The Pulp Stones: Morphological Analysis in Scanning Electron Microscopy and Spectroscopic Chemical Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. SEM and EDX Analysis
3. Results
3.1. Sample 01
3.2. Sample 02
3.3. Sample 03
3.4. Sample 04
3.5. Sample 05
3.6. Sample 06
3.7. Sample 07
3.8. EDX Chemical Quantification
4. Discussion
5. Conclusions
- −
- Most of the mineralizations found in the pulp chambers presented nodular morphology, while those located in the root canals presented a diffuse shape, resembling the anatomy of the root canals.
- −
- The topography of the nodules showed heterogeneous relief, revealing smooth and compact areas contrasting with the rugged and porous ones.
- −
- The chemical composition of the mineralizations varied depending on the location of the nodule in the pulp cavity and the relief of the analyzed area. Root mineralizations presented considerably lower calcium and phosphorus content than coronal nodules.
- −
- A careful study of the literature may lead to a conclusion that the high cellularity rate of the coronal pulp predisposes this region to nodular mineralizations around injured cells, whereas the presence of larger caliber vascular bundles and higher collagen fiber content in the root pulp determines a diffuse morphological pattern in this region.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goga, R.; Chandler, N.P.; Oginni, A.O. Pulp stones: A review. Int. Endod. J. 2008, 41, 457–468. [Google Scholar] [CrossRef]
- Jannati, R.; Afshari, M.; Moosazadeh, M.; Allahgholipour, S.Z.; Eidy, M.; Hajihoseini, M. Prevalence of pulp stones: A systematic review and meta-analysis. J. Evid. Based Med. 2019, 12, 133–139. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Wu, Y.C.; Su, C.C.; Chung, M.P.; Huang, R.Y.; Ting, P.Y.; Lai, C.K.; Chang, K.S.; Tsai, Y.C.; Shieh, Y.S. The prevalence and distribution of radiopaque, calcified pulp stones: A cone-beam computed tomography study in a northern Taiwanese population. J. Dent. Sci. 2018, 13, 138–144. