Implantoplasty Improves Clinical Parameters over a 2-Year Follow-Up: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Evaluation
2.3. Surgical Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 282–285. [Google Scholar] [CrossRef] [Green Version]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Merli, M.; Bernardelli, F.; Giulianelli, E.; Toselli, I.; Moscatelli, M.; Pagliaro, U.; Nieri, M. Inter-rater agreement in the diagnosis of mucositis and peri-implantitis. J. Clin. Periodontol. 2014, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implants 2014, 29, 325–345. [Google Scholar] [CrossRef] [Green Version]
- Khoshkam, V.; Del Amo, F.S.L.; Monje, A.; Lin, G.H.; Chan, H.L.; Wang, H.L. Long-term Radiographic and Clinical Outcomes of Regenerative Approach for Treating Peri-implantitis: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implants 2016, 31, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, M.; Craig, J.; Balkin, B.E.; Suzuki, J.B. Peri-implantitis: A Comprehensive Overview of Systematic Reviews. J. Oral Implantol. 2018, 44, 225–247. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin. Oral Implants Res. 2000, 11 (Suppl. 1), 146–155. [Google Scholar] [CrossRef]
- Rimondini, L.; Cicognani Simoncini, F.; Carrassi, A. Micro-morphometric assessment of titanium plasma-sprayed coating removal using burs for the treatment of peri-implant disease. Clin. Oral Implants Res. 2000, 11, 129–138. [Google Scholar] [CrossRef]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: Clinical outcome. Clin. Oral Implants Res. 2005, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Luso, S.; Mueller, C.; Ender, A.; Attin, T.; Stawarczyk, B.; Schmidin, P. Titanium Implant Characteristics After Implantoplasty: An In Vitro Study on Two Different Kinds of Instrumentation. Int. J. Oral Maxillofac. Implants 2019, 34, 1299–1305. [Google Scholar] [CrossRef]
- Ramel, C.F.; Lussi, A.; Ozcan, M.; Jung, R.E.; Hammerle, C.H.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B.; Haas, R.; Mailath-Pokorny, G.; Furhauser, R.; Watzek, G.; Busenlechner, D.; Muller-Kern, M.; Kloodt, C. Periimplantitis Treatment: Long-Term Comparison of Laser Decontamination and Implantoplasty Surgery. Implant Dent. 2016, 25, 646–649. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S278–S285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral Implants Res. 2007, 18, 161–170. [Google Scholar] [CrossRef]
