Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management
Abstract
1. Introduction
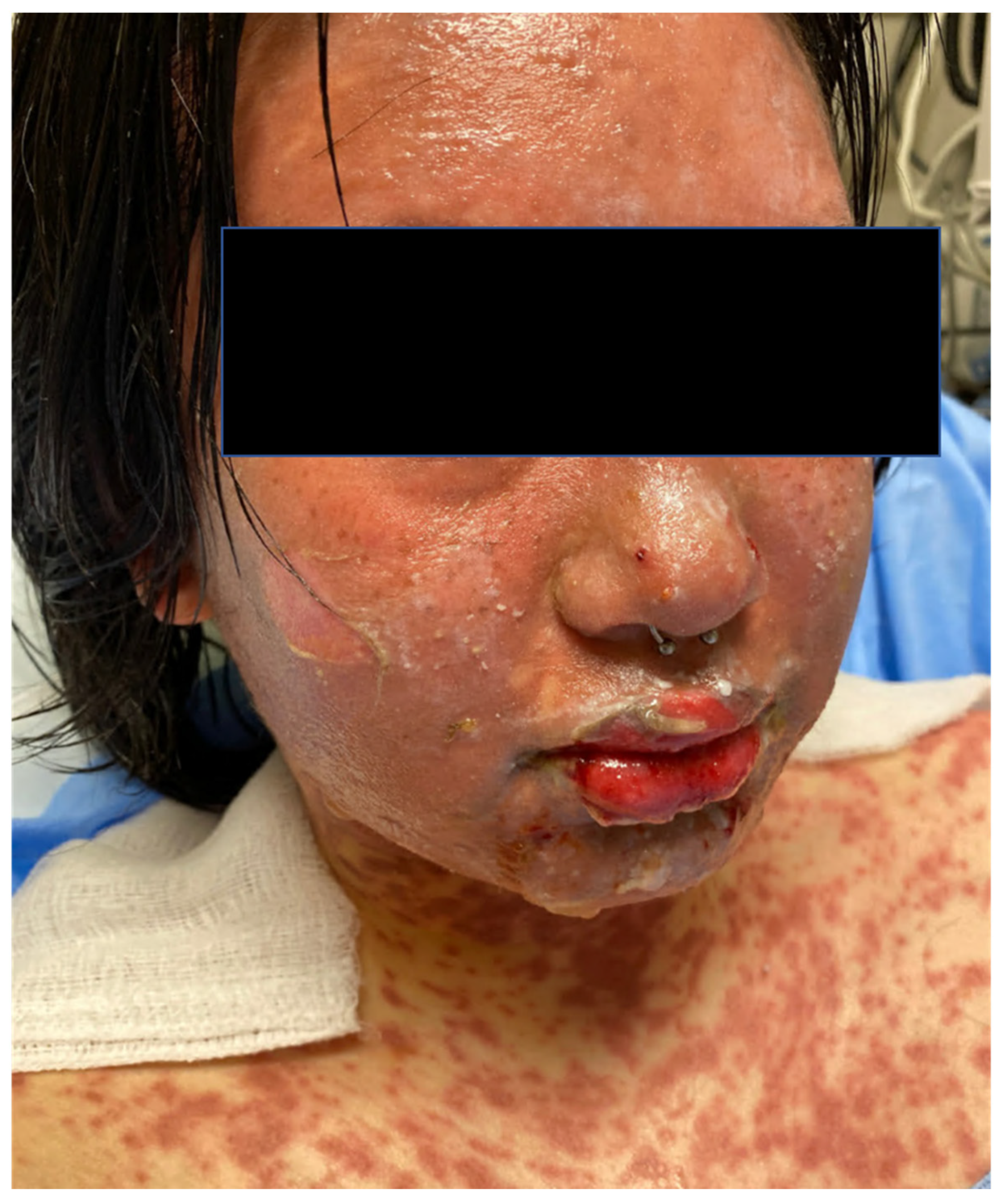
2. Clinical Features
3. Pathophysiology
4. Differential Diagnosis
5. Management
6. Materials and Methods
7. Clinical Updates
7.1. Updates on Diagnosis
7.1.1. Potential Biomarkers
7.1.2. Diagnostic Subclassification in Pediatric Patients
7.2. Updates on Management
7.2.1. Non-Pharmacologic Treatment
7.2.2. Pharmacologic Treatment
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Duong, T.A.; Valeyrie-Allanore, L.; Wolkenstein, P.; Chosidow, O. Severe cutaneous adverse reactions to drugs. Lancet 2017, 390, 1996–2011. [Google Scholar] [CrossRef]
- Frey, N.; Jossi, J.; Bodmer, M.; Bircher, A.; Jick, S.; Meier, C.R.; Spoendlin, J. The Epidemiology of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in the UK. J. Investig. Dermatol. 2017, 137, 1240–1247. [Google Scholar] [CrossRef]
- Hsu, D.; Brieva, J.; Silverberg, N.B.; Silverberg, J. Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J. Investig. Dermatol. 2016, 136, 1387–1397. [Google Scholar] [CrossRef]
- Yang, M.-S.; Lee, J.Y.; Kim, J.; Kim, G.-W.; Kim, B.-K.; Kim, J.-Y.; Park, H.-W.; Cho, S.-H.; Min, K.-U.; Kang, H.-R. Incidence of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Nationwide Population-Based Study Using National Health Insurance Database in Korea. PLoS ONE 2016, 11, e0165933. [Google Scholar] [CrossRef]
- Hsu, D.Y.; Brieva, J.; Silverberg, N.B.; Paller, A.S.; Silverberg, J.I. Pediatric Stevens-Johnson syndrome and toxic epidermal necroly-sis in the United States. J. Am. Acad. Dermatol. 2017, 76, 811–817.e4. [Google Scholar] [CrossRef] [PubMed]
- Paulmann, M.; Mockenhaupt, M. Severe skin reactions: Clinical picture, epidemiology, etiology, pathogenesis, and treatment. Allergo J. Int. 2019, 28, 311–326. [Google Scholar] [CrossRef]
- Lim, V.M.; Do, A.; Berger, T.G.; Nguyen, A.H.; Deweese, J.; Malone, J.D.; Jordan, K.; Hom, F.; Tuffanelli, L.; Fillari, P.; et al. A decade of burn unit experience with Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: Clinical pathological diagnosis and risk factor awareness. Burns 2016, 42, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Lissia, M.; Mulas, P.; Bulla, A.; Rubino, C. Toxic epidermal necrolysis (Lyell’s disease). Burns 2010, 36, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Richer, V.; Bouffard, D.; Belisle, A.; Duranceau, L.; Perreault, I.; Provost, N. Acute blistering diseases on the burn ward: Beyond Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis. Burns 2013, 39, 1290–1296. [Google Scholar] [CrossRef]
- Sekula, P.; Dunant, A.; Mockenhaupt, M.; Naldi, L.; Bavinck, J.N.B.; Halevy, S.; Kardaun, S.; Sidoroff, A.; Liss, Y.; Schumacher, M.; et al. Comprehensive Survival Analysis of a Cohort of Patients with Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2013, 133, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Paggiaro, A.O.; Silva, E.; Filho, M.L.; de Carvalho, V.F.; Isaac, C.; Gemperli, R. The Role of Biological Skin Substitutes in Ste-vens-Johnson Syndrome: Systematic Review. Plast. Surg. Nurs. Off. J. Am. Soc. Plast. Reconstr. Surg. Nurses 2018, 38, 121–127. [Google Scholar]
- Charlton, O.A.; Harris, V.; Phan, K.; Mewton, E.; Jackson, C.; Cooper, A. Toxic Epidermal Necrolysis and Steven-Johnson Syn-drome: A Comprehensive Review. Adv. Wound Care 2020, 9, 426–439. [Google Scholar] [CrossRef]
