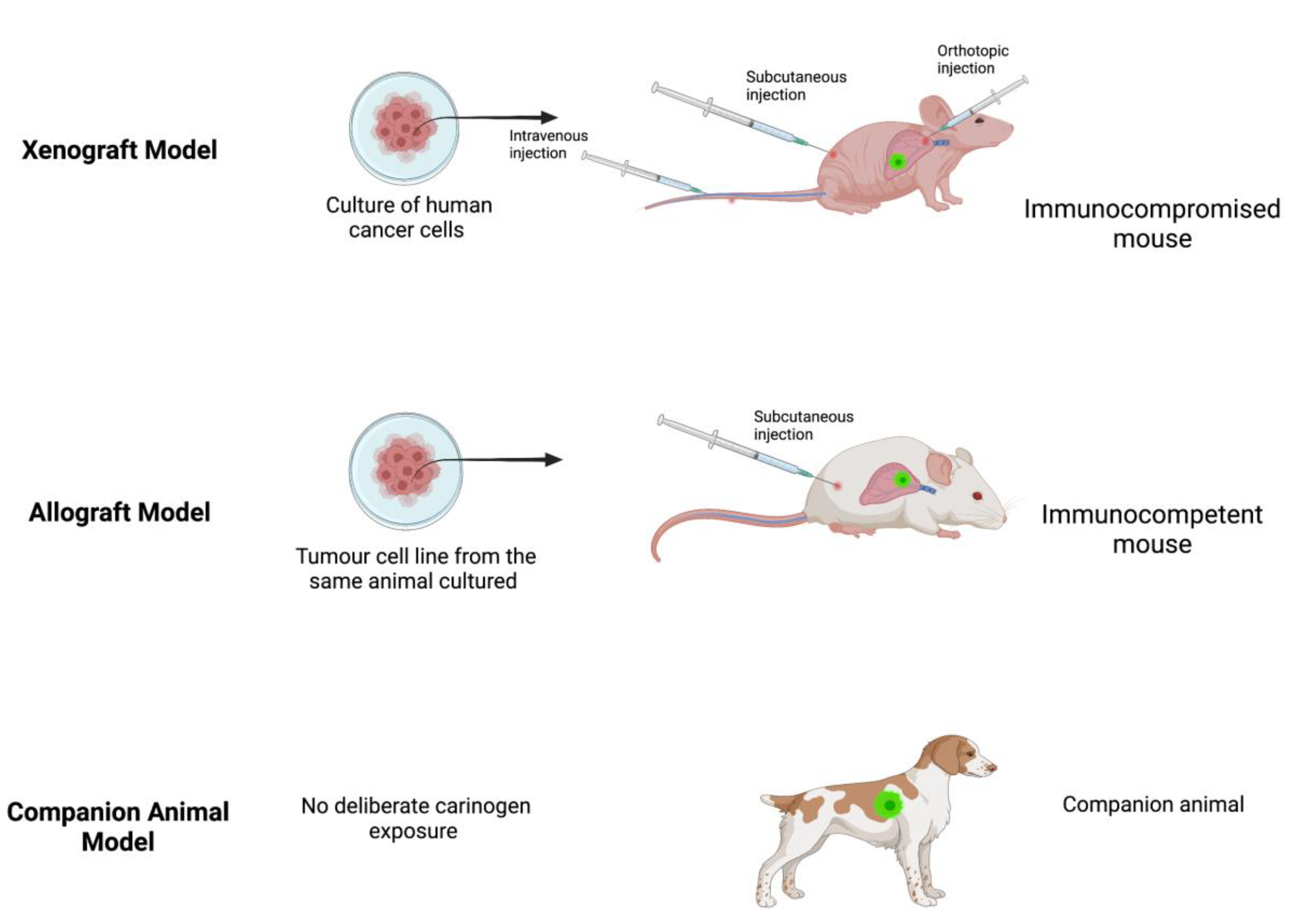
2. Xenograft Model
3. Allograft Model
4. Companion Animals
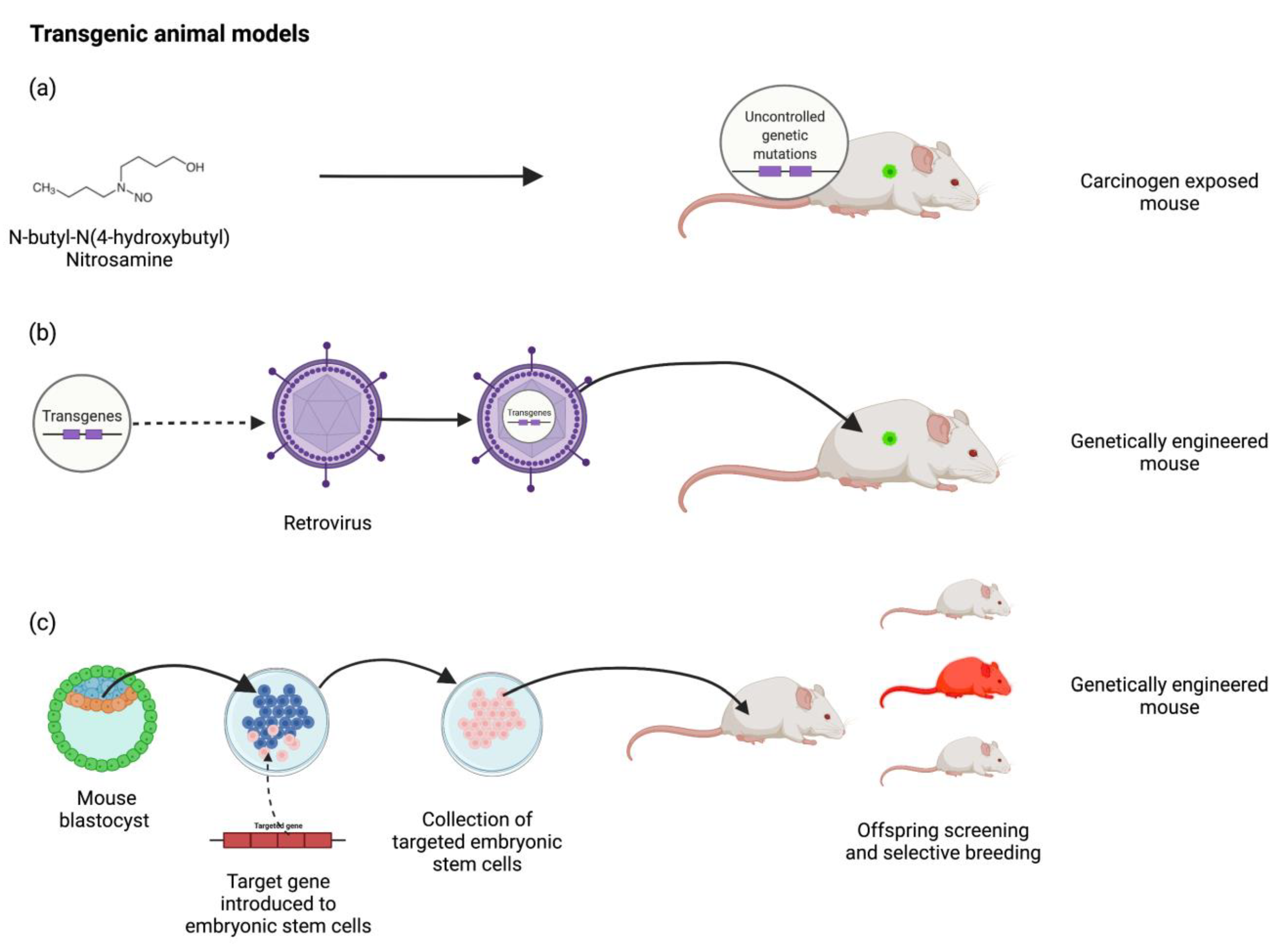
5. Transgenic Models
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Scott, A.M.; Hricak, H.; Abdel-Wahab, M.; Paez, D.; Lette, M.M.; Vargas, H.A.; Kingham, T.P.; Atun, R. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers in 200 countries: A simulation-based analysis. Lancet Oncol. 2020, 21, 1077–1088. [Google Scholar] [CrossRef]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2020, 124, 13–26. [Google Scholar] [CrossRef]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006, 105, 660–664. Available online: https://pubs.asahq.org/anesthesiology/article/105/4/660/6830/Can-Anesthetic-Technique-for-Primary-Breast-Cancer (accessed on 5 February 2022). [CrossRef] [PubMed]
- Afsharimani, B.; Cabot, P.J.; Parat, M.-O. Morphine Use in Cancer Surgery. Front. Pharmacol. 2011, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Does regional analgesia reduce the risk of cancer recurrence? a hypothesis. Eur. J. Cancer Prev. 2008, 17, 269–272. Available online: https://journals.lww.com/eurjcancerprev/Fulltext/2008/06000/Does_regional_analgesia_reduce_the_risk_of_cancer.13.aspx (accessed on 5 February 2022). [CrossRef]
- Li, Z.; Zheng, W.; Wang, H.; Cheng, Y.; Fang, Y.; Wu, F.; Sun, G.; Sun, G.; Lv, C.; Hui, B. Application of Animal Models in Cancer Research: Recent Progress and Future Prospects. Cancer Manag. Res. 2021, 13, 2455–2475. [Google Scholar] [CrossRef] [PubMed]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sathian, B.; Asim, M.; Banerjee, I.; Pizarro, A.B.; Roy, B.; Van Teijlingen, E.R.; Nascimento, I.J.B.D.; Alhamad, H.K. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal J. Epidemiol. 2020, 10, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.; Bartunek, P. Zebrafish models of cancer—New insights of modeling of human cancer in a non-mammalian vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Afsharimani, B.; Doornebal, C.W.; Cabot, P.J.; Hollmann, M.W.; Parat, M.-O. Comparison and analysis of the animal models used to study the effect of morphine on tumour growth and metastasis. J. Cereb. Blood Flow Metab. 2014, 172, 251–259. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/bph.12589 (accessed on 1 March 2022). [CrossRef] [PubMed]
