Effects of Sex and Seasonal Climatic Changes on the Risk of Incidence of Anti-EGFR Therapy-Induced Rash in Cancer Patients: A Retrospective Study
Abstract
:1. Introduction
2. Methods
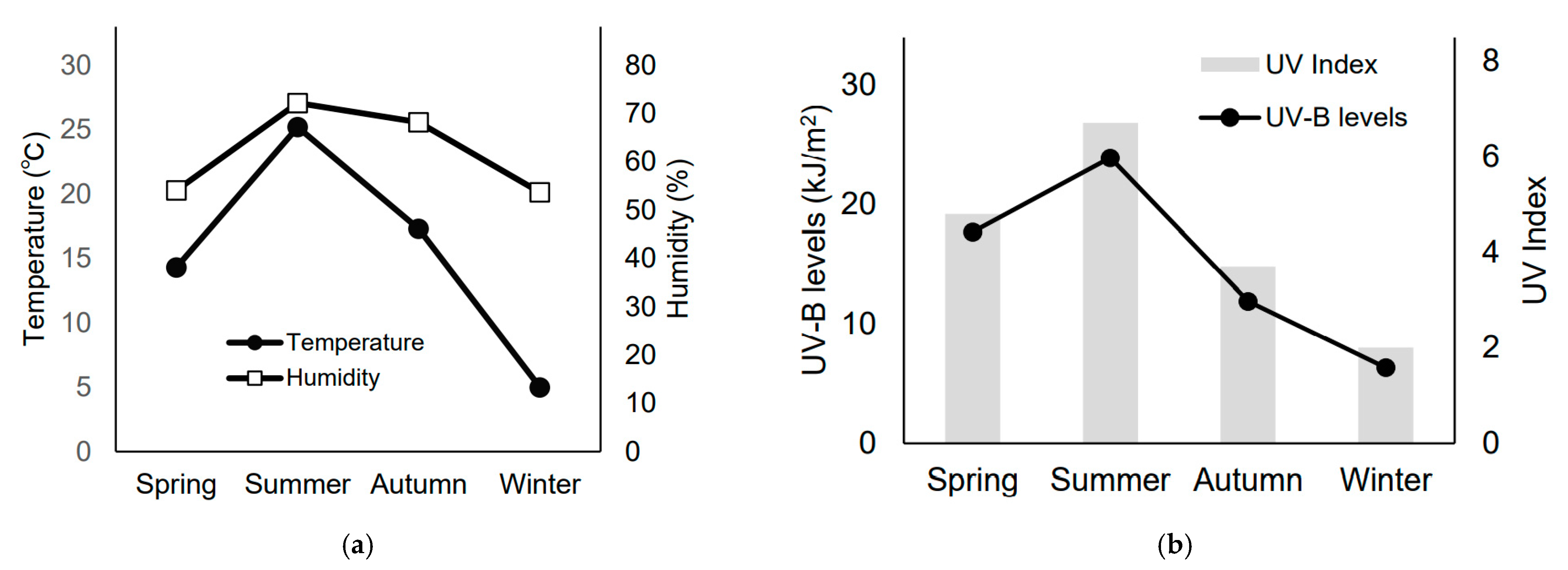
2.1. Season Definition and Patients
2.2. Evaluation Method
2.3. Statistical Analysis
3. Results
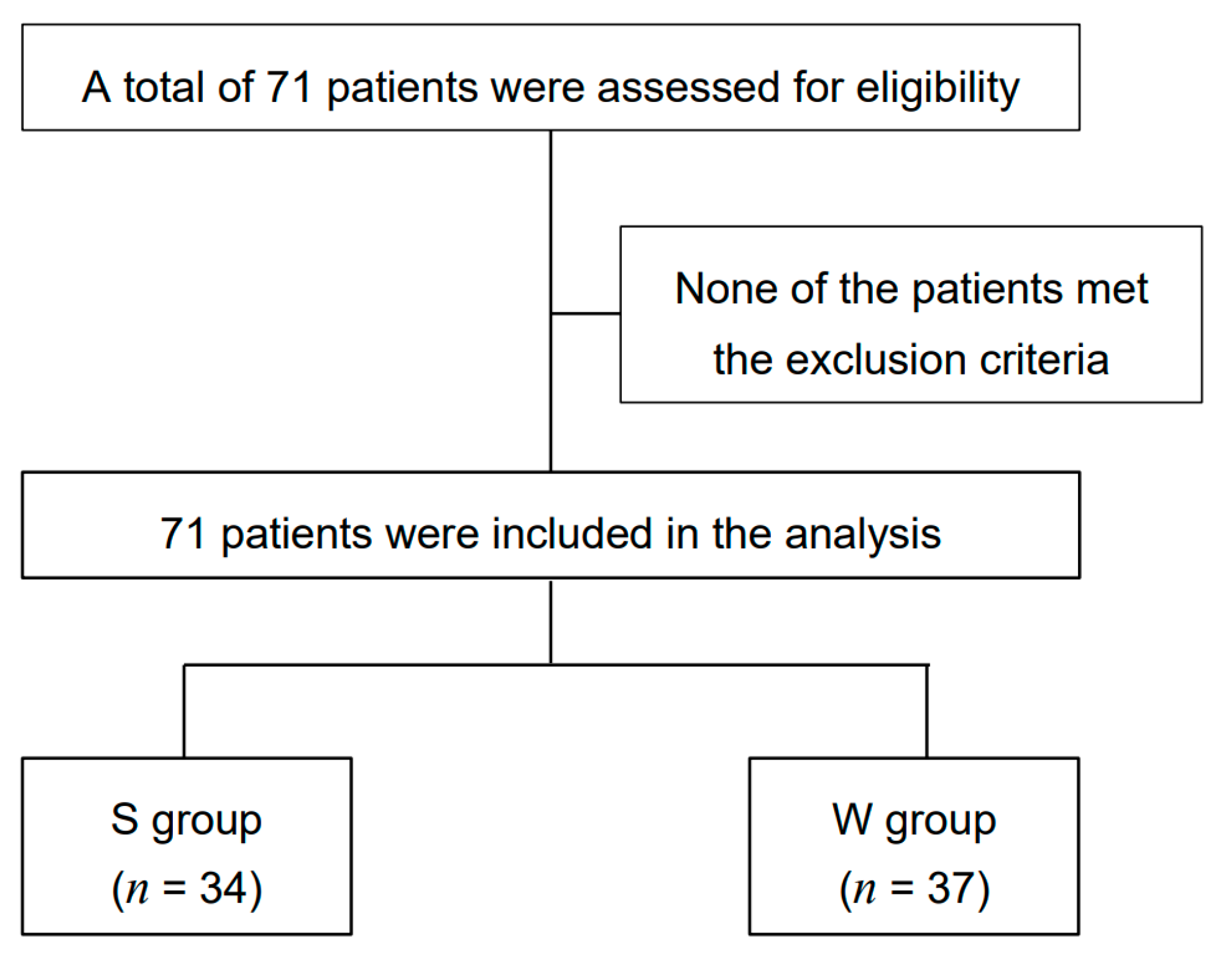
3.1. Patient Characteristics
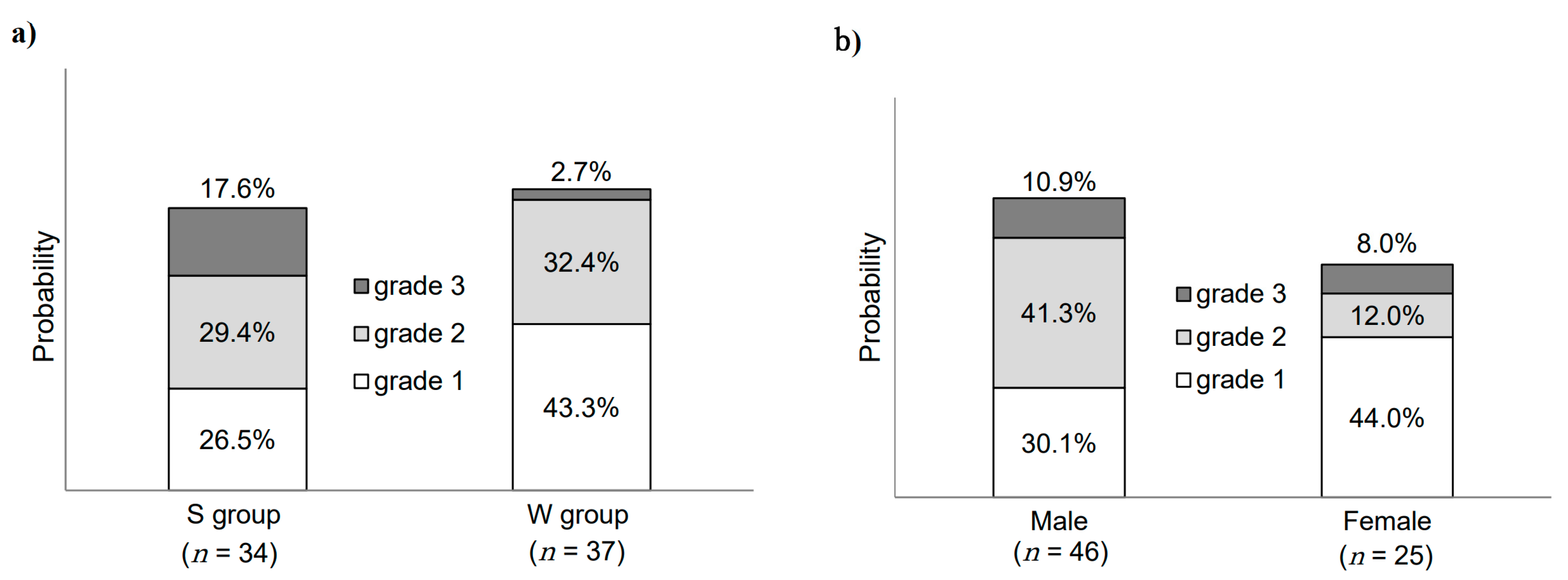
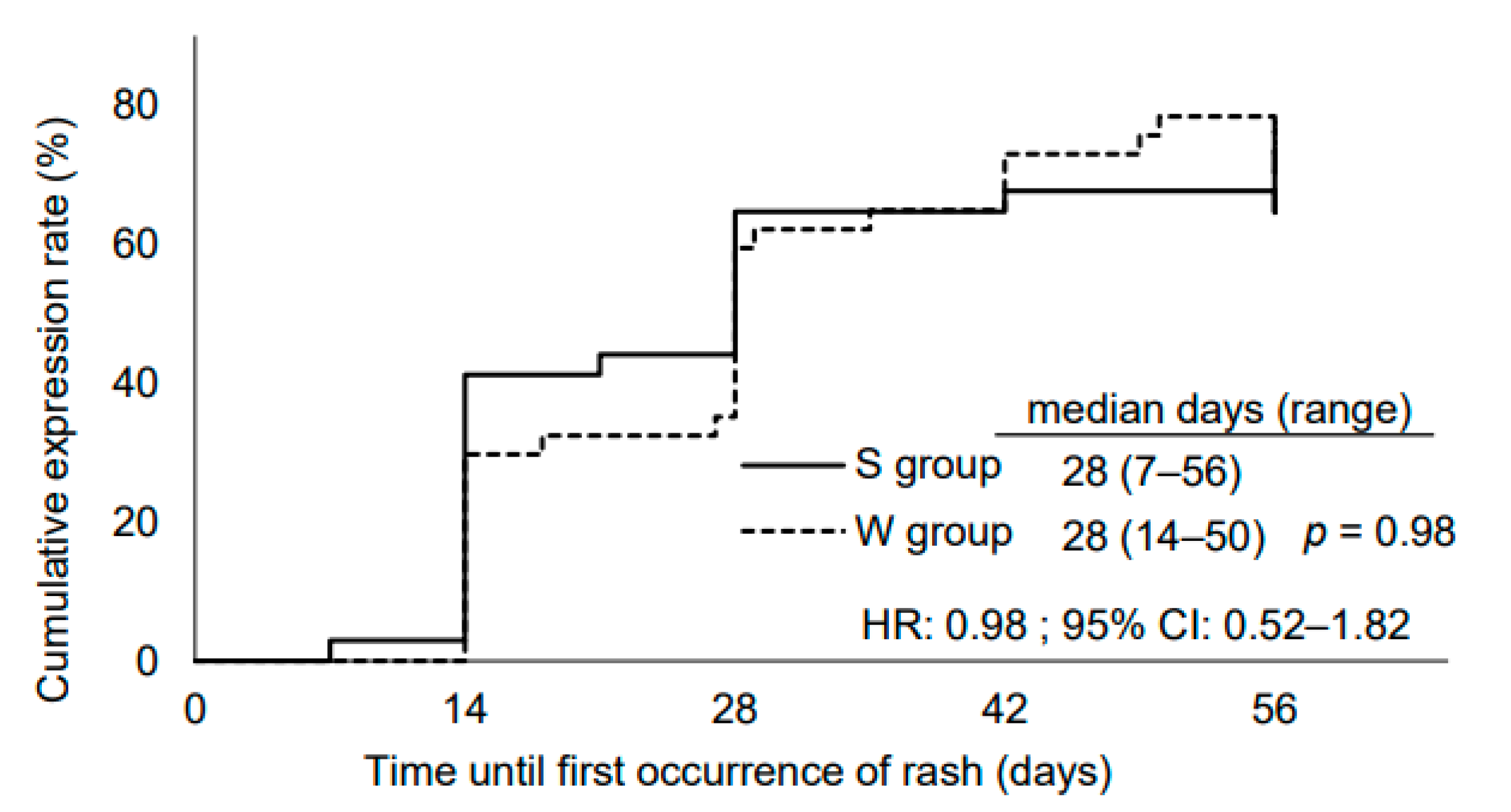
3.2. Seasonal Differences in Skin Toxicities
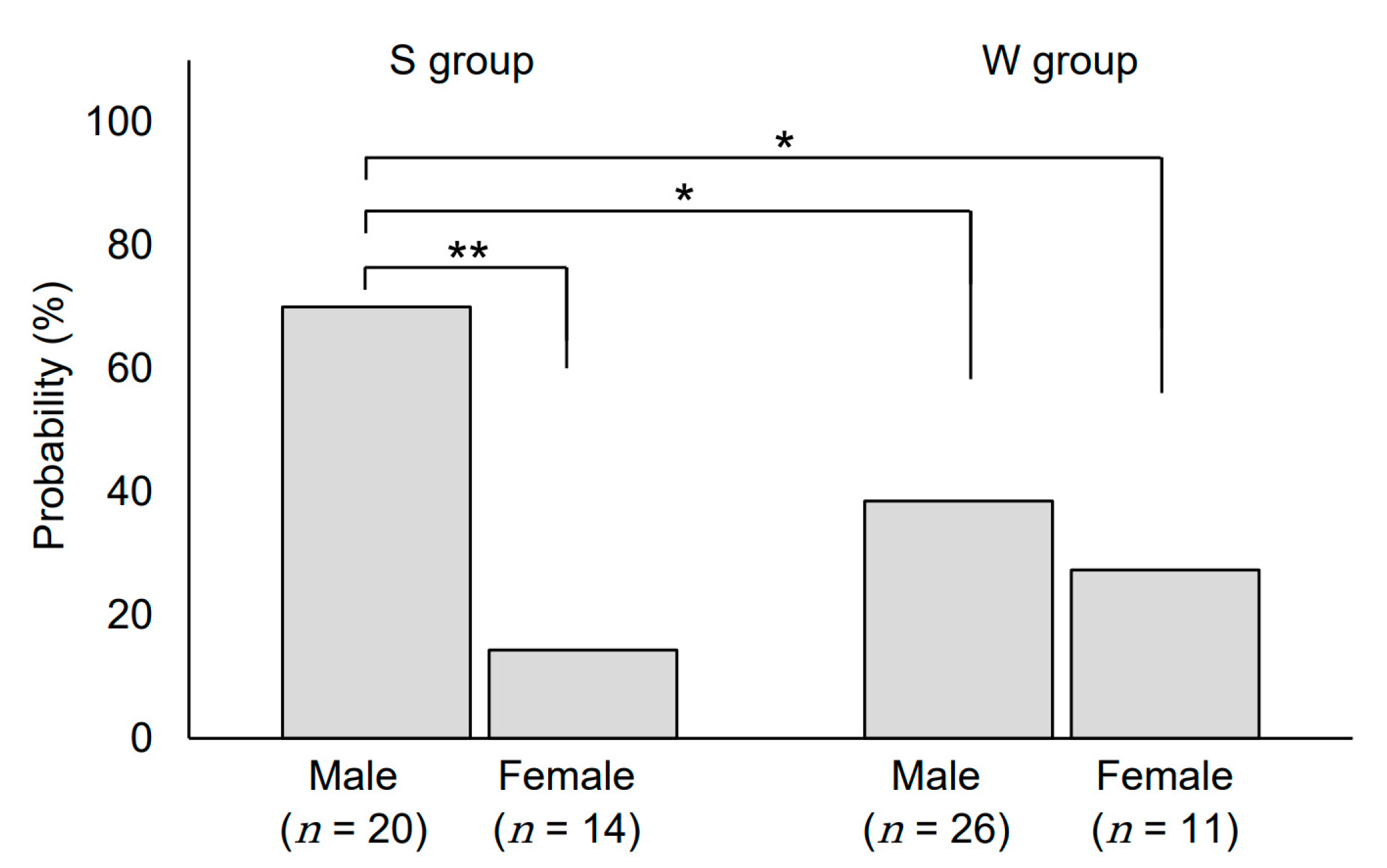