- Guvenir, H.; Arikoglu, T.; Vezir, E.; Misirlioglu, E.D. Clinical Phenotypes of Severe Cutaneous Drug Hypersensitivity Reactions. Curr. Pharm. Des. 2019, 25, 3840–3854. [Google Scholar] [CrossRef] [PubMed]
- Paulmann, M.; Mockenhaupt, M. Fever in Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis in Pediatric Cases. Pediatr. Infect. Dis. J. 2017, 36, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Alerhand, S.; Cassella, C.; Koyfman, A. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in the Pediatric Popula-tion: A Review. Pediatric Emerg. Care 2016, 32, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, P.; Mockenhaupt, M.; Panzer, R.; Emmert, S. Erythema multiforme, Stevens-Johnson syndrome/Toxic Epidermal Necrolysis—Diagnosis and treatment. JDDG J. Ger. Soc. Dermatol. 2020, 18, 547–553. [Google Scholar] [CrossRef]
- Shanbhag, S.; Chodosh, J.; Fathy, C.; Goverman, J.; Mitchell, C.; Saeed, H.N. Multidisciplinary care in Stevens-Johnson syndrome. Ther. Adv. Chronic Dis. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Shanbhag, S.S.; Gokani, A.; Kedar, R.; Bahuguna, C.; Sangwan, V.S. Chronic Ocular Sequelae of Stevens-Johnson Syndrome in Children: Long-term Impact of Appropriate Therapy on Natural History of Disease. Am. J. Ophthalmol. 2018, 189, 17–28. [Google Scholar] [CrossRef]
- Gregory, D.G. New Grading System and Treatment Guidelines for the Acute Ocular Manifestations of Stevens-Johnson Syn-drome. Ophthalmology 2016, 123, 1653–1658. [Google Scholar] [CrossRef]
- Aghdam, M.K.; Ahmadiafshar, A.; Eftekhari, K. Toxic epidermal necrolysis syndrome following single-dose diclofenac suppos-itory, a case report. J. Compr. Pediatrics 2020, 11, e100496. [Google Scholar]
- Alajmi, A.; Jfri, A.; Gomolin, A.; Jafarian, F. A pediatric case of Stevens-Johnson syndrome/Toxic Epidermal Necrolysis with rap-id response to intravenous cyclosporine. JAAD Case Rep. 2020, 6, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Maloney, N.J.; Ravi, V.; Cheng, K.; Bach, D.Q.; Worswick, S. Stevens-Johnson syndrome and Toxic Epidermal Necrolysis-like reac-tions to checkpoint inhibitors: A systematic review. Int. J. Dermatol. 2020, 59, e183–e188. [Google Scholar] [CrossRef]
- Simonsen, A.B.; Kaae, J.; Ellebaek, E.; Svane, I.M.; Zachariae, C. Cutaneous adverse reactions to anti–PD-1 treatment—A systematic review. J. Am. Acad. Dermatol. 2020, 83, 1415–1424. [Google Scholar] [CrossRef]
- Madabhavi, I.; Revannasiddaiah, S.; Patel, A.; Anand, A. Toxic epidermal necrolysis with the use of tamoxifen. BMJ Case Rep. 2015, 2015, bcr2014209102. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Monteleone, C.; Schalock, P.C.; Truong, T.; Zhang, X.B.; Wagner, M.L. Rashes and other hypersensitivity reactions associ-ated with antiepileptic drugs: A review of current literature. Seizure 2019, 71, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Kashyap, A.; Undela, K. Valproic acid and Stevens-Johnson syndrome: A systematic review of descriptive studies. Int. J. Dermatol. 2019, 58, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Sabharwal, N.; Patti, R.; Kupfer, Y. Allopurinol-Induced Stevens-Johnson Syndrome. Am. J. Med. Sci. 2019, 357, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, C.; Putterman, E.; Micheletti, R.; Treat, J.; Castelo-Soccio, L. Retrospective review of drug-induced Stevens-Johnson syn-drome and Toxic Epidermal Necrolysis cases at a pediatric tertiary care institution. Pediatric Dermatol. 2020, 37, 461–466. [Google Scholar] [CrossRef]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Le Louet, H.; et al. ALDEN, an algorithm for assessment of drug causal-ity in stevens-johnson syndrome and Toxic Epidermal Necrolysis: Comparison with case-control analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Abe, R. Recent advances in managing and understanding Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. F1000Research 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Abe, R. Immunological response in Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. J. Dermatol. 2015, 42, 42–48. [Google Scholar] [CrossRef]
