Effectiveness and Safety of EGFR-TKI Rechallenge Treatment in Elderly Patients with Advanced Non-Small-Cell Lung Cancer Harboring Drug-Sensitive EGFR Mutations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. EGFR Mutation Analysis
2.3. Response Evaluation
2.4. Statistical Analysis
3. Results
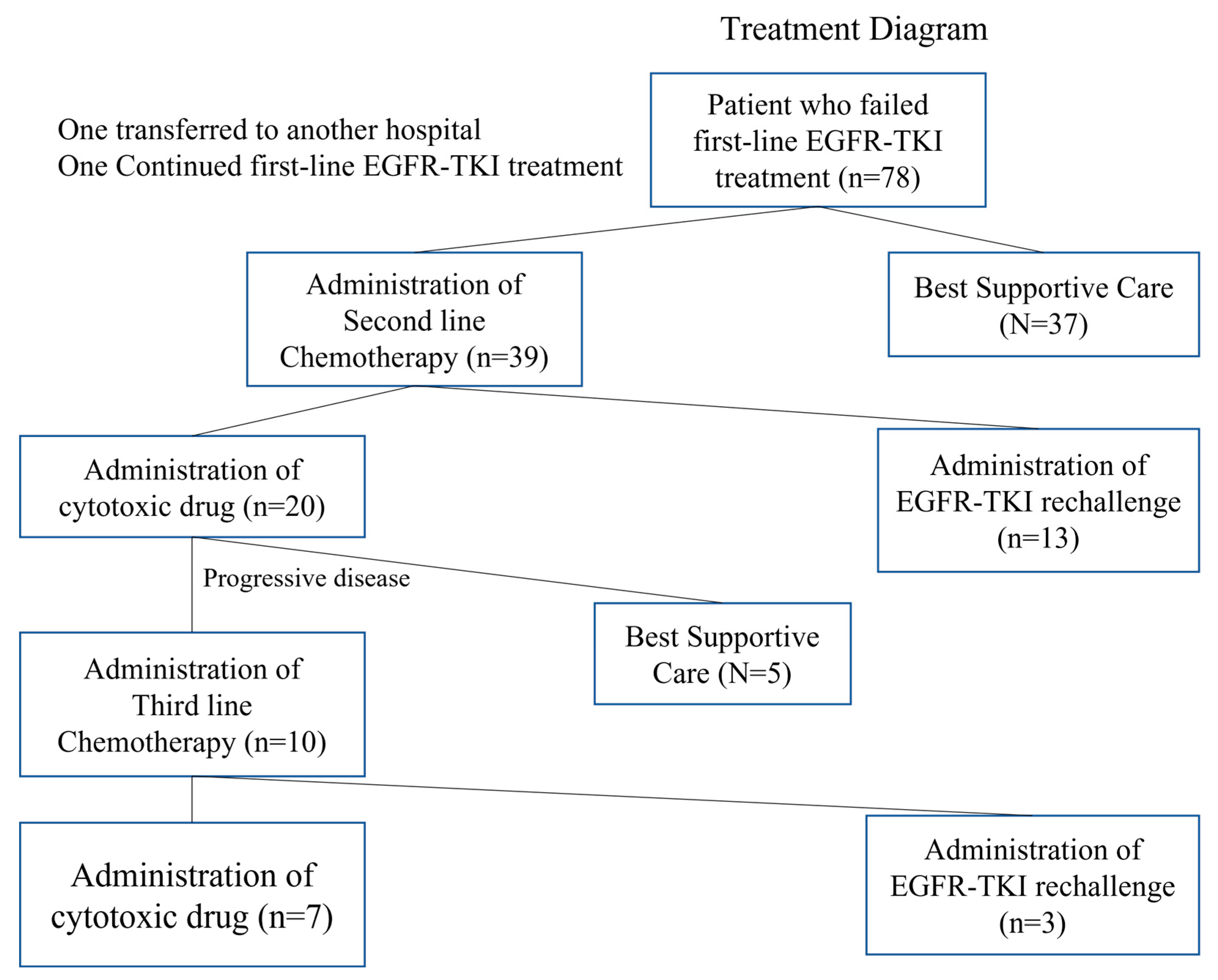
3.1. Patient Selection and Characteristics
3.2. Treatment Efficacy
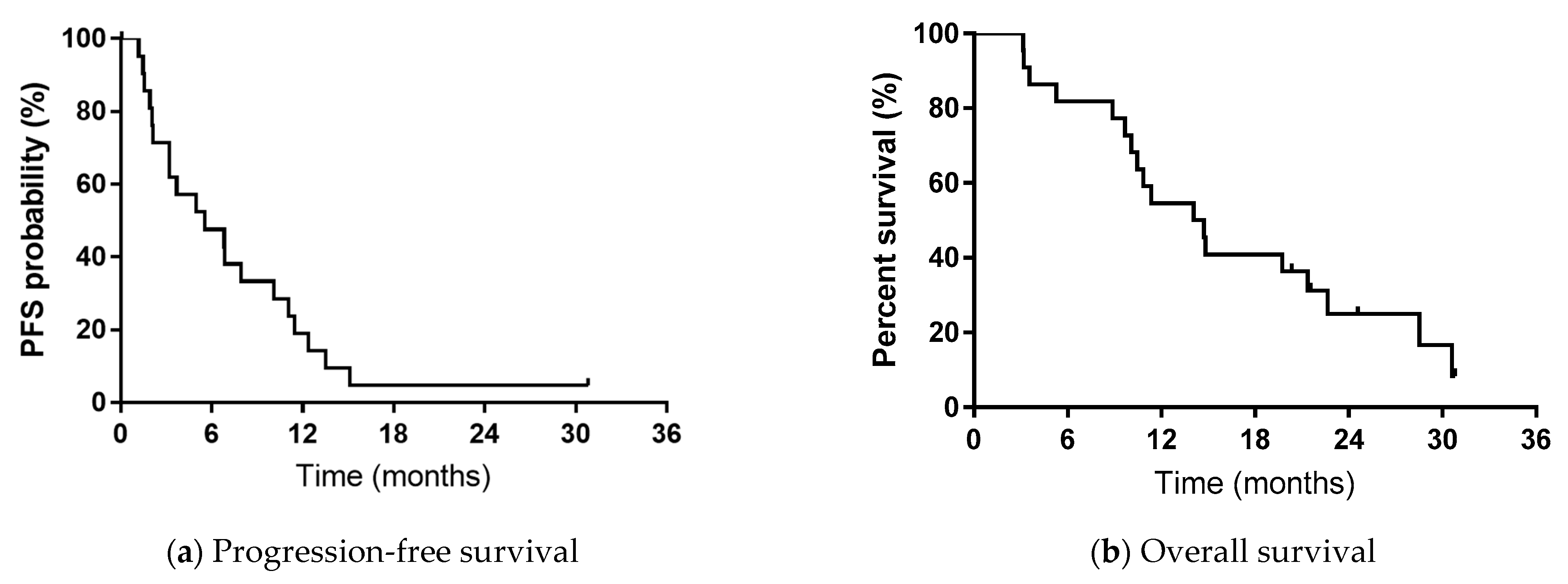
3.3. Survival Analysis
3.4. Toxicity and Adverse Events
3.5. Dose Change of EGFR-TKI
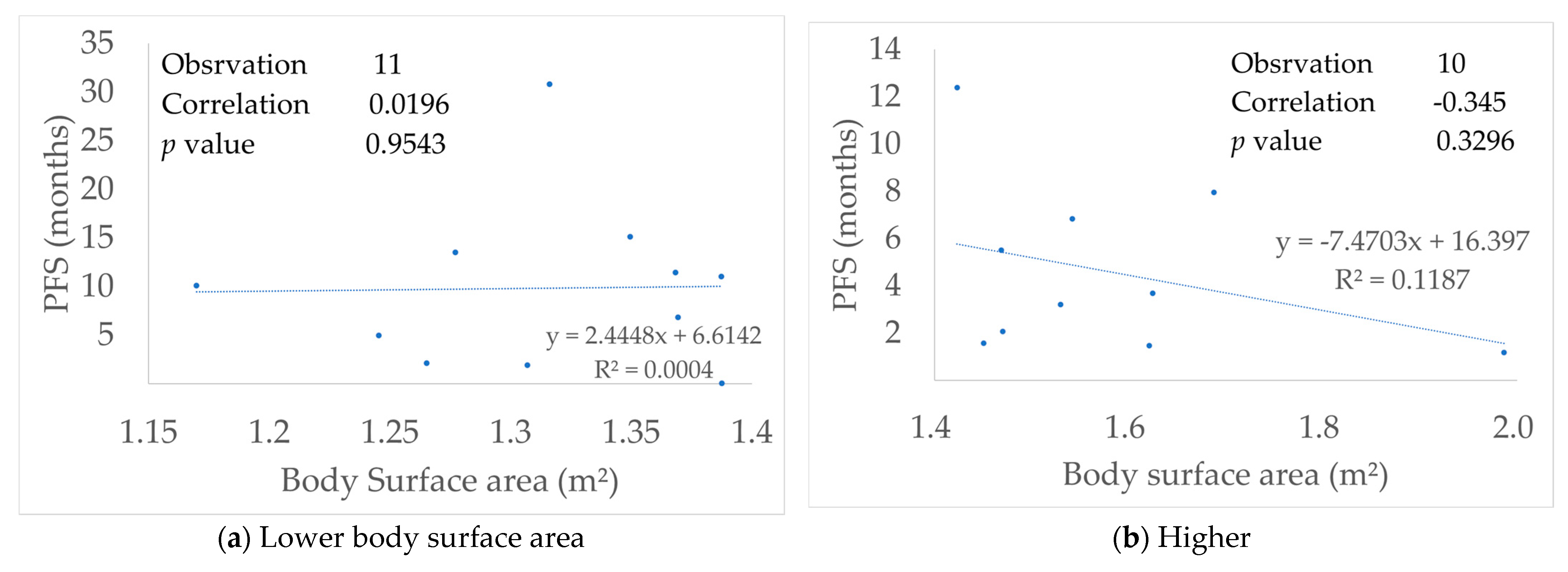
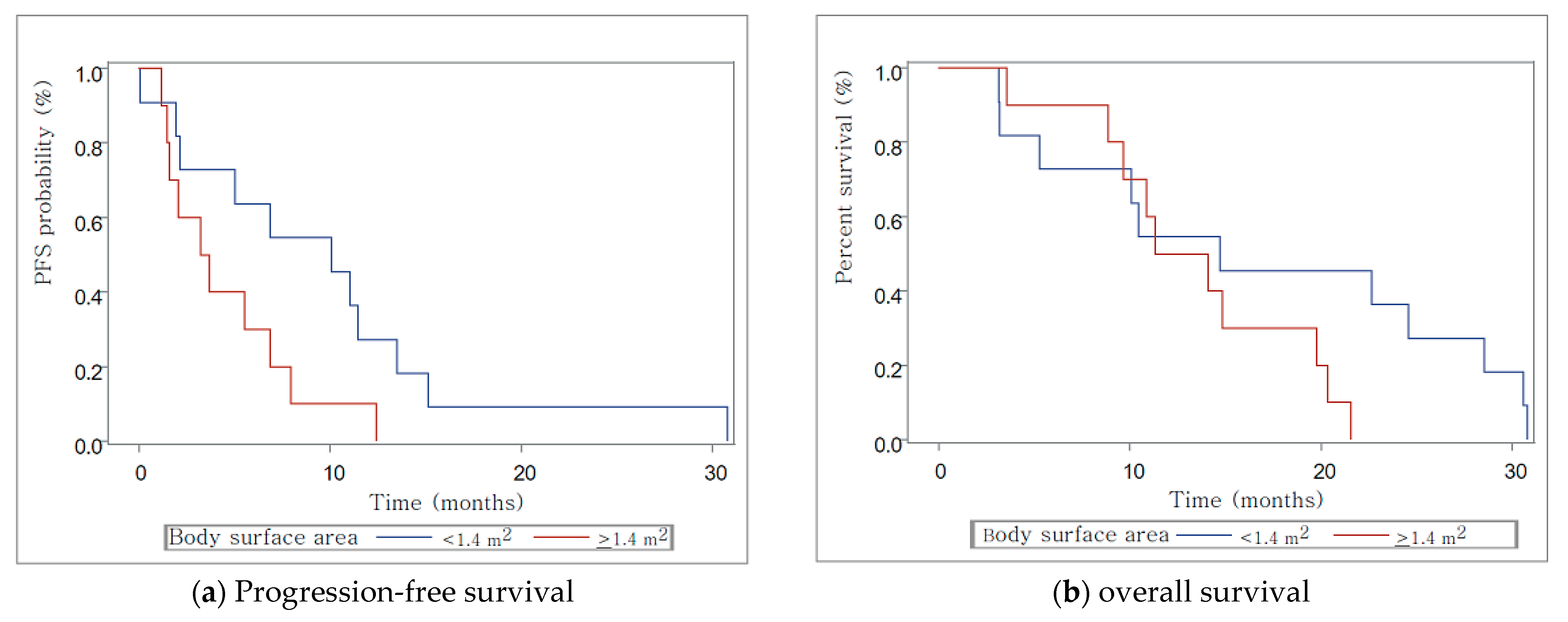
3.6. Relationship with Body Surface Area
3.7. Figures, Tables and Schemes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Davidoff, A.J.; Tang, M.; Seal, B.; Edelman, M.J. Chemotherapy and survival benefit in elderly patients with advanced non-small cell lung cancer. J. Clin. Oncol. 2010, 28, 2191–2197. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Ragin, C.C.; Belani, C.P.; Oton, A.B.; Gooding, W.E.; Taioli, E.; Ramalingam, S.S. Lung cancer in elderly patients: An analysis of the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2007, 25, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Fukuoka, M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Nukiwa, T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Paz-Ares, L. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomized phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef]
- Yang, J.C.H.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Sequist, L.V. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung ad-enocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Maemondo, M.; Minegishi, Y.; Inoue, A.; Kobayashi, K.; Harada, M.; Okinaga, S.; Gemmah, A. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J. Thorac. Oncol. 2012, 7, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Katakami, N.; Masago, K.; Yoshioka, H.; Tomii, K.; Kaneda, T.; Mio, T. Customized chemotherapy based on epidermal growth factor receptor mutation status for elderly patients with advanced non-small-cell lung cancer: A phase II trial. BMC Cancer 2012, 12, 185. [Google Scholar] [CrossRef]