3.3. Sex-Related Differences in Skin Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Papules and/or pustules covering <10% BSA, which may or may not be associated with symptoms of pruritus or tenderness | Papules and/or pustules covering 10–30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; associated with psychosocial impact; limiting instrumental ADL | Papules and/or pustules covering >30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; limiting self-care ADL; associated with local superinfection with oral antibiotics indicated | Papules and/or pustules covering any % BSA, which may or may not be associated with symptoms of pruritus or tenderness and are associated with extensive superinfection with IV antibiotics indicated; life-threatening consequences | Death |
References
- Lordick, F.; Kang, Y.K.; Chung, H.C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Lynch, T.J.; Kim, E.S.; Eaby, B.; Garey, J.; West, D.P.; Lacouture, M.E. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: An evolving paradigm in clinical management. Oncologist 2007, 12, 610–621. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Mitchell, E.P.; Piperdi, B.; Pillai, M.V.; Shearer, H.; Iannotti, N.; Xu, F.; Yassine, M. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 1351–1357. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yuki, S.; Fukushima, H.; Sasaki, T.; Iwanaga, I.; Uebayashi, M.; Okuda, H.; Kusumi, T.; Miyagishima, T.; Koji, O.; et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study; J-STEPP. Future Oncol. 2015, 11, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Peeters, M.; Siena, S.; Humblet, Y.; Hendlisz, A.; Neyns, B.; Canon, J.L.; Van Laethem, J.L.; Maurel, J.; Richardson, G.; et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tejpar, S.; Vanbeckevoort, D.; Peeters, M.; Humblet, Y.; Gelderblom, H.; Vermorken, J.B.; Viret, F.; Glimelius, B.; Gallerani, E.; et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: The randomized EVEREST study. J. Clin. Oncol. 2012, 30, 2861–2868. [Google Scholar] [CrossRef]
- Terms About Time (Japan Meteorological Agency). Available online: https://www.jma.go.jp/jma/kishou/know/yougo_hp/toki.html (accessed on 17 December 2020).
- Historical Weather Data (Japan Meteorological Agency). Available online: http://www.data.jma.go.jp/gmd/risk/obsdl/index.php (accessed on 17 December 2020).