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.J.N.L.; Prado, M.C.; Queiroz, P.M.; Nejaim, Y.; Brasil, D.M.; Groppo, F.C.; Neto, F.H. Assessing pulp stones by cone-beam computed tomography. Clin. Oral Investig. 2017, 21, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehab. 2017, 44, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Deva, V.; Mogoantă, L.; Pancă, O.A.; Vătu, M.; Vătăman, M. Radiological and microscopic aspects of the denticles. Rom. J. Morphol. Embryo 2006, 47, 263–268. [Google Scholar]
- Schaffner, M.; Stich, H.; Lussi, A. Denticles: Dental pulp calculi. Swiss Dent. J. 2014, 124, 416–417. [Google Scholar]
- Foreman, P.C. Micromorphology of mineralization deposits in the pulps of human teeth. Int. Endod. J. 1984, 17, 183–191. [Google Scholar] [CrossRef]
- Le May, O.; Kaqueler, J.C. Electron probe micro-analysis of human dental pulp stones. Scann. Microsc. 1993, 7, 67–71; discussion 271–272. [Google Scholar]
- Moss-Salentijn, L.; Klyvert, M.H. Epithelially induced denticles in the pulps of recently erupted, noncarious human premolars. J. Endod. 1983, 9, 554–560. [Google Scholar] [CrossRef]
- Hillmann, G.; Geurtsen, W. Light-microscopical investigation of the distribution of extracellular matrix molecules and calcifications in human dental pulps of various ages. Cell Tissue Res. 1997, 289, 145–154. [Google Scholar] [CrossRef]
- Berès, F.; Isaac, J.; Mouton, L.; Rouzière, S.; Berdal, A.; Simon, S.; Dessombz, A. Comparative physicochemical analysis of pulp stone and dentin. J. Endod. 2016, 42, 432–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabardo, M.C.L.; Wambier, L.M.; Rocha, J.S.; Küchler, E.C.; de Lara, R.M.; Leonardi, D.P.; Sousa-Neto, M.D.; Baratto-Filho, F.; Michel-Crosato, E. Association between pulp stones and kidney stones: A systematic review and meta-analysis. J. Endod. 2019, 45, 1099–1105. [Google Scholar] [CrossRef]