- Adam, J.; Pichler, W.J.; Yerly, D. Delayed drug hypersensitivity: Models of T-cell stimulation. Br. J. Clin. Pharmacol. 2010, 71, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. Modes of presentation of chemical neoantigens to the immune system. Toxicology 2002, 181–182, 49–54. [Google Scholar] [CrossRef]
- Abe, R.; Shimizu, T.; Shibaki, A.; Nakamura, H.; Watanabe, H.; Shimizu, H. Toxic epidermal necrolysis and Stevens-Johnson syn-drome are induced by soluble fas ligand. Am. J. Pathol. 2003, 162, 1515–1520. [Google Scholar] [CrossRef]
- Chung, W.-H.; Hung, S.-I.; Yang, J.-Y.; Su, S.-C.; Huang, S.-P.; Wei, C.-Y.; Chin, S.-W.; Chiou, C.-C.; Chu, S.-C.; Ho, H.-C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Kuo, K.L.; Wang, C.W.; Lu, C.W.; Chung-Yee, H.R.; Lu, K.L.; Chang, W.C.; Chen, W.T.; Yun, F.; Teng, Y.C.; et al. Detecting Lesional Granulysin Levels for Rapid Di-agnosis of Cytotoxic T lymphocyte–Mediated Bullous Skin Disorders. J. Allergy Clin. Immunol. Pract. 2020, 9, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Yoshioka, N.; Murata, J.; Fujita, Y.; Shimizu, H. Granulysin as a marker for early diagnosis of the Stevens-Johnson syn-drome. Ann. Intern. Med. 2009, 151, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Abe, R.; Yoshioka, N.; Murata, J.; Fujita, Y.; Shimizu, H. Prolonged elevation of serum granulysin in drug-induced hy-persensitivity syndrome. Br. J. Dermatol. 2012, 167, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-C.; Mockenhaupt, M.; Wolkenstein, P.; Dunant, A.; le Gouvello, S.; Chen, C.-B.; Chosidow, O.; Valeyrie-Allanore, L.; Bellon, T.; Sekula, P.; et al. Interleukin-15 Is Associated with Severity and Mortality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2017, 137, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Shinkuma, S.; Hayashi, R.; Hama, N.; Watanabe, H.; Kinoshita, M.; Ogawa, Y.; Abe, R. 019 Serum RIP3 level as a severi-ty-predictive marker for Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2019, 139, S4. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, A.; Shinkuma, S.; Hayashi, R.; Hama, N.; Watanabe, H.; Kinoshita, M.; Ogawa, Y.; Abe, R. RIP3 as a diagnostic and severity marker for Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1768–1771.e7. [Google Scholar] [CrossRef]
- Saito, N.; Qiao, H.; Yanagi, T.; Shinkuma, S.; Nishimura, K.; Suto, A.; Fujita, Y.; Suzuki, S.; Nomura, T.; Nakamura, H.; et al. An annexin A1-FPR1 interaction contributes to necrop-tosis of keratinocytes in severe cutaneous adverse drug reactions. Sci. Transl. Med. 2014, 6, 245ra95. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.S.; Battle, L.R.; Emerson, A.N.; Gardner, J.M.; Shalin, S.C. Dermatologic Urgencies and Emergencies: What Every Pathologist Should Know. Arch. Pathol. Lab. Med. 2019, 143, 919–942. [Google Scholar] [CrossRef]