- Tateishi, K.; Ichiyama, T.; Hirai, K.; Agatsuma, T.; Koyama, S.; Hachiya, T.; Koizumi, T. Clinical outcomes in elderly patients administered gefitinib as first-line treatment in epidermal growth factor receptor-mutated non-small-cell lung cancer: Retrospective analysis in a Nagano Lung Cancer Research Group study. Med. Oncol. 2013, 30, 450. [Google Scholar] [CrossRef]
- Goto, K.; Nishio, M.; Yamamoto, N.; Chikamori, K.; Hida, T.; Maemondo, M.; Katakami, N.; Kozuki, T.; Yoshioka, H.; Seto, T.; et al. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer 2013, 82, 109–114. [Google Scholar] [CrossRef]
- Takahashi, K.; Saito, H.; Hasegawa, Y.; Ando, M.; Yamamoto, M.; Kojima, E.; Suzuki, R. First-line gefitinib therapy for elderly patients with non-small cell lung cancer harboring EGFR mutation: Central Japan Lung Study Group 0901. Cancer Chemother. Pharm. 2014, 74, 721–727. [Google Scholar] [CrossRef]
- Kuwako, T.; Imai, H.; Masuda, T.; Miura, Y.; Seki, K.; Yoshino, R.; Yamada, M. First-line gefitinib treatment in elderly patients (aged>/=75 years) with non-small cell lung cancer harboring EGFR mutations. Cancer Chemother. Pharmacol. 2015, 76, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; Obyrne, K.J.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, Y.; Fujita, Y.; Tamura, A.; Shirai, M.; Shibata, S.; Kawabata, T.; Shibayama, T.; Fukai, S.; Kawahra, M.; Saito, R. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 2010, 68, 269–272. [Google Scholar] [CrossRef]
- Imai, H.; Gunma-Ibaraki-Fukushima-Tochigi (GIFT) Group; Minemura, H.; Sugiyama, T.; Yamada, Y.; Kaira, K.; Kanazawa, K.; Kasai, T.; Kaburagi, T.; Minato, K. Efficacy and safety of cytotoxic drug chemotherapy after first-line EGFR-TKI treatment in elderly patients with non-small-cell lung cancer harboring sensitive EGFR mutations. Cancer Chemother. Pharmacol. 2018, 82, 119–127. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R. International Association for the Study of Lung Cancer International Staging Committee. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar]