- Movahhedian, N.; Haghnegahdar, A.; Owji, F. How the prevalence of pulp stone in a population predicts the risk for kidney stone. Iran. Endod. J. 2018, 13, 246–250. [Google Scholar]
- Bains, S.K.; Bhatia, A.; Singh, H.P.; Biswal, S.S.; Kanth, S.; Nalla, S. Prevalence of coronal pulp stones and its relation with systemic disorders in northern Indian central punjabi population. ISRN Dent. 2014, 22, 617590. [Google Scholar] [CrossRef]
- Yeluri, G.; Kumar, C.A.; Raghav, N. Correlation of dental pulp stones, carotid artery and renal calcifications using digital panoramic radiography and ultrasonography. Contemp. Clin. Dent. 2015, 6, S147–S151. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; Wang, W.; Pfander, D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J. Bone Min. Res. 2003, 18, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatyńska-Ulatowska, A.; Pietrzycka, K.; Koprowicz, A. Denticles of the pulp chamber—Diagnostics and management. Case studies. Pomeranian J. Life Sci. 2019, 65, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Sundell, J.R.; Stanley, H.R.; White, C.L. The relationship of coronal pulp stone formation to experimental operative procedures. Oral Surg. Oral Med. Oral Pathol. 1968, 25, 579–589. [Google Scholar] [CrossRef]
- Vibhute, N.A.; Vibhute, A.H.; Rajendra, D.T.; Bansal, P.P.; Mahalle, A. Hard Facts about Stones: Pulpal Calcifications: A Review. J. Patient Care 2016, 2, 105. [Google Scholar] [CrossRef]
- Consolaro, A. Biologia e Patologia da Polpa e Periápice Para as Especialidades Clínicas; Dental Press: Maringá, Brazil, 2018. [Google Scholar]
- Sener, S.; Cobankara, F.K.; Akgünlü, F. Calcifications of the pulp chamber: Prevalence and mplicated factors. Clin. Oral Investig. 2009, 13, 209–215. [Google Scholar] [CrossRef]