- Kerl, K.; Kerl, H. Severe cutaneous adverse drug reactions. Diagn. Histopathol. 2020, 27, 1–5. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, C.-B.; Wang, C.-W.; Hung, S.-I.; Chung, W.-H. Stevens-Johnson syndrome and Toxic Epidermal Necrolysis: Risk factors, causality assessment and potential prevention strategies. Expert Rev. Clin. Immunol. 2020, 16, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Doval, I.; LeCleach, L.; Bocquet, H.; Otero, X.L.; Roujeau, J.C. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch. Dermatol. 2000, 136, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Cavkaytar, O.; Kuyucu, S. An Update on the Management of Severe Cutaneous Drug Hypersensitivity Reactions. Curr. Pharm. Des. 2019, 25, 3881–3901. [Google Scholar] [CrossRef]
- Gallagher, R.M.; Kirkham, J.J.; Mason, J.R.; Bird, K.A.; Williamson, P.R.; Nunn, A.J.; Turner, M.A.; Smyth, R.L.; Pirmohamed, M. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE 2011, 6, e28096. [Google Scholar] [CrossRef] [PubMed]
- Coias, J.L.; Abbas, L.F.; Cardones, A.R. Management of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Review and Update. Curr. Dermatol. Rep. 2019, 8, 219–233. [Google Scholar] [CrossRef]
- Hu, C.-H.; Chang, N.-J.; Liu, E.-W.; Chuang, S.-S.; Chung, W.-H.; Yang, J.-Y. SCORTEN and impaired renal function related to mortality of Toxic Epidermal Necrolysis syndrome patients in the Asian population. J. Eur. Acad. Dermatol. Venereol. 2012, 27, 628–633. [Google Scholar] [CrossRef]
- Torres-Navarro, I.; Briz-Redón, Á.; Botella-Estrada, R. Accuracy of SCORTEN to predict the prognosis of Stevens-Johnson syn-drome/Toxic Epidermal Necrolysis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2066–2077. [Google Scholar] [CrossRef]
- Imahara, S.D.; Holmes, J.H.; Heimbach, D.M.; Engrav, L.E.; Honari, S.; Klein, M.B.; Gibran, N. SCORTEN Overestimates Mortality in the Setting of a Standardized Treatment Protocol. J. Burn. Care Res. 2006, 27, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, R.G.; Chiesa-Fuxench, Z.; Noe, M.H.; Stephen, S.; Aleshin, M.; Agarwal, A.; Boggs, J.; Cardones, A.R.; Chen, J.K.; Cotliar, J.; et al. Stevens-Johnson Syndrome/Toxic Epi-dermal Necrolysis: A Multicenter Retrospective Study of 377 Adult Patients from the United States. J. Investig. Dermatol. 2018, 138, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Noe, M.H.; Rosenbach, M.; Hubbard, R.A.; Mostaghimi, A.; Cardones, A.R.; Chen, J.K.; Cotliar, J.; Davis, M.D.P.; Dominguez, A.; Fox, L.P.; et al. Development and Validation of a Risk Prediction Model for In-Hospital Mortality Among Patients With Stevens-Johnson Syndrome/Toxic Epidermal Necroly-sis-ABCD-10. JAMA Dermatol. 2019, 155, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Torres-Navarro, I.; Briz-Redón, Á.; Botella-Casas, G.; Sahuquillo-Torralba, A.; Calle-Andrino, A.; de Unamuno-Bustos, B.; Piqueras-García, J.; Ginés, J.R.; Tapial, J.M.; de Miquel, V.A.; et al. Accuracy of SCORTEN and ABCD-10 to predict mortality and the influence of renal function in Stevens–Johnson syn-drome/Toxic Epidermal Necrolysis. J. Dermatol. 2020, 47, 1182–1186. [Google Scholar] [CrossRef]
- Creamer, D.; Walsh, S.A.; Dziewulski, P.; Exton, L.S.; Lee, H.Y.; Dart, J.K.G.; Setterfield, J.; Bunker, C.B.; Ardern-Jones, M.R.; Watson, K.M.T.; et al. UK guidelines for the management of Ste-vens-Johnson syndrome/Toxic Epidermal Necrolysis in adults 2016. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, e119–e153. [Google Scholar] [CrossRef]
- McCullough, M.; Burg, M.; Lin, E.; Peng, D.; Garner, W. Steven Johnson Syndrome and Toxic Epidermal Necrolysis in a burn unit: A 15-year experience. Burn 2017, 43, 200–205. [Google Scholar] [CrossRef] [PubMed]