- Nagai, Y.; Miyazawa, H.; Tanaka, T.; Udagawa, K.; Kato, M.; Fukuyama, S.; Hagiwara, K. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005, 65, 7276–7282. [Google Scholar] [CrossRef]
- Yatabe, Y.; Hida, T.; Horio, Y.; Kosaka, T.; Takahashi, T.; Mitsudomi, T. A Rapid, Sensitive Assay to Detect EGFR Mutation in Small Biopsy Specimens from Lung Cancer. J. Mol. Diagn. 2006, 8, 335–341. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Verweij, J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.-M.; Park, K.; Kim, S.-W.; Zhou, C.; Su, W.-C.; Wang, M.; Sun, Y.; et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Chang, G.-C.; Tseng, C.-H.; Hsu, K.-H.; Yu, C.-J.; Yang, C.-T.; Chen, K.-C.; Yang, T.-Y.; Tseng, J.-S.; Liu, C.-Y.; Liao, W.-Y.; et al. Predictive factors for EGFR -tyrosine kinase inhibitor retreatment in patients with EGFR -mutated non-small-cell lung cancer – A multicenter retrospective SEQUENCE study. Lung Cancer 2017, 104, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Lai, C.-H.; Rau, K.-M.; Huang, C.-H.; Chang, H.-C.; Chao, T.-Y.; Tseng, C.-C.; Fang, W.-F.; Chung, Y.-H.; Wang, Y.-H.; et al. Impact of clinical parameters and systemic inflammatory status on epidermal growth factor receptor-mutant non-small cell lung cancer patients readministration with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2016, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.L.; Heideman, D.A.; Würdinger, T.; Grünberg, K.; Groen, H.J.; Smit, E.F. Rationale and Study Design of the IRENE-Trial (NVALT-16): A Phase II Trial to Evaluate Iressa Rechallenge in Advanced NSCLC Patients With an Activating EGFR Mutation Who Responded to an EGFR-TKI Used As First-Line or Previous Treatment. Clin. Lung Cancer 2015, 16, 60–66. [Google Scholar] [CrossRef]
- Xia, G.-H.; Zeng, Y.; Fang, Y.; Yu, S.-R.; Wang, L.; Shi, M.-Q.; Sun, W.-L.; Huang, X.-E.; Chen, J.; Feng, J.