- Kannan, S.; Kannepady, S.K.; Muthu, K.; Jeevan, M.B.; Thapasum, A. Radiographic assessment of the prevalence of pulp stones in Malaysians. J. Endod. 2015, 41, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Bolean, M.; Simão, A.M.; Barioni, M.B.; Favarin, B.Z.; Sebinelli, H.G.; Veschi, E.A.; Janku, T.A.; Bottini, M.; Hoylaerts, M.F.; Itri, R.; et al. Biophysical aspects of biomineralization. Biophys. Rev. 2017, 9, 747–760. [Google Scholar] [CrossRef] [Green Version]
- Caviedes-Bucheli, J.; Correa-Ortíz, J.A.; García, L.V.; López-Torres, R.; Lombana, N.; Muñoz, H.R. The effect of cavity preparation on substance P expression in human dental pulp. J. Endod. 2005, 31, 857–859. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Azuero-Holguin, M.M.; Correa-Ortiz, J.A.; Aguilar-Mora, M.V.; Pedroza-Flores, J.D.; Ulate, E.; Lombana, N.; Munoz, H.R. Effect of experimentally induced occlusal trauma on substance p expression in human dental pulp and periodontal ligament. J. Endod. 2011, 37, 627–630. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.Q.; Jiang, L.; Peng, W.; Ritchie, H.H. Hypoxia promotes mineralization of human dental pulp cells. J. Endod. 2011, 37, 799–802. [Google Scholar] [CrossRef]
- Burke, F.M.; Samarawickrama, D.Y. Progressive changes in the pulpo-dentinal complex and their clinical consequences. Gerodontology 1995, 12, 57–66. [Google Scholar] [CrossRef]
- Ikawa, M.; Komatsu, H.; Ikawa, K.; Mayanagi, H.; Shimauchi, H. Age-related changes in the human pulpal blood flow measured by laser Doppler flowmetry. Dent. Traumatol. 2003, 19, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Tranasi, M.; Sberna, M.T.; Zizzari, V.; D’Apolito, G.; Mastrangelo, F.; Salini, L.; Stuppia, L.; Tetè, S. Microarray evaluation of age-related changes in human dental pulp. J. Endod. 