- McPherson, T.; Exton, L.S.; Biswas, S.; Creamer, D.; Dziewulski, P.; Newell, L.; Tabor, K.L.; Wali, G.N.; Walker, G.; Walker, R.; et al. British Association of Dermatologists’ guide-lines for the management of Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis in children and young people. Br. J. Dermatol. 2019, 181, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Tapia, B.; Padial, A.; Sánchez-Sabaté, E.; Alvarez-Ferreira, J.; Morel, E.; Blanca, M.; Bellón, T. Involvement of CCL27-CCR10 interactions in drug-induced cutaneous reactions. J. Allergy Clin. Immunol. 2004, 114, 335–340. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Y.; Luo, Z.Y.; Gao, Q.; Luo, D.Q.; Zhang, X. Diverse expression of TNF-α and CCL27 in serum and blister of Ste-vens-Johnson syndrome/Toxic Epidermal Necrolysis. Clin. Transl. Allergy 2018, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hama, N.; Nishimura, K.; Hasegawa, A.; Yuki, A.; Kume, H.; Adachi, J.; Kinoshita, M.; Ogawa, Y.; Nakajima, S.; Nomura, T.; et al. Galectin-7 as a potential biomarker of Ste-vens-Johnson syndrome/Toxic Epidermal Necrolysis: Identification by targeted proteomics using causative drug-exposed peripheral blood cells. J. Allergy Clin. Immunol. Pract. 2019, 7, 2894–2897.e7. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, W.J.; Yoon, J.H.; Ji, J.H.; Morgan, M.J.; Cho, H.; Kim, Y.C.; Kim, Y.-S. Upregulated RIP3 Expression Potentiates MLKL Phosphoryla-tion-Mediated Programmed Necrosis in Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2015, 135, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Canavan, T.N.; Mathes, E.F.; Frieden, I.; Shinkai, K. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: A systematic review. J. Am. Acad. Dermatol. 2015, 72, 239–245.e4. [Google Scholar] [CrossRef]
- Gámez-González, L.B.; Peña-Varela, C.; Ramírez-López, J.M.; Yamazaki-Nakashimada, M.A. Adenoviral-induced rash and mu-cositis: Expanding the spectrum of reactive infectious mucocutaneous eruption. Pediatric Dermatol. 2020, 38, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Hook, K. Two pediatric cases of influenza B-induced rash and mucositis: Stevens-Johnson syndrome or expansion of the Mycoplasma pneumoniae -induced rash with mucositis (MIRM) spectrum? Pediatr. Dermatol. 2019, 36, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Mayor-Ibarguren, A.; Feito-Rodriguez, M.; González-Ramos, J.; del Rosal-Rabes, T.; González-Sainz, F.J.; Sánchez-Orta, A.; de Lucas-Laguna, R. Mucositis Secondary to Chlamydia pneumoniae Infection: Expanding the Mycoplasma pneumoniae–Induced Rash and Mu-cositis Concept. Pediatric Dermatol. 2017, 34, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ramien, M.; Goldman, J.L. Pediatric SJS-TEN: Where are we now? F1000Research 2020, 9, 982. [Google Scholar] [CrossRef]
- Ramien, M.L.; Bahubeshi, A.; Eichenfield, L.; Lara-Corrales, I.; Nopper, A.J.; Pope, E.; Levy, M.L.; Shear, N.H. Redefining severe cutaneous reactions in children. Pediatric Dermatol. 2018, 35, 716–717. [Google Scholar]
- Ramien, M.; Bruckner, A.L. Mucocutaneous Eruptions in Acutely Ill Pediatric Patients—Think of Mycoplasma pneumoniae (and Other Infections) First. JAMA Dermatol. 2020, 156, 124. [Google Scholar] [CrossRef]