-F. Effect of EGFR-TKI retreatment following chemotherapy for advanced non-small cell lung cancer patients who underwent EGFR-TKI. Cancer Biol. Med. 2014, 11, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yu, X.; He, C.; Zhang, B.; Zhang, Y. Re-administration after the failure of gefitinib or erlotinib in patients with advanced non-small cell lung cancer. J. Thorac. Dis. 2013, 5, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Kobayashi, K.; Maemondo, M.; Sugawara, S.; Oizumi, S.; Isobe, H.; North-East Japan Study Group. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann. Oncol. 2013, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Azuma, K.; Ishii, H.; Bessho, A.; Hosokawa, S.; Fukamatsu, N.; Okamoto, H. Low-Dose Erlotinib Treatment in Elderly or Frail Patients With EGFR Mutation-Positive Non-Small Cell Lung Cancer A Multicenter Phase 2 Trial. JAMA Oncol. 2020, 6, e201250. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, S.; Takeda, K.; Nakagawa, K.; Takada, M.; Katakami, N.; Matsui, K.; Fukuoka, M. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: Results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J. Clin. Oncol. 2006, 24, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Yano, S. Clinical significance of epidermal growth factor receptor tyrosine kinase inhibitors: Sensitivity and resistance. Respir. Investig. 2014, 52, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Papadimitrakopoulou, V.A. Osimertinib or platinumpemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.; Kenmotsu, H.; Serizawa, M.; Koh, Y.; Wakuda, K.; Ono, A.; Takahashi, T. Frequency of EGFR T790M mutation and multimutational profiles of rebiopsy samples from non-small cell lung cancer developing acquired resistance to EGFR tyrosine kinase inhibitors in Japanese patients. BMC Cancer 2016, 16, 864. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Gaspar, C.; Pina, M.; Azevedo, I.; Rodrigues, A. Real-World T790M Mutation Frequency and Impact of Rebiopsy in Patients With EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, H.C.; Kim, Y.; Jang, S.H.; Lee, K.Y.; Lee, S.Y.; Lee, S.H.; Lee, S.Y.; Yoon, S.H.; Ryu, J.; et al. Clinical impact of rebiopsy among patients with epidermal growth factor receptor-mutant lung adenocarcinoma in a real-world clinical setting. Thorac. Cancer 2021, 12, 890–898. [Google Scholar] [CrossRef]