- Kikuchi, K.; Kobayashi, H.; Le Fur, I.; Tschachler, E.; Tagami, H. The winter season affects more severely the facial skin than the forearm skin: Comparative biophysical studies conducted in the same Japanese females in later summer and winter. Exog. Dermatol. 2002, 1, 32–38. [Google Scholar] [CrossRef]
- Uenishi, T.; Sugiura, H.; Uehara, M. Changes in the seasonal dependence of atopic dermatitis in Japan. J. Dermatol. 2001, 28, 244–247. [Google Scholar] [CrossRef]
- Engebretsen, K.A.; Johansen, J.D.; Kezic, S.; Linneberg, A.; Thyssen, J.P. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Lankheet, N.A.; Huitema, A.D.; Mallo, H.; Adriaansz, S.; Haanen, J.B.; Schellens, J.H.; Beijnen, J.H.; Blank, C.U. The effect of seasonal variation and secretion of sunitinib in sweat on the development of hand-foot syndrome. Eur. J. Clin. Pharmacol. 2013, 69, 2065–2072. [Google Scholar] [CrossRef]
- Jatoi, A.; Green, E.M.; Rowland, K.M., Jr.; Sargent, D.J.; Alberts, S.R. Clinical predictors of severe cetuximab-induced rash: Observations from 933 patients enrolled in north central cancer treatment group study N0147. Oncology 2009, 77, 120–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, D.A. Hormone epidermal growth factor interactions in development. Horm Res. 1990, 33, 69–74. [Google Scholar] [CrossRef]
- Mullenders, L.H.; Hazekamp-van Dokkum, A.M.; Kalle, W.H.; Vrieling, H.; Zdzienicka, M.Z.; van Zeeland, A.A. UV-induced photolesions, their repair and mutations. Mutat. Res. 1993, 299, 271–276. [Google Scholar] [CrossRef]
- Haratake, A.; Uchida, Y.; Schmuth, M.; Tanno, O.; Yasuda, R.; Epstein, J.H.; Elias, P.M.; Holleran, W.M. UVB-induced alterations in permeability barrier function: Role for epidermal hyperproliferation and thymocyte-mediated response. J. Investig. Dermatol. 1997, 108, 769–775. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Mori, K.; Taguchi, H.; Nishizaka, T.; Takema, Y. UVB sensitivity correlates with cutaneous barrier function in the skin of Japanese females. Photodermatol. Photoimmunol. Photomed. 2019, 35, 284–285. [Google Scholar] [CrossRef]
- UV Data Collection (Japan Meteorological Agency). Available online: http://www.data.jma.go.jp/gmd/env/uvhp/info_uv.html (accessed on 17 December 2020).
- World Health Organization. Global Solar UV Index: A Practical Guide. A joint recommendation of the World Health Organization, World Meteorological Organization, United Nations Environment Programme, and the International Commission on Non-Ionizing Radiation Protection. 2002. Available online: https://www.who.int/uv/publications/en/UVIGuide.pdf (accessed on 17 December 2020).
- Narbutt, J.; Philipsen, P.A.; Harrison, G.I.; Morgan, K.A.; Lawrence, K.P.; Baczynska, K.A.; Grys, K.; Rogowski-Tylman, M.; Olejniczak-Staruch, I.; Tewari, A.; et al. Sunscreen applied at ≥2 mg cm−2 during a sunny holiday prevents erythema, a biomarker of ultraviolet radiation-induced DNA damage and suppression of acquired immunity. Br. J. Dermatol. 2019, 180, 604–614. [Google Scholar] [CrossRef] [Green Version]
- Görig, T.; Diehl, K.; Greinert, R.; Breitbart, E.W.; Schneider, S. Prevalence of sun-protective behaviour and intentional sun tanning in German adolescents and adults: Results of a nationwide telephone survey. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 225–235. [Google Scholar] [CrossRef]