- Ramien, M.L. Reactive infectious mucocutaneous eruption: Mycoplasma pneumoniae -induced rash and mucositis and other parainfectious eruptions. Clin. Exp. Dermatol. 2021, 46, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Kamath, S.; Hughes, M.; Harter, N.; Luu, M. Evaluation of Etanercept for Treatment of Reactive Infectious Mucocu-taneous Eruption. JAMA Dermatol. 2021, 157, 230–232. [Google Scholar] [CrossRef]
- Gupta, L.K.; Martin, A.M.; Agarwal, N.; D’Souza, P.; Das, S.; Kumar, R.; Pande, S.; Das, N.K.; Kumareshan, M.; Kumar, P.; et al. Guidelines for the management of Stevens-Johnson syndrome/Toxic Epidermal Necrolysis: An Indian perspective. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Cohen, P.R. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Compre-hensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv. Ther. 2017, 34, 1235–1244. [Google Scholar] [CrossRef]
- Liotti, L.; Caimmi, S.; Bottau, P.; Bernardini, R.; Cardinale, F.; Saretta, F.; Francesca, M.; Giuseppe, C.; Fabrizio, F.; Carlo, C. Clinical features, outcomes and treatment in chil-dren with drug induced Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. Acta Biomed. 2019, 90, 52–60. [Google Scholar] [PubMed]
- Palmieri, T.L.; Greenhalgh, D.G.; Saffle, J.R.; Spence, R.J.; Peck, M.D.; Jeng, J.C.; Mozingo, D.W.; Yowler, C.J.; Sheridan, R.L.; Ahrenholz, D.H.; et al. A multicenter review of toxic epidermal necroly-sis treated in U.S. burn centers at the end of the twentieth century. J. Burn Care Rehabil. 2002, 23, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Dorafshar, A.H.; Dickie, S.R.; Cohn, A.B.; Aycock, J.K.; O’Connor, A.; Tung, A.; Gottlieb, L. Antishear therapy for Toxic Epidermal Necrolysis: An alternative treatment approach. Plast. Reconstr. Surg. 2008, 122, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jaller, J.A.; McLellan, B.N.; Balagula, Y. Wound Management in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Curr. Dermatol. Rep. 2020, 9, 58–72. [Google Scholar] [CrossRef]
- Chafranska, L.; Saunte, D.M.; Behrendt, N.; Nygaard, U.; Christensen, R.J.; Sand, C.; Jemec, G.B. Pediatric Toxic Epidermal Necrolysis treated successfully with infliximab. Pediatric Dermatol. 2019, 36, 342–345. [Google Scholar] [CrossRef]
- Nassim, J.S.; Karim, S.A.; Grenier, P.; Schmidt, B.; Jones, K.M. Infantile Toxic Epidermal Necrolysis: Successful treatment of an 8-week-old with intravenous immunoglobulin and amniotic membrane transplant. Pediatric Dermatol. 2021, 38, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhong, S.; Tu, P.; Li, R.; Wang, M. Rapid remission of Stevens-Johnson syndrome by combination therapy using etanercept and intravenous immunoglobulin and a review of the literature. Dermatol. Ther. 2019, 32, e12832. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, J.; Belzile, E.; Duhamel, A.; Rault, P.; Buteau, C.; Debruycker, J.-J.; Bussières, J.-F. Pediatric SJS/TEN Subdued by a Combination of Dexamethasone, Cyclosporine, and Etanercept. J. Cutan. Med. Surg. 2019, 23, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Michaels, B.; del Rosso, J.Q. The role of systemic corticosteroid therapy in erythema multiforme major and stevens-johnson syndrome a review of past and current opinions. J. Clin. Aesthetic Dermatol. 2009, 2, 51. [Google Scholar]