- Ninomaru, T.; Hata, A.; Kokan, C.; Okada, H.; Tomimatsu, H.; Ishida, J. Higher osimertinib introduction rate achieved by multiple repeated rebiopsy after acquired resistance to first/second generation EGFR-TKIs. Thorac. Cancer 2021, 12, 746–751. [Google Scholar] [CrossRef]
- Mountzios, G.; Koumarianou, A.; Bokas, A.; Mavroudis, D.; Samantas, E.; Fergadis, E.; Linardou, H.; Katsaounis, P.; Athanasiadis, E.; Karamouzis, M.; et al. A Real-World, Observational, Prospective Study to Assess the Molecular Epidemiology of Epidermal Growth Factor Receptor (EGFR) Mutations upon Progression on or after First-Line Therapy with a First- or Second-Generation EGFR Tyrosine Kinase Inhibitor in EGFR Mutation-Positive Locally Advanced or Metastatic Non-Small Cell Lung Cancer: The ‘LUNGFUL’ Study. Cancers 2021, 13, 3172. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, L.; Xie, F.; Zheng, X.; Li, Y.; Zhu, L.; Sun, J. Next-generation sequencing of tissue and circulating tumor DNA: Resistance mechanisms to EGFR targeted therapy in a cohort of patients with advanced non-small cell lung cancer. Cancer Med. 2021, 10, 4697–4709. [Google Scholar] [CrossRef]
- Soria, J.C.; Wu, Y.L.; Nakagawa, K.; Kim, S.W.; Yang, J.J.; Ahn, M.J.; Mok, T.S. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutationpositive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): A phase 3 randomised trial. Lancet Oncol. 2015, 16, 990–998. [Google Scholar] [CrossRef]
- Abe, T.; Takeda, K.; Ohe, Y.; Kudoh, S.; Ichinose, Y.; Okamoto, H.; Tamura, T. Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: The intergroup trial JCOG0803/WJOG4307L. J. Clin. Oncol. 2015, 33, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Zalcman, G.; Oster, J.P.; Westeel, V.; Pichon, E.; Lavolé, A. Carboplatinand weekly paclitaxel doublet chemotherapy compared with mono-therapy in elderly patients with advanced non-small-cell lung cancer: IFCT- 0501 randomised, phase 3 trial. Lancet 2011, 378, 1079–1088. [Google Scholar] [CrossRef]
- Tamiya, M.; Tamiya, A.; Suzuki, H.; Moriizumi, K.; Nakahama, K.; Taniguchi, Y.; Kunimasa, K.; Kimura, M.; Inoue, T.; Kuhara, H.; et al. Which Is Better EGFR-TKI Followed by Osimertinib: Afatinib or Gefitinib/Erlotinib? Anticancer Res. 2019, 39, 3923–3929. [Google Scholar] [CrossRef]