- Onaciu, A.; Munteanu, R.; Munteanu, V.C.; Gulei, D.; Raduly, L.; Feder, R.-I.; Pirlog, R.; Atanasov, A.G.; Korban, S.S.; Irimie, A.; et al. Spontaneous and Induced Animal Models for Cancer Research. Diagnostics 2020, 10, 660. Available online: https://www.mdpi.com/2075-4418/10/9/660/htm (accessed on 1 March 2022). [CrossRef] [PubMed]
- Kopetz, S.; Lemos, R.; Powis, G. The Promise of Patient-Derived Xenografts: The Best Laid Plans of Mice and Men. Clin. Cancer Res. 2012, 18, 5160–5162. [Google Scholar] [CrossRef]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Schuh, J.C. Trials, Tribulations, and Trends in Tumor Modeling in Mice. Toxicol. Pathol. 2004, 32, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.J.; Lin, A.F.; Arendt, L.M.; Klebba, I.; Jones, A.D.; Rudnick, J.A.; DiMeo, T.A.; Gilmore, H.; Jefferson, D.M.; Graham, R.A.; et al. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res. 2010, 12, R87. Available online: https://link.springer.com/articles/10.1186/bcr2755 (accessed on 1 March 2022). [CrossRef] [PubMed]
- Rashid, O.; Takabe, K. Animal models for exploring the pharmacokinetics of breast cancer therapies. Expert Opin. Drug Metab. Toxicol. 2014, 11, 221–230. Available online: https://www.tandfonline.com/doi/abs/10.1517/17425255.2015.983073 (accessed on 15 March 2022). [CrossRef]
- Wen, J.; Ding, Y.; Zheng, S.; Li, X.; Xiao, Y. Sevoflurane suppresses glioma cell proliferation, migration, and invasion both in vitro and in vivo partially via regulating KCNQ1OT1/miR-146b-5p/STC1 axis. Cancer Biother Radiopharm. 2020. [CrossRef] [PubMed]
- Ren, J.; Wang, X.; Wei, G.; Meng, Y. Exposure to desflurane anesthesia confers colorectal cancer cells metastatic capacity through deregulation of miR-34a/LOXL3. Eur. J. Cancer Prev. 2020, 30, 143–153. [Google Scholar] [CrossRef]