2009, 35, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T. Determinants of pathologic mineralization. Crit. Rev. Eukar. Gene 2008, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T. Biomineralization-an active or passive process? Connect. Tissue Res. 2012, 53, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Martel, J.; Cheng, W.Y.; He, C.C.; Ojcius, D.M.; Young, J.D. Membrane vesicles nucleate mineralo-organic nanoparticles and induce carbonate apatite precipitation in human body fluids. J. Biol. Chem. 2013, 18, 30571–30584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; Yamamoto, T.; Tsuchiya, E.; Hongo, H.; Tsuboi, K.; Kudo, A.; Abe, M.; Yoshida, T.; Nagai, T.; Khadiza, N.; et al. Ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization. Jpn. Dent. Sci. Rev. 2017, 53, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef]
- Huitema, L.F.; Vaandrager, A.B. What triggers cell-mediated mineralization? Front. Biosci. 2007, 1, 2631–2645. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Roszkowska, M.; Strzelecka-Kiliszek, A.; Magne, D.; Pikula, S.; Bessueille, L. Membranes and pathophysiological mineralization. Postępy Biochem. 2016, 62, 511–517. [Google Scholar]
- Le May, O.; Kaqueler, J.C. Scanning electron microscopic study of pulp stones in human permanent teeth. Scanning Microsc. 1991, 5, 257–267. [Google Scholar]
- Taylor, E.R.; Stoller, M.L. Vascular theory of the formation of Randall plaques. Urolithiasis 2015, 43, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Yiu, A.J.; Callaghan, D.; Sultana, R.; Bandyopadhyay, B.C. Vascular Calcification and Stone Disease: A New Look towards the Mechanism. J. Cardiovasc. Dev. Dis. 2015, 2, 141–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caviedes-Bucheli, J.; Gomez-Sosa, J.F.; Azuero-Holguin, M.M.; Ormeño-Gomez, M.; Pinto-Pascual, V.; Munoz, H.R. Angiogenic mechanisms of human dental pulp and their relationship with substance P expression in response to occlusal trauma. Int. Endod. J. 2017, 50, 339–351. [Google Scholar] [CrossRef]