- Zimmermann, S.; Sekula, P.; Venhoff, M.; Motschall, E.; Knaus, J.; Schumacher, M.; Mockenhaupt, M. Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017, 153, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Li, Y.-C.; Chen, T.-J. The efficacy of intravenous immunoglobulin for the treatment of Toxic Epidermal Necrolysis: A systematic review and meta-analysis. Br. J. Dermatol. 2012, 167, 424–432. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lim, Y.L.; Thirumoorthy, T.; Pang, S.M. The role of intravenous immunoglobulin in Toxic Epidermal Necrolysis: A ret-rospective analysis of 64 patients managed in a specialized centre. Br. J. Dermatol. 2013, 169, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Scherrer, L.A. Efficacy and safety of cyclosporine in Stevens-Johnson syndrome and Toxic Epidermal Necrolysis. Dermatol. Ther. 2019, 32, e12758. [Google Scholar] [CrossRef] [PubMed]
- González-Herrada, C.; Rodríguez-Martín, S.; Cachafeiro, L.; Lerma, V.; González, O.; Lorente, J.A.; Rodríguez-Miguel, A.; González-Ramos, J.; Roustan, G.; Ramírez, E.; et al. Cyclosporine Use in Epidermal Necrolysis Is Associated with an Important Mortality Reduction: Evidence from Three Different Approaches. J. Investig. Dermatol. 2017, 137, 2092–2100. [Google Scholar] [CrossRef]
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Venkatanarayanan, N.; Ho, C.Y.X.; Yeo, W.-S. A meta-analysis of cyclosporine treatment for Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis. J. Inflamm. Res. 2018, 11, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Hsu, C.-Y.; Chien, Y.-N.; Lee, W.-R.; Huang, Y.-C. Efficacy of cyclosporine for the treatment of Stevens-Johnson syndrome and Toxic Epidermal Necrolysis: Systemic review and meta-analysis. Dermatol. Sin. 2017, 35, 131–137. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Huang, I.H.; Chao, Y.C.; Li, H.; Hsieh, T.S.; Wang, H.H.; Huang, Y.T.; Chen, C.Y.; Cheng, Y.C.; Kuo, P.H.; et al. Treating Toxic Epidermal Necrolysis with systemic immunomodulating therapies: A systematic review and network meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 390–397. [Google Scholar] [CrossRef]
- Fernandez, A.P.; Kerdel, F.A. The use of i.v. IG therapy in dermatology. Dermatol. Ther. 2007, 20, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, J.; Guo, Q.; Feng, Y.; Gao, Y.; Guo, L.; Hou, Y.; An, J.; Wang, X.; Yan, B.; et al. Successful treatment of Toxic Epidermal Necrolysis using plasmaphere-sis: A prospective observational study. J. Crit. Care 2017, 42, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, S.; Li, S.; Pan, Y.; Ding, Y. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and Toxic Epidermal Necrolysis: A systemic review. J. Dermatol. Treat. 2020, 31, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-W.; Yang, L.-Y.; Chen, C.-B.; Ho, H.-C.; Hung, S.-I.; Yang, C.-H.; Chang, C.-J.; Su, S.-C.; Hui, R.C.-Y.; Chin, S.-W.; et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J. Clin. Investig. 2018, 128, 985–996. [Google Scholar] [CrossRef]
| Diagnosis Based on BSA (%) | |
|---|---|
| SJS | <10% |
| SJS/TEN Overlap | 10–30% |
| TEN | >30% |
| Common Drug Triggers of SJS/TEN | |
|---|---|
| Anti-epileptics | Antibiotics |
| ○ Lamotrigine | ○ TMP-SMX |
| ○ Phenytoin | ○ Aminopenicillins |
| ○ Carbamazepine | ○ Tetracyclines |
| ○ Valproic Acid | ○ Cephalosporins |
| ○ Phenobarbital | Immune Checkpoint Inhibitors |
| NSAIDs | ○ Nivolumab |
| Allopurinol | ○ Pembrolizumab |
| Nevirapine | |
| Differential Diagnosis of SJS/TEN | |
|---|---|
| Erythema multiforme major | Pemphigus vulgaris |
| Staphylococcal scalded skin syndrome | Bullous pemphigoid |
| Generalized fixed drug eruption (BFDE) | Linear IgA bullous dermatosis |
| Acute generalized exanthematous pustulosis | Paraneoplastic pemphigus |
| Phototoxic eruptions | Acute or subacute cutaneous lupus with epidermal necrosis (Rowell syndrome) |