- Loe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Lang, N.P.; Tonetti, M.S. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral Health Prev. Dent. 2003, 1, 7–16. [Google Scholar]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Lang, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T. Working Group 4 of Seventh European Workshop on P. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 178–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englezos, E.; Cosyn, J.; Koole, S.; Jacquet, W.; De Bruyn, H. Resective Treatment of Peri-implantitis: Clinical and Radiographic Outcomes After 2 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S152–S157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemtov-Yona, K.; Rittel, D.; Levin, L.; Machtei, E.E. Effect of dental implant diameter on fatigue performance. Part I: Mechanical behavior. Clin. Implant Dent. Relat. Res. 2014, 16, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Costa-Berenguer, X.; Garcia-Garcia, M.; Sanchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellon, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implants Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: A 7-year follow-up observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef]
- Schwarz, F.; John, G.; Becker, J. The influence of implantoplasty on the diameter, chemical surface composition, and biocompatibility of titanium implants. Clin. Oral Investig. 2017, 21, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Oh, W.S.; Ong, H.S.; Fu, J.H.; Steigmann, M.; Sierraalta, M.; Wang, H.L. Impact of implantoplasty on strength of the implant-abutment complex. Int. J. Oral maxillofac. Implants. 2013, 28, 1530–1535. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Junior, J.S.A.; Dedavid, B.A.; Shibli, J.A. Analysis of Implant Strength After Implantoplasty in Three Implant-Abutment Connection Designs: An In Vitro Study. Int. J. Oral Maxillofac. Implants. 2016, 31, e65–e70. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Silva, G.L.; Cortelli, J.R.; Costa, J.E.; Costa, F.O. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J. Clin. Periodontol. 2006, 33, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Strom, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implants Res. 2009, 20, 169–174. [Google Scholar] [CrossRef]
- Costa, F.O.; Ferreira, S.D.; Cortelli, J.R.; Lima, R.P.E.; Cortelli, S.C.; Cota, L.O.M. Microbiological profile associated with peri-implant diseases in individuals with and without preventive maintenance therapy: A 5-year follow-up. Clin. Oral Investig. 2019, 23, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- Dalago, H.R.; Filho, G.S.; Rodrigues, M.A.; Renvert, S.; Bianchini, M.A. Risk indicators for Peri-implantitis. A cross-sectional study with 916 implants. Clin. Oral Implants Res. 2017, 28, 144–150. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 292–304. [Google Scholar] [CrossRef] [PubMed]