- Chen, Q.; Bei, J.J.; Liu, C.; Feng, S.B.; Zhao, W.B.; Zhou, Z.; Yu, Z.P.; Du, X.J.; Hu, H.Y. HMGB1 induces secretion of matrix vesicles by macrophages to enhance ectopic mineralization. PLoS ONE 2016, 11, 156686. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninomiya, M.; Ohishi, M.; Kido, J.; Ohsaki, Y.; Nagata, T. Immunohistochemical localization of osteopontin in human pulp stones. J. Endod. 2001, 27, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Aparecida, A.H.; Fook, M.V.L.; Santos, M.L.; Guastaldi, A.C. Estudo da influência dos íons K+, Mg2+, SO4(2−) e CO3(2−) na cristalização biomimética de fosfato de cálcio amorfo (ACP) e conversão a fosfato octacálcico (OCP). Quim. Nova 2007, 30, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Aoba, T.; Ebisu, S.; Yagi, T. A study of the mineral phase of pulp calcification. J. Oral Pathol. 1980, 9, 129–136. [Google Scholar] [CrossRef]
- Trowbridge, H.O. Pulp biology: Progress during the past 25 years. Aust. Endod. J. 2003, 29, 5–12. [Google Scholar] [CrossRef]
- Arys, A.; Jedwab, J.; Pireaux, J.J.; Philippart, C.; Dourow, N. Brushite in the pulp of primary molars. J. Oral Pathol. Med. 1989, 18, 371–376. [Google Scholar] [CrossRef]
- Danilchenko, S.N.; Kuznetsov, V.N.; Stanislavov, A.S.; Kalinkevich, A.N.; Starikov, V.V.; Moskalenko, R.A.; Kalinichenko, T.G.; Kochenko, A.V.; Lü, J.; Shang, J.; et al. The mineral component of human cardiovascular deposits: Morphological, structural and crystal-chemical characterization. Cryst. Res. Technol. 2013, 48, 153–162. [Google Scholar] [CrossRef]