- Chang, Q.; Wu, J.; An, Y.; Liu, H.; Sun, Y. Propofol suppresses proliferation, migration, invasion, and tumor growth of liver cancer cells via suppressing cancer susceptibility candidate 9/phosphatase and tensin homolog/AKT serine/threonine kinase/mechanistic target of rapamycin kinase axis. Hum. Exp. Toxicol. 2022, 41, 09603271211065972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lian, Y.; Xie, K.; Cai, Y.; Pan, Y.; Zhu, Y. Ropivacaine suppresses tumor biological characteristics of human hepatocellular carcinoma via inhibiting IGF-1R/PI3K/AKT/mTOR signaling axis. Bioengineered 2021, 12, 9162–9173. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Sun, T.; Liu, J.; Wang, X.; Zhao, G.; Shi, J.; Chen, Y. Isoflurane activates AMP-activated protein kinase to inhibit proliferation, and promote apoptosis and autophagy in cervical carcinoma both in vitro and in vivo. J. Recept. Signal Transduct. 2021, 41, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Ottewell, P.D.; Coleman, R.E.; Holen, I. From genetic abnormality to metastases: Murine models of breast cancer and their use in the development of anticancer therapies. Breast Cancer Res. Treat. 2005, 96, 101–113. Available online: https://link.springer.com/article/10.1007/s10549-005-9067-x (accessed on 15 March 2022). [CrossRef] [PubMed]
- Wigmore, T.; Farquhar-Smith, P. Opioids and cancer: Friend or foe? Curr. Opin. Support. Palliat. Care 2016, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lavon, H.; Matzner, P.; Benbenishty, A.; Sorski, L.; Rossene, E.; Haldar, R.; Ben-Eliyahu, S. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br. J. Anaesth. 2018, 120, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Crowley, P.; Foley, A.; Xue, C.; Connolly, C.; Gallagher, H.; Buggy, D. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br. J. Anaesth. 2018, 121, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Crowley, P.D.; Foley, A.G.; Gallagher, H.C.; Iwasaki, M.; Ma, D.; Buggy, D.J. Effect of Perioperative Lidocaine and Cisplatin on Metastasis in a Murine Model of Breast Cancer Surgery. Anticancer Res. 2018, 38, 5599–5606. Available online: https://ar.iiarjournals.org/content/38/10/5599 (accessed on 15 March 2022). [CrossRef] [PubMed]