| SJS/TEN vs. EM | ||
|---|---|---|
| SJS/TEN | EM | |
| Characteristic Lesions | Atypical target lesions: macules with central clearing and 2 poorly demarcated components | Typical target lesions: papules with a dark center and 3 well-demarcated, concentric components |
| Large sheets of painful desquamation in later lesions | ||
| Distribution | Typically begins on the face and trunk with centrifugal spread | Face and acral skin, rare involvement of trunk |
| Triggers | Drugs (see Table 2) | Infection (most commonly HSV and M. pneumonia) |
| Mucosal Involvement | Very common—most cases have involvement of ≥2 mucosal surfaces | Rare—typically only one mucosal surface involved if present |
| Recurrence | Rarely seen with removal and avoidance of causative drug | Frequently seen |
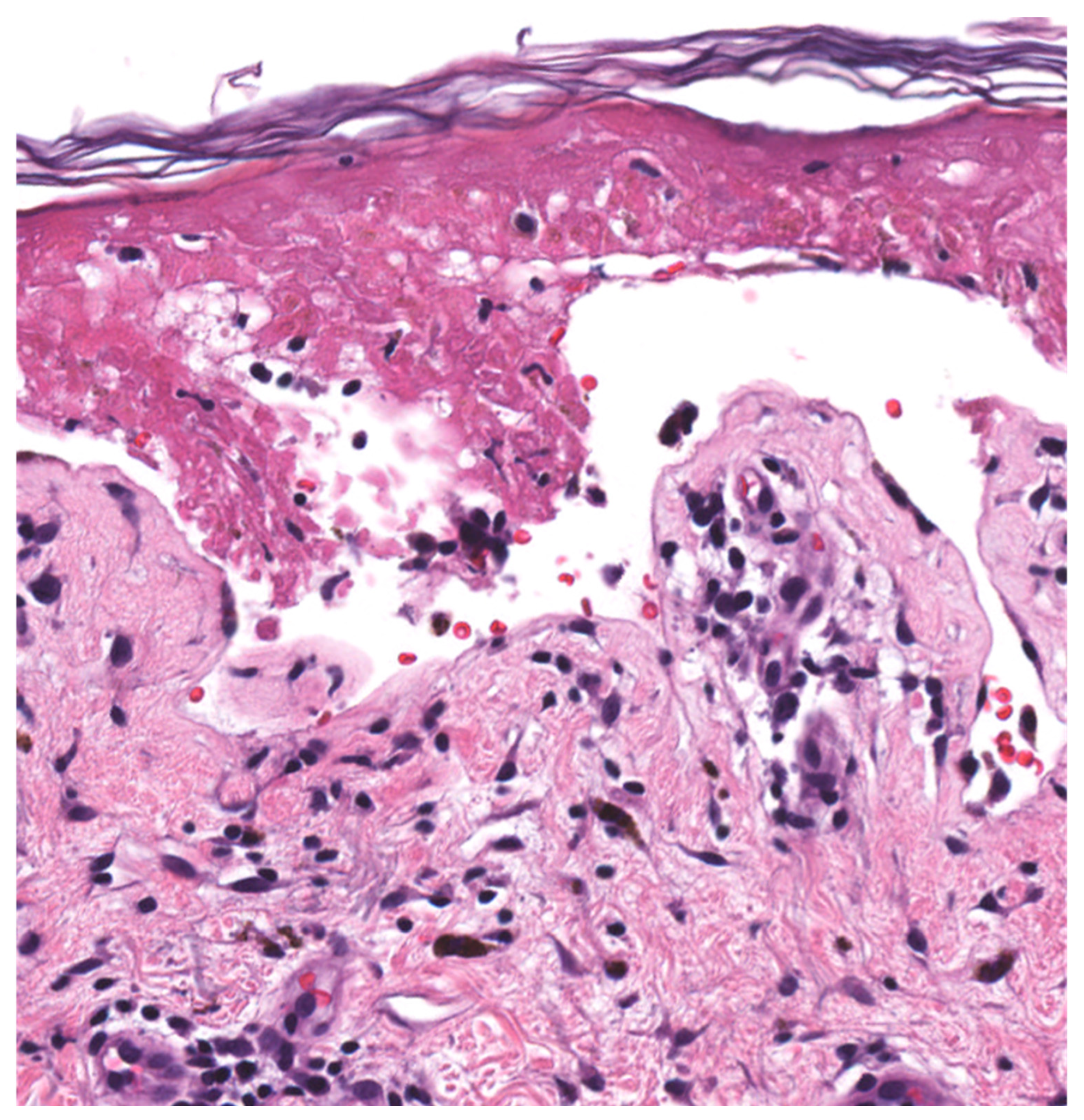
| Histopathology (Figure 2) | Early Stage Basal layer liquefaction with vacuolar interface changes, scattered necrotic keratinocytes, and interface lymphocytes | |
| Late Stage * Subepidermal split with full-thickness epidermal necrosis | ||
| SCORTEN | ABCD-10 | ||
|---|---|---|---|
| Parameter | Weight | Parameter | Weight |
| Age ≥ 40 years | 1 | Age ≥ 50 years | 1 |
| Malignancy—Yes | 1 | Serum Bicarbonate < 20 mmol/L | 1 |
| BSA detached > 10% | 1 | Active Cancer—Yes | 2 |
| Serum bicarbonate < 20 mmol/L | 1 | Dialysis prior to admission—Yes | 3 |
| Serum urea nitrogen > 28 mg/dL | 1 | BSA Involvement > 10% | 1 |
| Serum glucose > 252 mg/dL | 1 | ||
| Tachycardia ≥ 120 bpm | 1 | ||
| Maximum score possible | 7 | 8 | |
| Estimated Mortality in Patients with SJS/TEN | |||
|---|---|---|---|
| SCORTEN Score | Estimated Mortality (%) | ABCD-10 Score | Estimated Mortality (%) |
| 0–1 | 3.2 | 0 | 2.3 |
| 2 | 12.1 | 1 | 5.4 |
| 3 | 35.3 | 2 | 12.3 |
| 4 | 58.3 | 3 | 25.5 |
| >5 | >90 | 4 | 45.7 |
| 5 | 67.4 | ||
| >6 | 83.6 | ||
| Common Drug Triggers of SJS/TEN | |
|---|---|
| Non-Specific for SJS/TEN | Specific for SJS/TEN |
| ○ Granulysin ○ CCL-27 | ○ Galectin-7 ○ RIP3 |
| Dosing Regimen for SJS/TEN of Selected Drugs | |
|---|---|
| IVIg | 3 g/kg, divided over 3 days [90] |
| TNF-alpha inhibitors | - Infliximab: 5 mg/kg as a single dose [92] - Etanercept: Single 50 mg dose [92] |
| Cyclosporine | 2.5–5 mg/kg/day for 7–10 days, followed by gradual taper [87,88] |
| Corticosteroids | Prednisone 0.5–1 mg/kg/day or pulse methylprednisolone 1 mg/kg/d for 3 days [81] |
| Relative Cost of Selected Drugs ** | |
|---|---|
| IVIG | $1932 for a treatment course * |
| Etanercept | $1386 for a single 50 mg subcutaneous dose |
| Infliximab | $4900 * |
| Cyclosporine | ~$336 for a 3-week course/taper at $16 per day |
| Prednisone | <$20 for 2–3-week taper at $1 per day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frantz, R.; Huang, S.; Are, A.; Motaparthi, K. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina 2021, 57, 895. https://doi.org/10.3390/medicina57090895
Frantz R, Huang S, Are A, Motaparthi K. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina. 2021; 57(9):895. https://doi.org/10.3390/medicina57090895
Chicago/Turabian StyleFrantz, Robert, Simo Huang, Abhirup Are, and Kiran Motaparthi. 2021. "Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management" Medicina 57, no. 9: 895. https://doi.org/10.3390/medicina57090895
APA StyleFrantz, R., Huang, S., Are, A., & Motaparthi, K. (2021). Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina, 57(9), 895. https://doi.org/10.3390/medicina57090895