- Simpson, D.R. Problems of the composition and structure of the bone minerals. Clin. Orthop. Relat. Res. 1972, 86, 260–286. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Uitto, J. Ectopic mineralization disorders of the extracellular matrix of connective tissue: Molecular genetics and pathomechanisms of aberrant calcification. Matrix Biol. 2014, 33, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaRusso, J.; Li, Q.; Jiang, Q.; Uitto, J. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum. J. Investig. Dermatol. 2009, 129, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
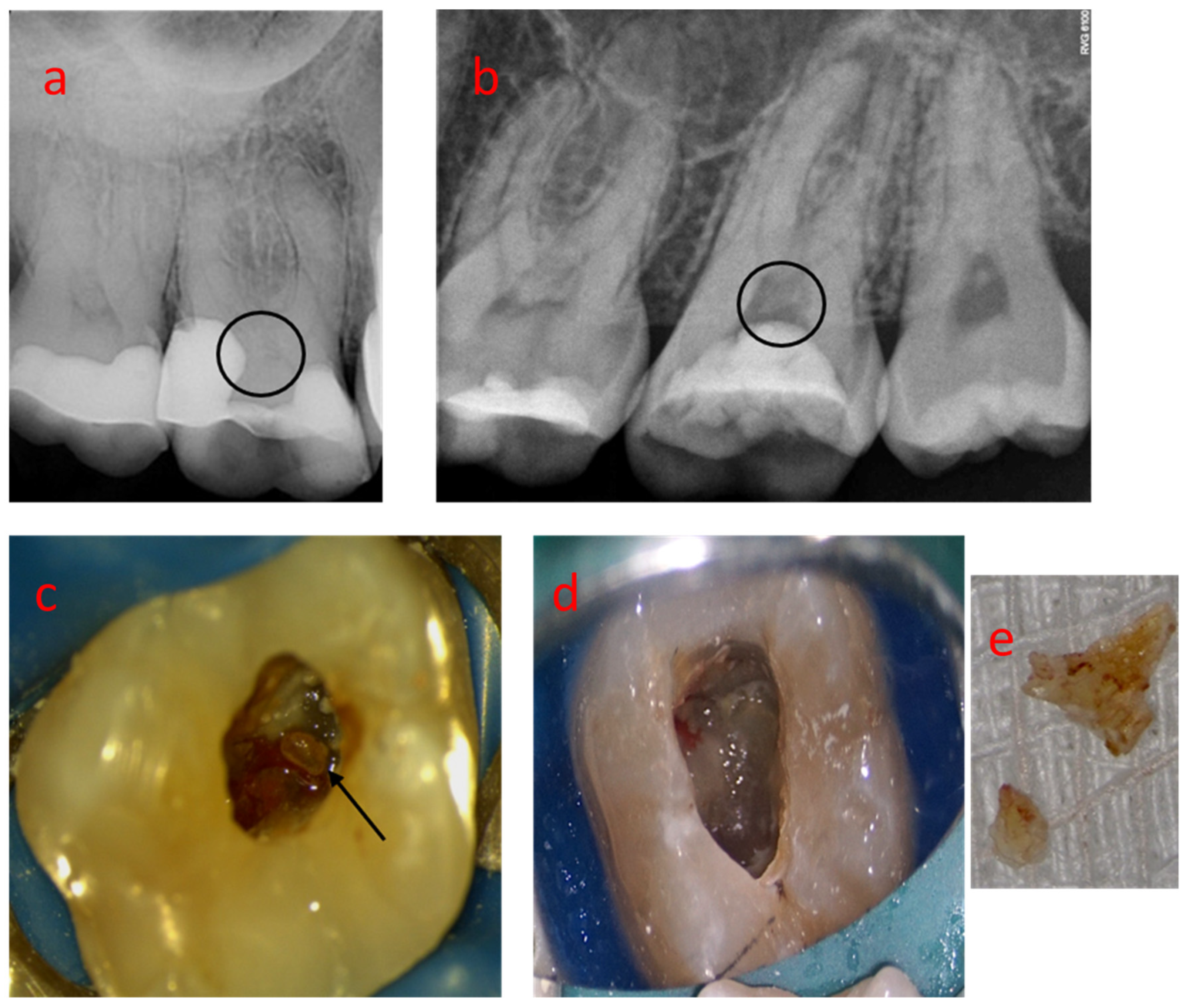
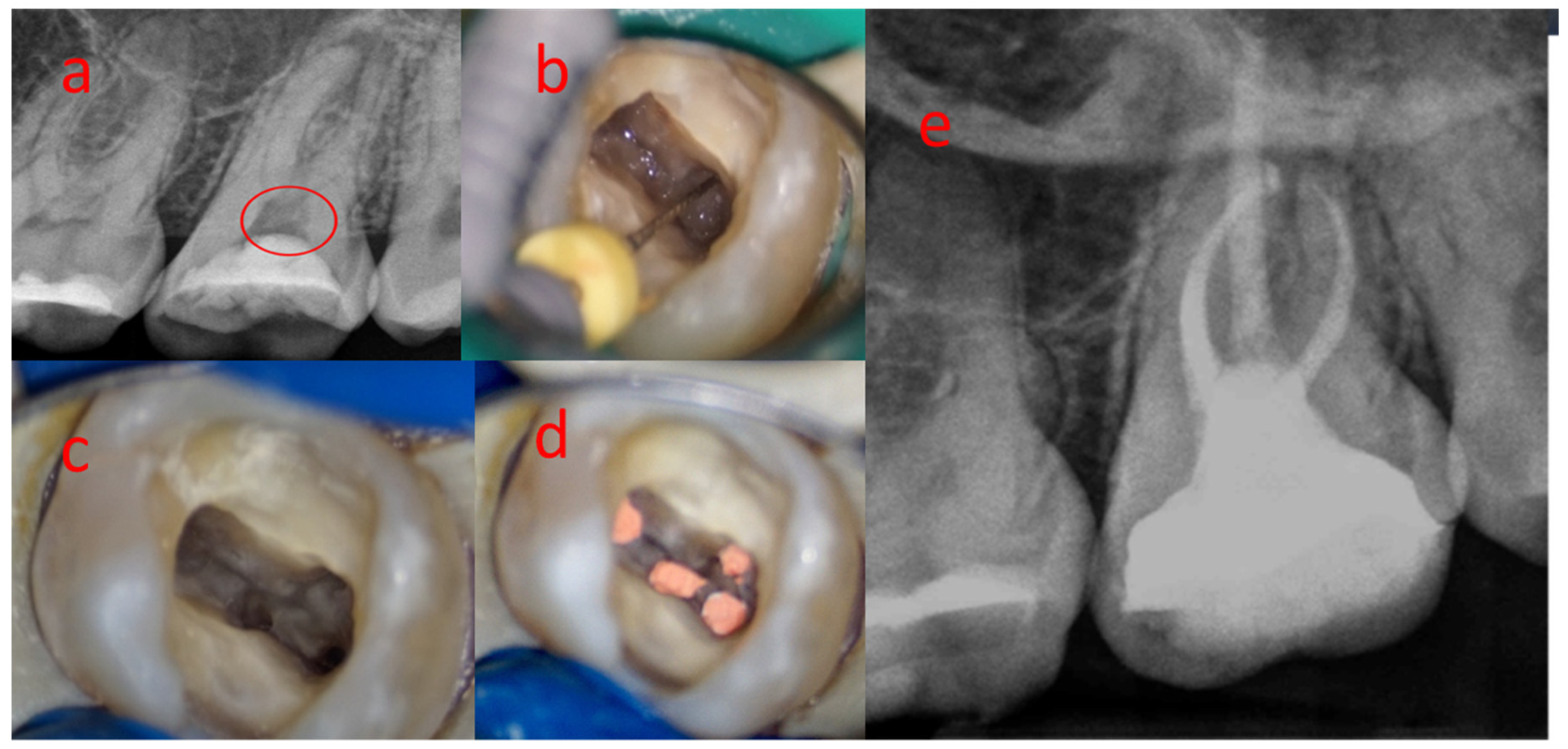

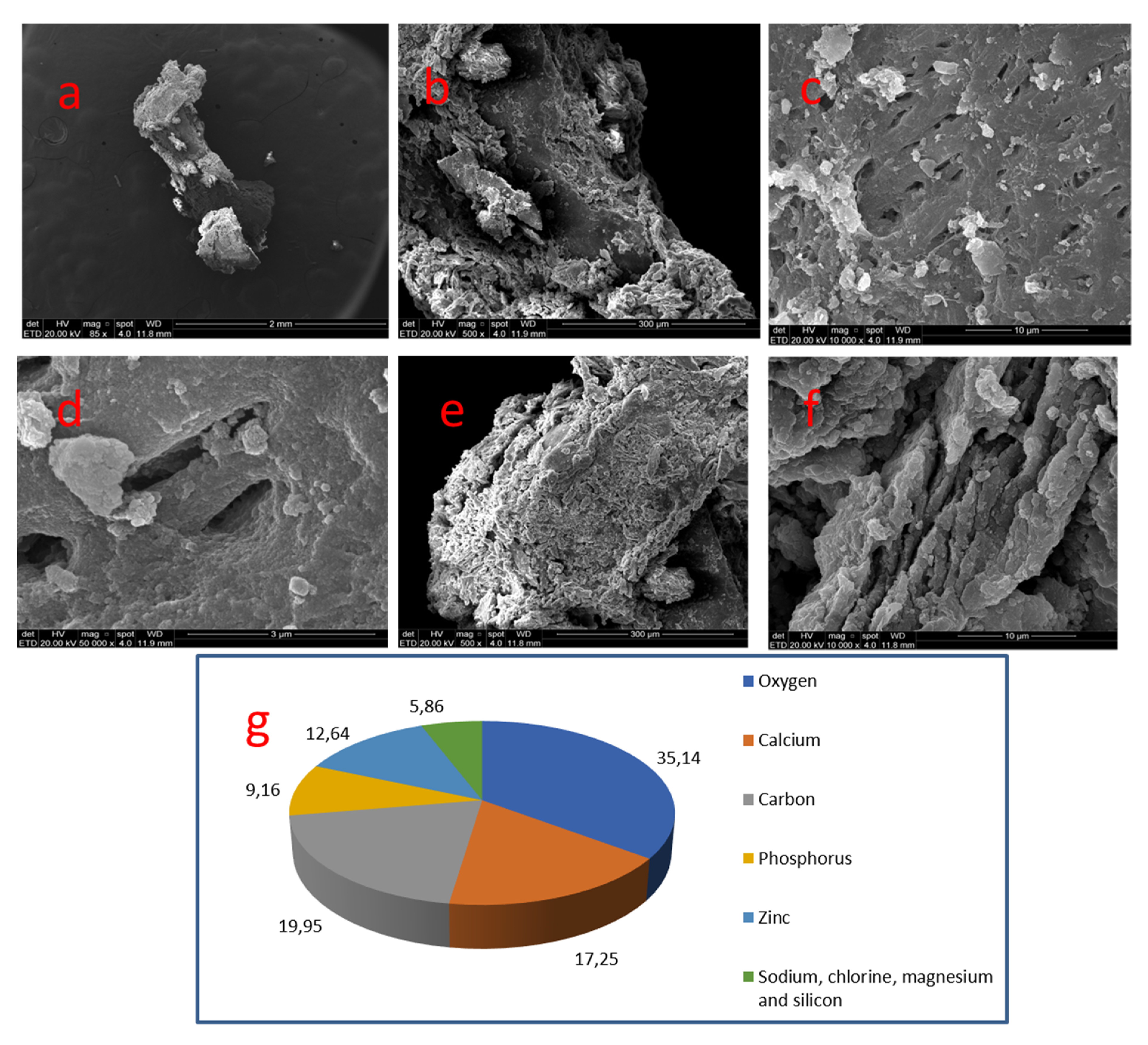
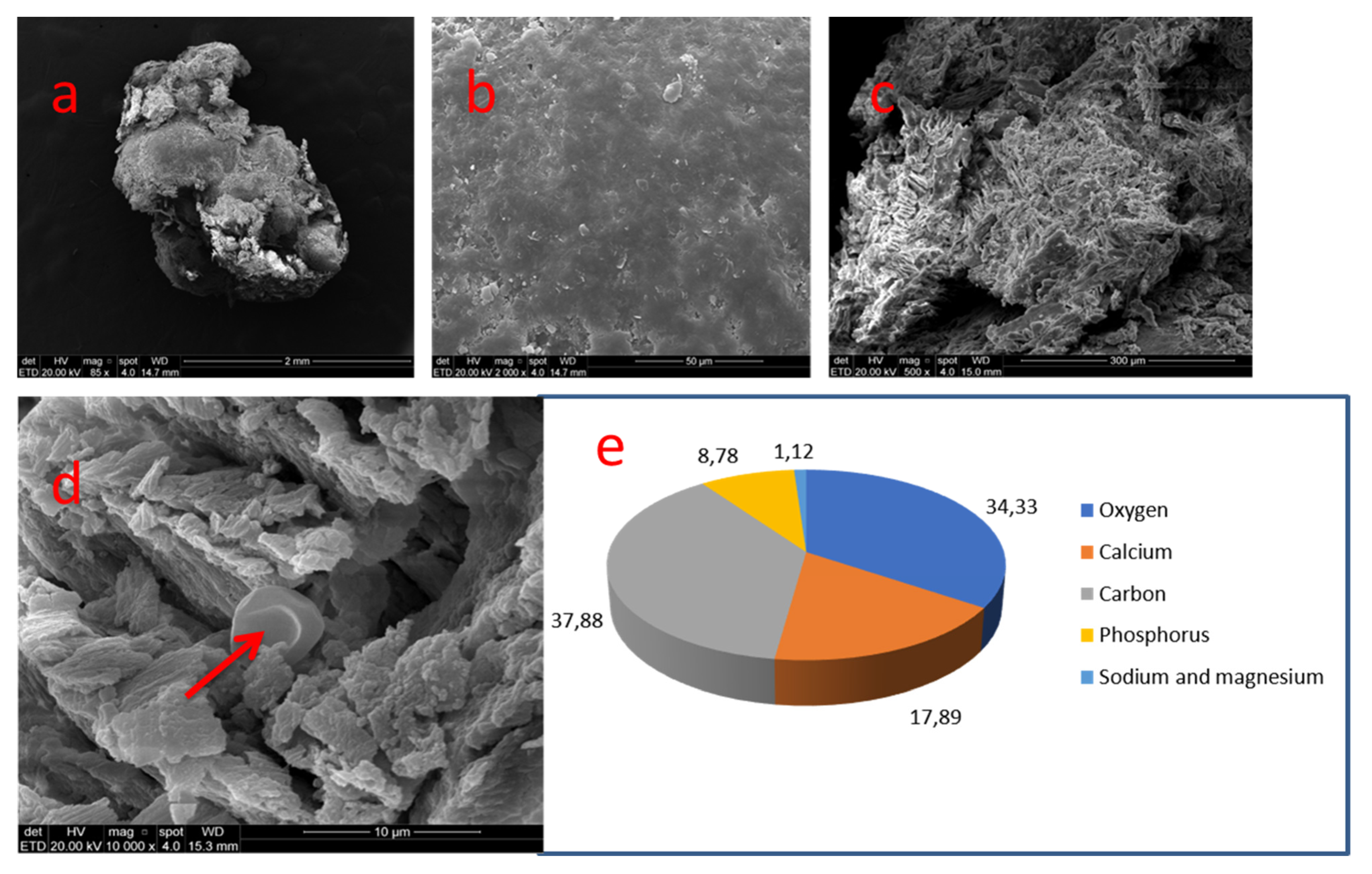

| Nº Nodule | Location | Dentin Wall Relationship | Morphology | Size | Predominant Topography |
|---|---|---|---|---|---|
| 01 | Coronal pulp | Free | Difuse (ovoid fragment) | 2.00 mm (diameter) | Irregular |
| 02 | Coronal pulp | Adhered | Nodular (elongated ovoid) | 2.00 × 0.5 mm | Irregular |
| 03 | Coronal pulp | Free | Nodular (ovoid) | 2.00 mm (diameter) | Irregular |
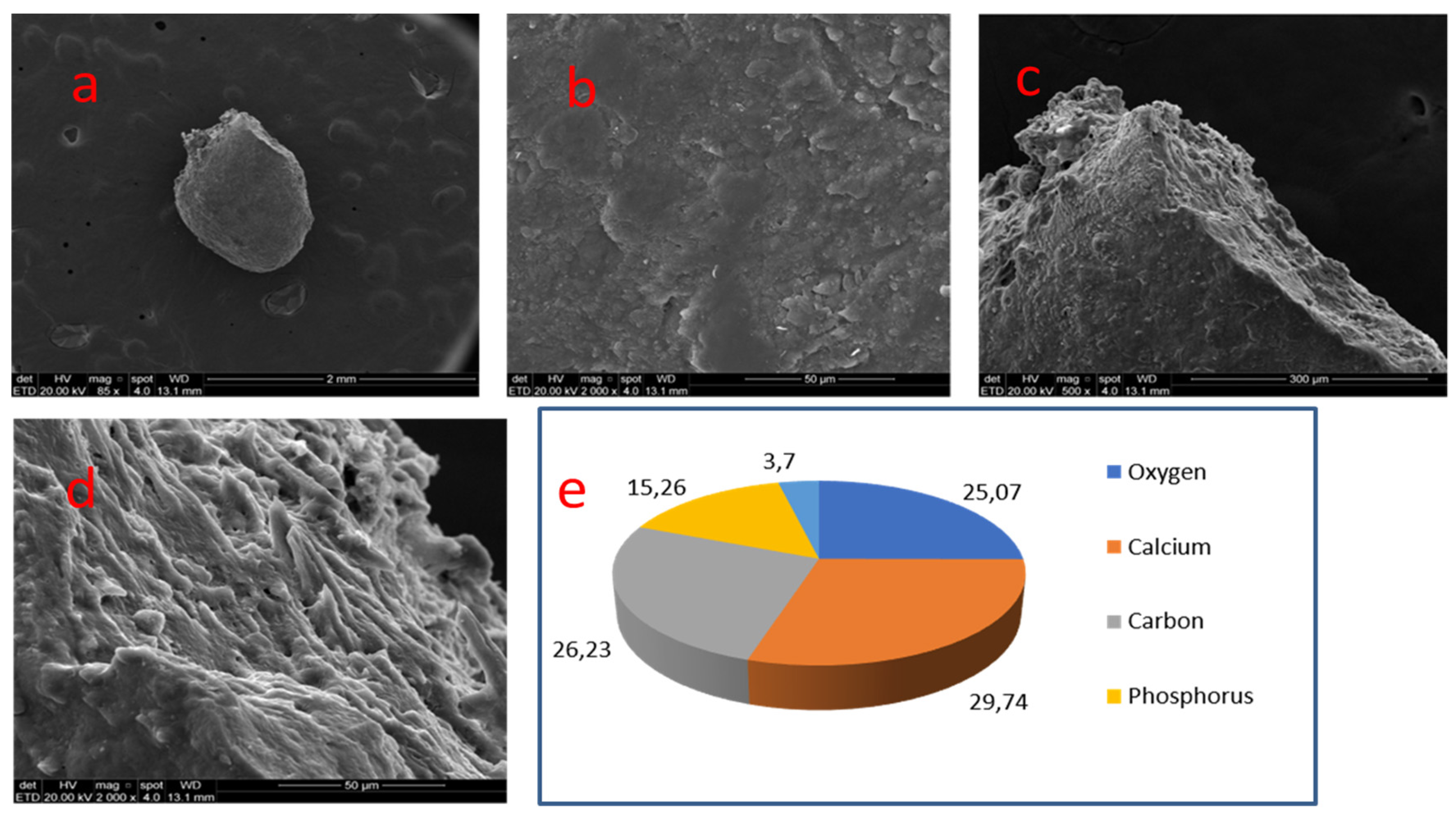
| 04 | Coronal pulp | Undefined | Nodular (ovoid) | 1.0 mm (diameter) | Smooth and compact |
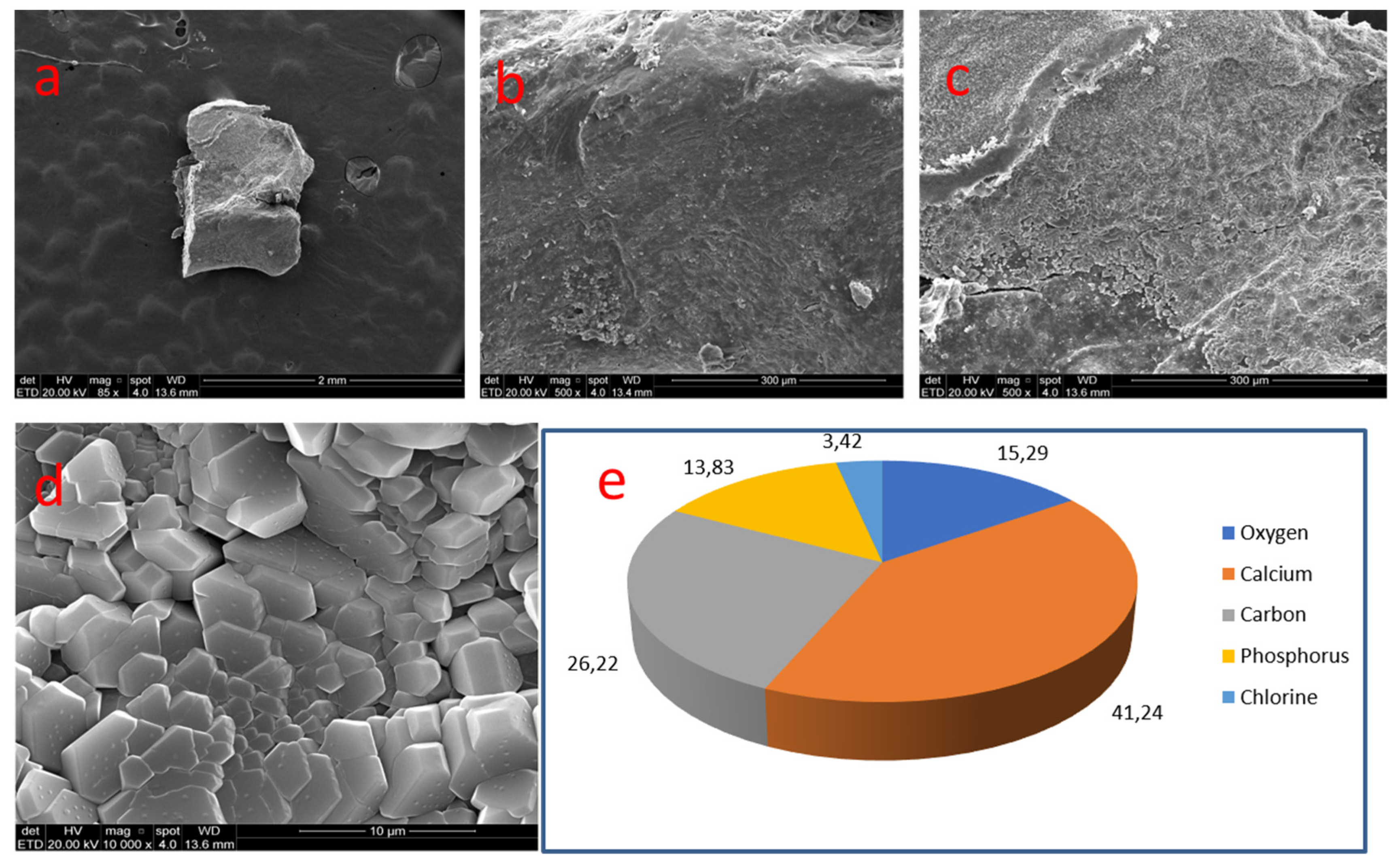
| 05 | Coronal pulp | Undefined | Nodular (rectangular) | 1.0 mm (diameter) | Smooth and compact |
| 06 | Root canal | Adhered | Cylindric (string) | 4.0 mm (length) | Irregular grooved surface |
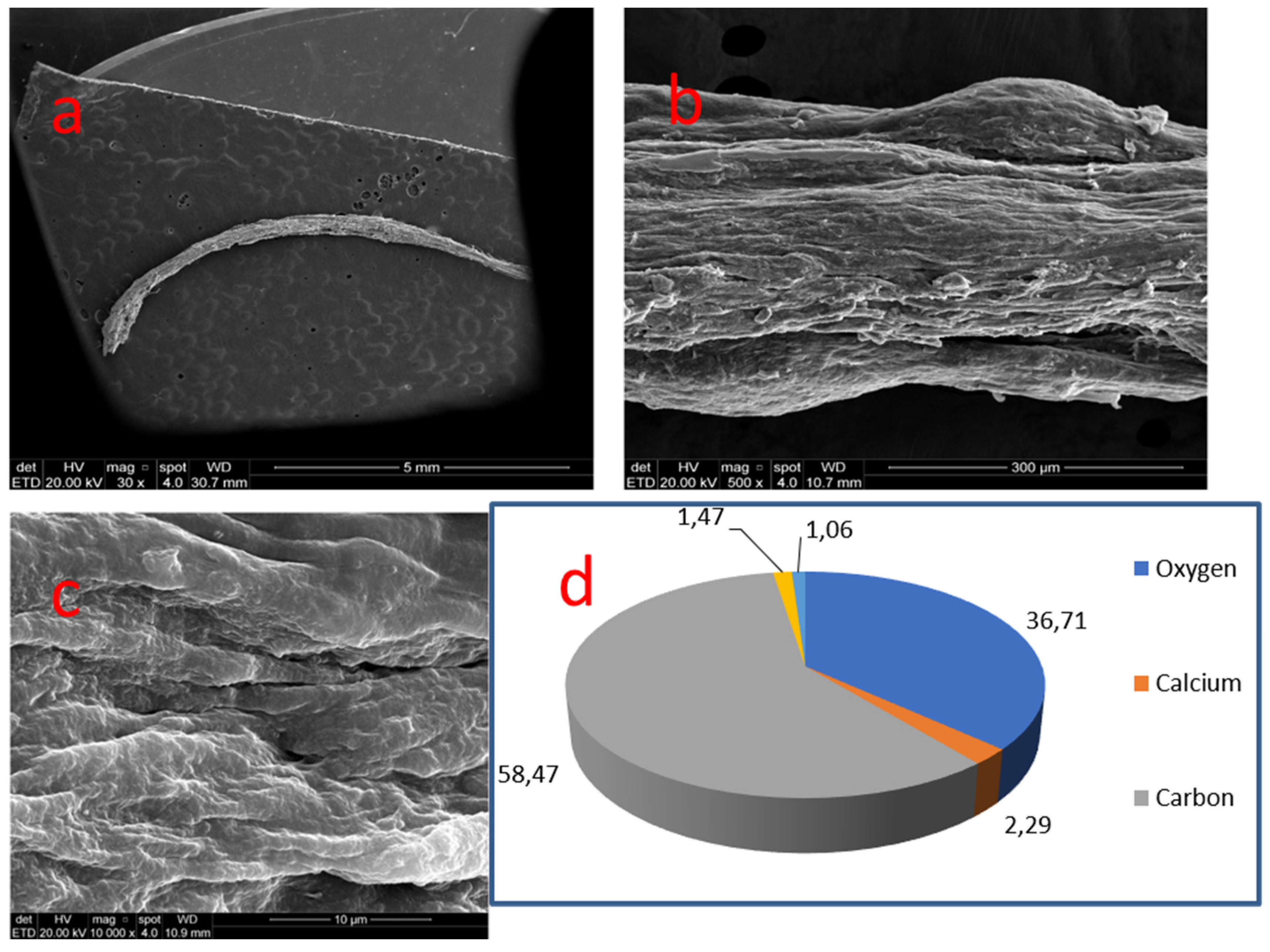
| 07 | Root canal | Free | Cylindric (fillet) | 7.0 mm (length) | Irregular grooved surface |
| Nodule No | Age of the Patient | Location | Topography of the Analyzed Area | 1 | 2 | 3 | 4 | 5 | 6 | Ca/P Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 30 | Coronal | Smooth and porous | O 41.5% | Ca 24.93% | C 17.95% | P 11.58% | Zn, Na, Cl and Mg 4.04% | - | 2.15 |
| 02 | 44 | Coronal | Smooth | O 35.14% | C 19.95% | Ca 17.25% | Zn 12.64% | P 9.16% | Na, Mg and Si 5.86% | 1.88 |