- Wall, T.P.; Crowley, P.D.; Sherwin, A.; Foley, A.G.; Buggy, D.J. Effects of Lidocaine and Src Inhibition on Metastasis in a Murine Model of Breast Cancer Surgery. Cancers 2019, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Crowley, P.D.; Foley, A.G.; Gallagher, H.C.; Iwasaki, M.; Ma, D.; Buggy, D.J. Effect of Perioperative Lidocaine, Propofol and Steroids on Pulmonary Metastasis in a Murine Model of Breast Cancer Surgery. Cancers 2019, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G. Spontaneous tumors in dogs and cats: Models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990, 9, 125–136. Available online: https://link.springer.com/article/10.1007/BF00046339 (accessed on 15 March 2022). [CrossRef] [PubMed]
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Cancer 2008, 8, 147–156. Available online: https://www.nature.com/articles/nrc2273 (accessed on 7 February 2022). [CrossRef] [PubMed]
- Regua, A.T.; Arrigo, A.; Doheny, D.; Wong, G.L.; Lo, H.W. Transgenic mouse models of breast cancer. Cancer Lett. 2021, 516, 73–83. Available online: https://pubmed.ncbi.nlm.nih.gov/34090924/ (accessed on 7 February 2022). [CrossRef]
- Lampreht Tratar, U.; Horvat, S.; Cemazar, M. Transgenic Mouse Models in Cancer Research. Front. Oncol. 2018, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Eisener-Dorman, A.F.; Lawrence, D.A.; Bolivar, V.J. Cautionary insights on knockout mouse studies: The gene or not the gene? Brain Behav. Immun. 2009, 23, 318–324. [Google Scholar] [CrossRef]
- Nguyen, J.; Luk, K.; Vang, D.; Soto, W.; Vincent, L.; Robiner, S.; Saavedra, R.; Li, Y.; Gupta, P.; Gupta, K. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br. J. Anaesth. 2014, 113, i4–i13. Available online: https://academic.oup.com/bja/article/113/suppl_1/i4/2919828 (accessed on 1 April 2022). [CrossRef]
- Tarnowski, B.; Guruswami, S.; Wagner, U.; George, D.; Pandya, S.; Piparo, M.; Heiskanen, M. Improvements and additions to caMOD: Cancer Models Database. Cancer Res. 2007, 67, 3864. Available online: https://aacrjournals.org/cancerres/article/67/9_Supplement/3864/537992/Improvements-and-additions-to-caMOD-Cancer-Models (accessed on 1 April 2022).