- Aglietta, M.; Siciliano, V.I.; Rasperini, G.; Cafiero, C.; Lang, N.P.; Salvi, G.E. A 10-year retrospective analysis of marginal bone-level changes around implants in periodontally healthy and periodontally compromised tobacco smokers. Clin. Oral Implants Res. 2011, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Implantoplasty Versus Glycine Air Abrasion for the Surgical Treatment of Peri-implantitis: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implants 2020, 35, 197–206. [Google Scholar] [CrossRef]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar]
- Quirynen, M.; van der Mei, H.C.; Bollen, C.M.; Schotte, A.; Marechal, M.; Doornbusch, G.I.; Naert, I.; Busscher, H.J.; van Steenberghe, D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J. Dent. Res. 1993, 72, 1304–1309. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Iglhaut, G.; Becker, J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: A randomized controlled clinical study. J. Clin. Periodontol. 2011, 38, 276–284. [Google Scholar] [CrossRef]
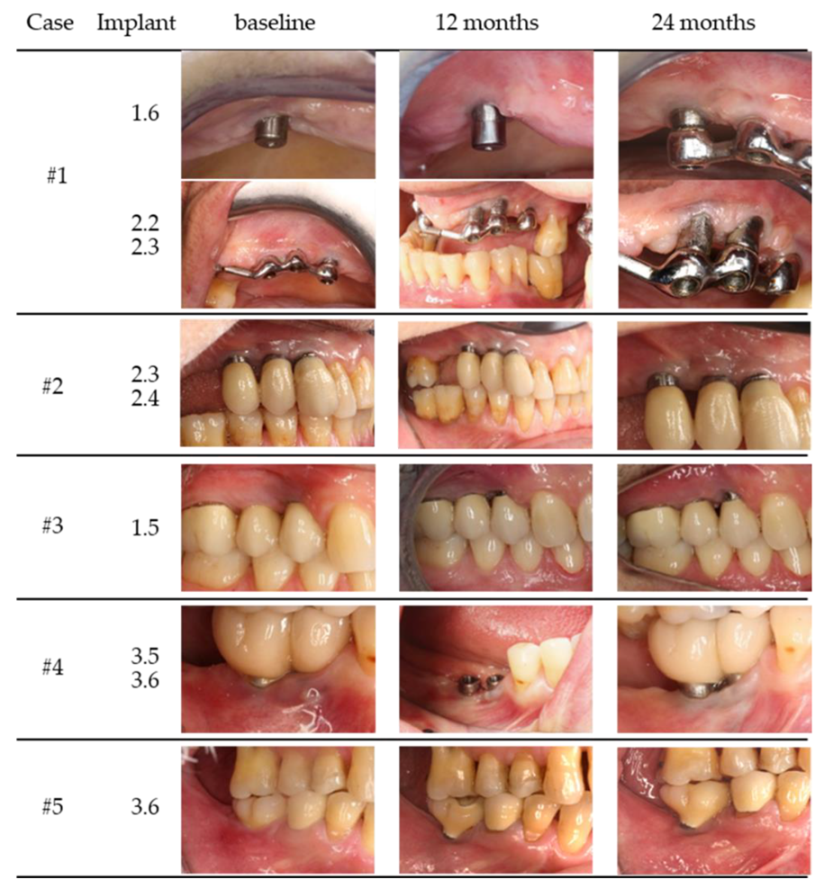
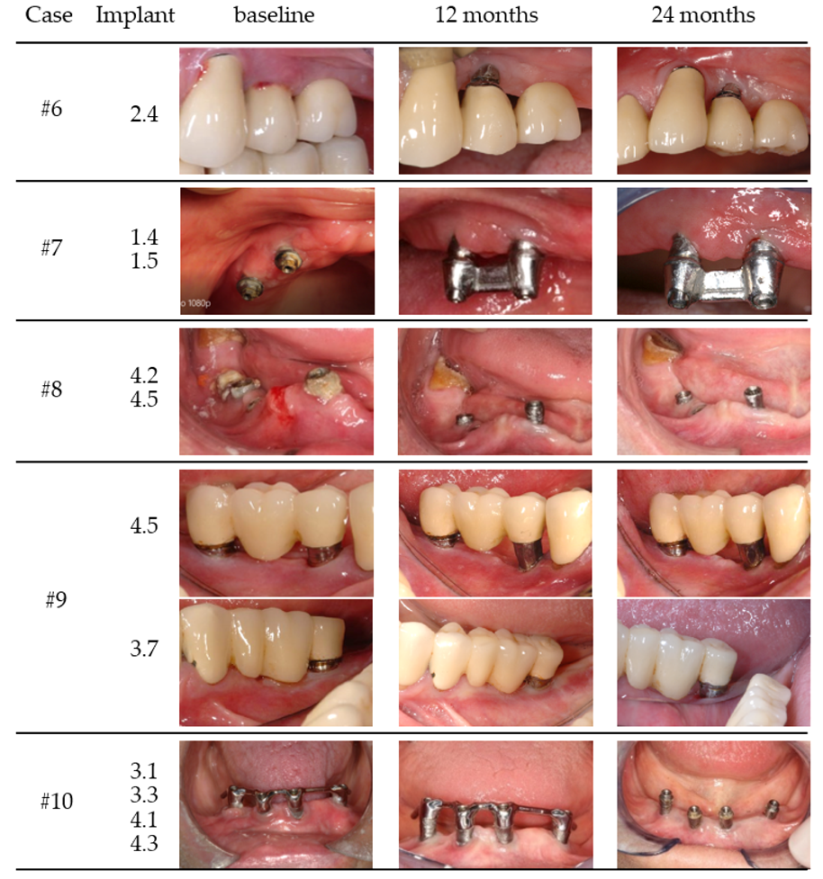
| Case | Gender/Age | Smoking Status (Y/N) | Periodontal Disease (Y/N) | Health Status | Implant Position | Years in Function | Prosthesis/Fully (FE) or Partially (PE) Edentulous | Total Number of Implants (Position) |
|---|---|---|---|---|---|---|---|---|
| #1 | female/62 year | N | Y | Healthy | 1.6 | 6 | bar overdenture/PE | 6 |
| 2.2 | 8 | (1.3, 1.4, 1.6, 2.2, 2.3, 2.6) | ||||||
| 2.3 | ||||||||
| #2 | female/66 year | N | Y | Healthy | 2.3 | 9 | cemented single unit/PE | 10 |
| 2.4 | 9 | (1.4, 1.5, 1.6, 2.3, 2.4, 2.5, 3.6, 3.7, 4.6, 4.7) | ||||||
| #3 | male/47 year | Y | Y | Heathy | 1.5 | 5 | cemented single unit/PE | 2 |
| (5 cig/d) | (1.4, 1.5) | |||||||
| #4 | female/51 year | N | N | Healthy | 3.5 | 6 | screw type fixed partial/PE | 2 |
| 3.6 | 6 | (3.5, 3.6) | ||||||
| #5 | male/60 year | N | Y | Healthy | 3.6 | 4 | screw type single unit/PE | 2 |
| (3.6, 4.6) | ||||||||
| #6 | male/65 year | N | Y | Healthy | 2.4 | 7 | Cemented bridge/PE | 4 (1.4, 1.5, 2.3, 2.4) |
| #7 | female/75 year | N | Y | Anti-HT | 1.4 | 6 | bar overdenture/FE | 6 |
| 1.5 | 6 | (1.5, 1.4, 2.4, 2.5, 3.2, 4.2) | ||||||
| #8 | male/43 year | N | Y | Healthy | 4.2 | 5 | hybrid overdenture/FE | 13 |