- Hirano, R.; Uchino, J.; Ueno, M.; Fujita, M.; Watanabe, K. Low-dose Epidermal Growth Factor Receptor (EGFR)-Tyrosine Kinase Inhibition of EGFR Mutation-positive Lung Cancer: Therapeutic Benefits and Associations Between Dosage, Efficacy and Body Surface Area. Asian Pac. J. Cancer Prev. 2016, 17, 785–789. [Google Scholar] [CrossRef][Green Version]
- Sonehara, K.; Kobayashi, T.; Tateishi, K.; Morozumi, N.; Yoshiike, F.; Hachiya, T.; Ono, Y.; Takasuna, K.; Agatsuma, T.; Masubuchi, T.; et al. Clinical analysis ofEGFR-positive non-small cell lung cancer patients treated with first-line afatinib: A Nagano Lung Cancer Research Group. Thorac. Cancer 2019, 10, 1078–1085. [Google Scholar] [CrossRef]
- Nakagawa, K.; Garon, E.B.; Seto, T.; Nishio, M.; Aix, S.P.; Paz-Ares, L.; Dakhil, S. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1655–1669. [Google Scholar] [CrossRef]
- Tan, D.S.-W.; Leighl, N.B.; Riely, G.J.; Yang, J.C.-H.; Sequist, L.V.; Wolf, J.; Seto, T.; Felip, E.; Aix, S.P.; Jonnaert, M.; et al. Safety and efficacy of nazartinib (EGF816) in adults with EGFR-mutant non-small-cell lung carcinoma: A multicentre, open-label, phase 1 study. Lancet Respir. Med. 2020, 8, 561–572. [Google Scholar] [CrossRef]
- Ahn, M.J.; Han, J.Y.; Lee, K.H.; Kim, S.W.; Kim, D.W.; Lee, Y.G.; Cho, B.C. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol. 2019, 20, 1681–1690. [Google Scholar] [CrossRef]
- Wang, H.; Pan, R.; Zhang, X.; Si, X.; Wang, M.; Zhang, L. Abivertinib in patients with T790M-positive advanced NSCLC and its subsequent treatment with osimertinib. Thorac. Cancer 2020, 11, 594–602. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Camidge, D.R.; Yang, C.-T.; Zhou, J.; Guo, R.; Chiu, C.-H.; Chang, G.-C.; Shiah, H.-S.; Chen, Y.; Wang, C.-C.; et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial. J. Thorac. Oncol. 2020, 15, 1907–1918. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Number of Patients | (%) |
|---|---|---|
| Sex | ||
| Male | 7 | 32 |
| Female | 15 | 68 |