| 03 | 36 | Coronal | Smooth | C 37.88% | O 34.33% | Ca 17.89% | P 8.78% | Na and Mg 3.7% | - | 2.03 |
| 04 | 45 | Coronal | Smooth | Ca 29.74% | C 26.23% | O 25.07% | P 15.26% | Zn, Na and Mg 3.7% | - | 1.94 |
| 05 | 60 | Coronal | Smooth | Ca 41.24% | C 26.22% | O 15.29% | P 13.83% | Cl 3.42% | - | 2.98 |
| 06 | 61 | Radicular | Smooth | C 59.57% | O 34.02% | Ca 3.3% | Cl, Na, Mg, S and Si 1.95% | P 1.16% | - | 2.84 |
| 07 | 24 | Radicular | Porous | C 58.47% | O 36.71% | Ca 2.29% | P 1.47% | Cl, Na, Mg, S and Si 1.06% | - | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palatyńska-Ulatowska, A.; Fernandes, M.C.; Pietrzycka, K.; Koprowicz, A.; Klimek, L.; Souza, R.A.; Pradebon, M.; de Figueiredo, J.A.P. The Pulp Stones: Morphological Analysis in Scanning Electron Microscopy and Spectroscopic Chemical Quantification. Medicina 2022, 58, 5. https://doi.org/10.3390/medicina58010005
Palatyńska-Ulatowska A, Fernandes MC, Pietrzycka K, Koprowicz A, Klimek L, Souza RA, Pradebon M, de Figueiredo JAP. The Pulp Stones: Morphological Analysis in Scanning Electron Microscopy and Spectroscopic Chemical Quantification. Medicina. 2022; 58(1):5. https://doi.org/10.3390/medicina58010005
Chicago/Turabian StylePalatyńska-Ulatowska, Aleksandra, Marcos Cook Fernandes, Krystyna Pietrzycka, Agata Koprowicz, Leszek Klimek, Ronaldo Araújo Souza, Marieli Pradebon, and José Antonio Poli de Figueiredo. 2022. "The Pulp Stones: Morphological Analysis in Scanning Electron Microscopy and Spectroscopic Chemical Quantification" Medicina 58, no. 1: 5. https://doi.org/10.3390/medicina58010005
APA StylePalatyńska-Ulatowska, A., Fernandes, M. C., Pietrzycka, K., Koprowicz, A., Klimek, L., Souza, R. A., Pradebon, M., & de Figueiredo, J. A. P. (2022). The Pulp Stones: Morphological Analysis in Scanning Electron Microscopy and Spectroscopic Chemical Quantification. Medicina, 58(1), 5. https://doi.org/10.3390/medicina58010005