| 4.5 | 5 | (1.1, 1.2, 1.4, 2.3, 2.4, 2.7, 2.8, 3.1, 3.4, 3.6, 4.2, 4.5, 4.6) | ||||||
| #9 | female/60 year | Y | Y | Anti-coagulation | 4.5 | 8 | cemented bridge/PE | 3 |
| (9 cig/d) | 3.7 | 9 | Single unit but bridge/PE | (3.7, 4.5, 4.7) | ||||
| #10 | female/80 year | N | Y | Healthy | 3.3 | 8 | bar overdenture/FE | |
| 3.1 | 4 | |||||||
| 4.1 | (3.3, 3.1, 4.3, 4.1) | |||||||
| 4.3 | ||||||||
| Mean ± SD | 6.95 ± 1.50 | |||||||
| Parameters | Baseline | 12 Months | 24 Months | |
|---|---|---|---|---|
| PD (mm) | 5.37 ± 0.86 | 2.9 ± 0.39 | 2.85 ± 0.45 | |
| min/max | 4.17/7.50 | 2.50/3.50 | 2.08/3.67 | |
| IC95% | [4.75; 5.98] | [2.63; 3.18] | [2.53; 3.18] | |
| p | 0.005 | |||
| p | 0.796 | |||
| p | 0.05 | |||
| treatment effect | −2.52 IC95% [−3.17; −1.86] | |||
| MR (mm) | 0.69 ± 0.99 | 1.96 ± 1.33 | 1.94 ± 1.48 | |
| min/max | 0/2.92 | 0.17/4.17 | 0.17/4.67 | |
| IC95% | [−0.01; 1.4] | [1; 2.91] | [0.88; 3] | |
| p | 0.005 | |||
| p | 0.673 | |||
| p | 0.005 | |||
| treatment effect | 1.25 IC95% [0.51; 1.98] | |||
| BoP (%) | 0.12 ± 0.06 | 0.01 ± 0.01 | 0.01 ± 0.01 | |
| min/max | 0.01/0.17 | 0.00/0.04 | 0.00/0.03 | |
| IC95% | [0.08; 0.17] | [0.00; 0.02] | [0.00; 0.02] | |
| p | 0.005 | |||
| p | 0.344 | |||
| p | 0.005 | |||
| treatment effect | −0.11 IC95% [−0.15; −0.07] | |||
| Sup (%) | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| min/max | 0.00/0.03 | 0.00/0.00 | 0.00/0.00 | |
| IC95% | [0.00; 0.02] | [0.00; 0.00] | [0.00; 0.00] | |
| p | 0.027 | |||
| p | 1.000 | |||
| p | 0.027 | |||
| treatment effect | −0.01 IC95% [−0.02; 0.00] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, O.; Sahrmann, P.; Ramos, J.; Caramelo, F.; Matos, S.; Baptista, I.P. Implantoplasty Improves Clinical Parameters over a 2-Year Follow-Up: A Case Series. Medicina 2022, 58, 113. https://doi.org/10.3390/medicina58010113
Martins O, Sahrmann P, Ramos J, Caramelo F, Matos S, Baptista IP. Implantoplasty Improves Clinical Parameters over a 2-Year Follow-Up: A Case Series. Medicina. 2022; 58(1):113. https://doi.org/10.3390/medicina58010113
Chicago/Turabian StyleMartins, Orlando, Philipp Sahrmann, João Ramos, Francisco Caramelo, Sérgio Matos, and Isabel Poiares Baptista. 2022. "Implantoplasty Improves Clinical Parameters over a 2-Year Follow-Up: A Case Series" Medicina 58, no. 1: 113. https://doi.org/10.3390/medicina58010113
APA StyleMartins, O., Sahrmann, P., Ramos, J., Caramelo, F., Matos, S., & Baptista, I. P. (2022). Implantoplasty Improves Clinical Parameters over a 2-Year Follow-Up: A Case Series. Medicina, 58(1), 113. https://doi.org/10.3390/medicina58010113