| Age (years), median (range) | 79.5 (75–87) | |
| Performance status | ||
| 0 | 7 | 32 |
| 1 | 11 | 50 |
| 2 | 4 | 18 |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| Clinical Stage at diagnosis | ||
| IIIB | 1 | 4 |
| IV | 20 | 92 |
| Postoperative recurrence | 1 | 4 |
| Histology | ||
| Adenocarcinoma | 22 | 100 |
| Other/not otherwise specified | 0 | 0 |
| Smoking history | ||
| Current or former | 6 | 28 |
| Never | 15 | 68 |
| Unknown | 1 | 4 |
| EGFR mutation | ||
| Exon 19 deletion | 10 | 45 |
| Exon 21 L858R | 11 | 50 |
| Exon 18 G719X | 1 | 4 |
| First-line EGFR-TKI | ||
| Gefitinib | 19 | 86 |
| Erlotinib | 3 | 14 |
| Afatinib | 0 | 0 |
| Rechallenge EGFR-TKI | ||
| Gefitinib→Gefitinib | 1 | 4 |
| Gefitinib→Erlotinib | 18 | 82 |
| Erlotinib→Erlotinib | 1 | 4 |
| Erlotinib→Gefitinib | 2 | 9 |
| Administration line of rechallenge EGFR-TKI | ||
| 2 | 13 | 59 |
| 3 | 5 | 22 |
| 4 | 4 | 18 |
| T790M mutation | ||
| Positive | 3 | 14 |
| Negative | 1 | 4 |
| Unknown | 18 | 72 |
| Median follow-up period [months] (range) | 14.6 (3.2–31.2) |
| Number of Patients (%) | ||
|---|---|---|
| Primary EGFR-TKI | Rechallenge EGFR-TKI | |
| Complete response | 0 (0) | 0 (0) |
| Partial response | 17 (77) | 5 (23) |
| Stable disease | 1 (4) | 12 (54) |
| Progressive disease | 4 (19) | 4 (19) |
| Not evaluable | 0 (0) | 1 (4) |
| Response rate (%) | 77 | 23 |
| Disease control rate a (%) | 81 | 77 |
| Dose reduction or alternative day administration | ||
| Yes/No | 10/12 | 15/7 |
| Regimen | Number of Patients | |
|---|---|---|
| Therapies before EGFR-TKI Rechallenge | Therapies after Failure of EGFR-TKI Rechallenge | |
| DTX | 5 | 0 |
| PEM | 3 | 7 |
| S-1 | 3 | 1 |
| CDDP-based combination chemotherapy | 0 | 0 |
| CBDCA-based combination chemotherapy | 2 | 0 |
| Immune check point inhibitor | 0 | 2 |
| EGFR-TKI rechallenge (Gefitinib, erlotinib, or afatinib) | - | 7 |
| Osimertinib * | - | 3 |
| Best supportive care | - | 9 |
| NCI-CTCAE Grade (Ver 4.0) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ≥3 (%) | |
| Nonhematologic adverse events | ||||||
| Fatigue | 2 | 0 | 0 | − | − | 0 (0) |