| Classification | Description | Example |
|---|---|---|
| Spontaneous companion animals | Spontaneous cancers in household pets | Mammary carcinoma in dogs |
Spontaneous Transgenic
| Genetically engineered animals with specific mutations the precipitate the development of cancer during their normal lifespan | Mice with a rat C3(1) simian virus 40 large tumour antigen fusion gene |
Induced Transgenic
| Inducing genetic mutation via environmental triggers that precipitate cancer development | N-butyl-N-(4-hydroxybutyl) nitrosamine exposed mice & Tetracycline induced Cre recombinase gene expression system |
Induced Allograft
| Transplantation of cancer cells between animals of the same species that may (syngenic) or may not (non-syngenic) be genetically identical | 4T1 mouse cancer cells transplanted into Bagg Albino (BALB/c) mice |
Induced Xenograft
| Human cancer cells that may be commercially obtained or patient-specific (PDX) transplanted into other animals | Athymic nude mice Severely compromised immunodeficient (SCID) mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howle, R.; Moorthy, A.; Buggy, D.J. Investigating the Influence of Anaesthesiology for Cancer Resection Surgery on Oncologic Outcomes: The Role of Experimental In Vivo Models. Medicina 2022, 58, 1380. https://doi.org/10.3390/medicina58101380
Howle R, Moorthy A, Buggy DJ. Investigating the Influence of Anaesthesiology for Cancer Resection Surgery on Oncologic Outcomes: The Role of Experimental In Vivo Models. Medicina. 2022; 58(10):1380. https://doi.org/10.3390/medicina58101380
Chicago/Turabian StyleHowle, Ryan, Aneurin Moorthy, and Donal J. Buggy. 2022. "Investigating the Influence of Anaesthesiology for Cancer Resection Surgery on Oncologic Outcomes: The Role of Experimental In Vivo Models" Medicina 58, no. 10: 1380. https://doi.org/10.3390/medicina58101380
APA StyleHowle, R., Moorthy, A., & Buggy, D. J. (2022). Investigating the Influence of Anaesthesiology for Cancer Resection Surgery on Oncologic Outcomes: The Role of Experimental In Vivo Models. Medicina, 58(10), 1380. https://doi.org/10.3390/medicina58101380