- Gaiser, M.R.; Lorenzen, S.; Merx, K.; Trojan, J.; Ocvirk, J.; Ettrich, T.J.; Al-Batran, S.E.; Schulz, H.; Homann, N.; Feustel, H.P.; et al. Evaluation of EGFR inhibitor-mediated acneiform skin toxicity within the double-blind randomized EVITA trial: A thorough gender-specific analysis using the WoMo score. Cancer Med. 2019, 8, 4169–4175. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.J.; Chang, P.; Lai, K.P.; Chen, L.; Chang, C. The role of androgen and androgen receptor in skin-related disorders. Arch. Dermatol. Res. 2012, 304, 499–510. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Ashcroft, G.S. Sex steroids and cutaneous wound healing: The contrasting influences of estrogens and androgens. Climacteric 2007, 10, 276–288. [Google Scholar] [CrossRef]
- Demir, A.; Uslu, M.; Arslan, O.E. The effect of seasonal variation on sexual behaviors in males and its correlation with hormone levels: A prospective clinical trial. Cent. Eur. J. Urol. 2016, 69, 285–289. [Google Scholar] [CrossRef]
- Southren, A.L.; Tochimoto, S.; Carmody, N.C.; Isurugi, K. Plasma production rates of testosterone in normal adult men and women and in patients with the syndrome of feminizing testes. J. Clin. Endocrinol. Metab. 1965, 25, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
| S Group (n = 34) | W Group (n = 37) | p-Value | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex Male Female | 20 14 | 58.8 41.2 | 26 11 | 70.3 29.7 | 0.32 |
| Type of cancer Colorectal cancer Head and neck cancer | 27 7 | 79.4 20.6 | 31 6 | 83.8 16.2 | 0.87 |
| Therapy regimen Cetuximab-based regimen Panitumumab-based regimen | 20 14 | 58.8 41.2 | 23 14 | 62.2 37.8 | 0.77 |
| Moisturizer prescription Yes No | 34 0 | 100.0 0.0 | 37 | 100.0 0.0 | - |
| Minocycline prescription Yes No | 33 1 | 97.1 2.9 | 36 1 | 97.3 2.7 | 0.51 |
| History of allergies Yes No | 4 30 | 11.8 88.2 | 6 31 | 16.2 83.8 | 0.84 |
| Median (range) | Median (range) | ||||
| Age (years) | 65 (35–78) | 64 (35–78) | 0.45 | ||
| RDI of anti-EGFR mAb | 0.73 (0.30–1.00) | 0.74 (0.39–1.07) | 0.80 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, T.; Fujita, Y.; Imai, H.; Matsumoto, H.; Yamazaki, M.; Hiruta, E.; Suzuki, Y.; Ojima, H.; Hosaka, H.; Minato, K.; et al. Effects of Sex and Seasonal Climatic Changes on the Risk of Incidence of Anti-EGFR Therapy-Induced Rash in Cancer Patients: A Retrospective Study. Medicina 2021, 57, 801. https://doi.org/10.3390/medicina57080801
Arai T, Fujita Y, Imai H, Matsumoto H, Yamazaki M, Hiruta E, Suzuki Y, Ojima H, Hosaka H, Minato K, et al. Effects of Sex and Seasonal Climatic Changes on the Risk of Incidence of Anti-EGFR Therapy-Induced Rash in Cancer Patients: A Retrospective Study. Medicina. 2021; 57(8):801. https://doi.org/10.3390/medicina57080801
Chicago/Turabian StyleArai, Takahiro, Yukiyoshi Fujita, Hisao Imai, Hiroe Matsumoto, Miho Yamazaki, Eriko Hiruta, Yuka Suzuki, Hitoshi Ojima, Hisashi Hosaka, Koichi Minato, and et al. 2021. "Effects of Sex and Seasonal Climatic Changes on the Risk of Incidence of Anti-EGFR Therapy-Induced Rash in Cancer Patients: A Retrospective Study" Medicina 57, no. 8: 801. https://doi.org/10.3390/medicina57080801
APA StyleArai, T., Fujita, Y., Imai, H., Matsumoto, H., Yamazaki, M., Hiruta, E., Suzuki, Y., Ojima, H., Hosaka, H., Minato, K., & Saito, T. (2021). Effects of Sex and Seasonal Climatic Changes on the Risk of Incidence of Anti-EGFR Therapy-Induced Rash in Cancer Patients: A Retrospective Study. Medicina, 57(8), 801. https://doi.org/10.3390/medicina57080801