| Paronychia | 0 | 8 | 0 | − | − | 0 (0) |
| Pruritus | 2 | 0 | 0 | − | − | 0 (0) |
| Rash acneiform | 7 | 7 | 0 | 0 | 0 | 0 (0) |
| Dyspnea | 3 | 0 | 0 | 0 | 0 | 0 (0) |
| Anorexia | 4 | 1 | 0 | 0 | 0 | 0 (0) |
| Diarrhea | 4 | 2 | 1 | 0 | 0 | 1 (4.5) |
| Mucositis | 1 | 3 | 0 | 0 | 0 | 0 (0) |
| Nausea | 2 | 0 | 0 | 0 | 0 | 0 (0) |
| Pain | 1 | 0 | 0 | − | − | 0 (0) |
| Infection | 0 | 0 | 1 | 0 | 0 | 1 (4.5) |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Thromboembolic event | 0 | 1 | 0 | 0 | 0 | 0 (0) |
| Hematologic or laboratory adverse events | ||||||
| Anemia | 3 | 0 | 0 | 0 | 0 | 0 (0) |
| ALT increased | 4 | 1 | 0 | 0 | − | 0 (0) |
| AST increased | 4 | 1 | 0 | 0 | − | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Imai, H.; Sugiyama, T.; Minemura, H.; Kanazawa, K.; Kasai, T.; Minato, K.; Kaira, K.; Kaburagi, T. Effectiveness and Safety of EGFR-TKI Rechallenge Treatment in Elderly Patients with Advanced Non-Small-Cell Lung Cancer Harboring Drug-Sensitive EGFR Mutations. Medicina 2021, 57, 929. https://doi.org/10.3390/medicina57090929
Yamada Y, Imai H, Sugiyama T, Minemura H, Kanazawa K, Kasai T, Minato K, Kaira K, Kaburagi T. Effectiveness and Safety of EGFR-TKI Rechallenge Treatment in Elderly Patients with Advanced Non-Small-Cell Lung Cancer Harboring Drug-Sensitive EGFR Mutations. Medicina. 2021; 57(9):929. https://doi.org/10.3390/medicina57090929
Chicago/Turabian StyleYamada, Yutaka, Hisao Imai, Tomohide Sugiyama, Hiroyuki Minemura, Kenya Kanazawa, Takashi Kasai, Koichi Minato, Kyoichi Kaira, and Takayuki Kaburagi. 2021. "Effectiveness and Safety of EGFR-TKI Rechallenge Treatment in Elderly Patients with Advanced Non-Small-Cell Lung Cancer Harboring Drug-Sensitive EGFR Mutations" Medicina 57, no. 9: 929. https://doi.org/10.3390/medicina57090929
APA StyleYamada, Y., Imai, H., Sugiyama, T., Minemura, H., Kanazawa, K., Kasai, T., Minato, K., Kaira, K., & Kaburagi, T. (2021). Effectiveness and Safety of EGFR-TKI Rechallenge Treatment in Elderly Patients with Advanced Non-Small-Cell Lung Cancer Harboring Drug-Sensitive EGFR Mutations. Medicina, 57(9), 929. https://doi.org/10.3390/medicina57090929






