Current Evidence about Developmental Dysplasia of the Hip in Pregnancy
Abstract
1. Introduction
2. Classifications of the Female Pelvis, Based on Bone and Ligament Morphology, and Their Relationships to Pelvic Osteotomy
2.1. Pelvis Morphology
2.2. Pelvic Osteotomy
2.3. Effects of a Pelvic Osteotomy on the Birth Mechanism
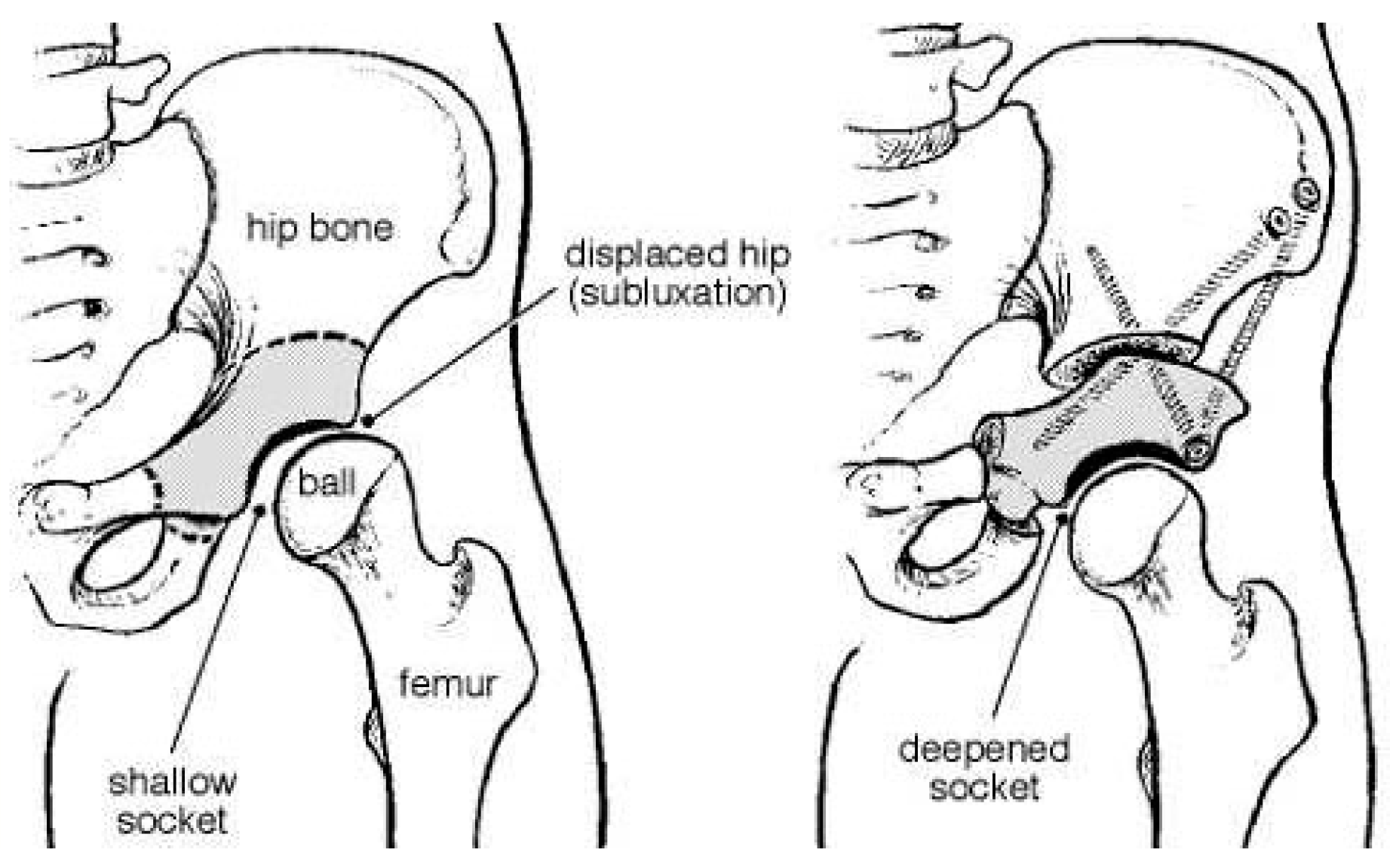
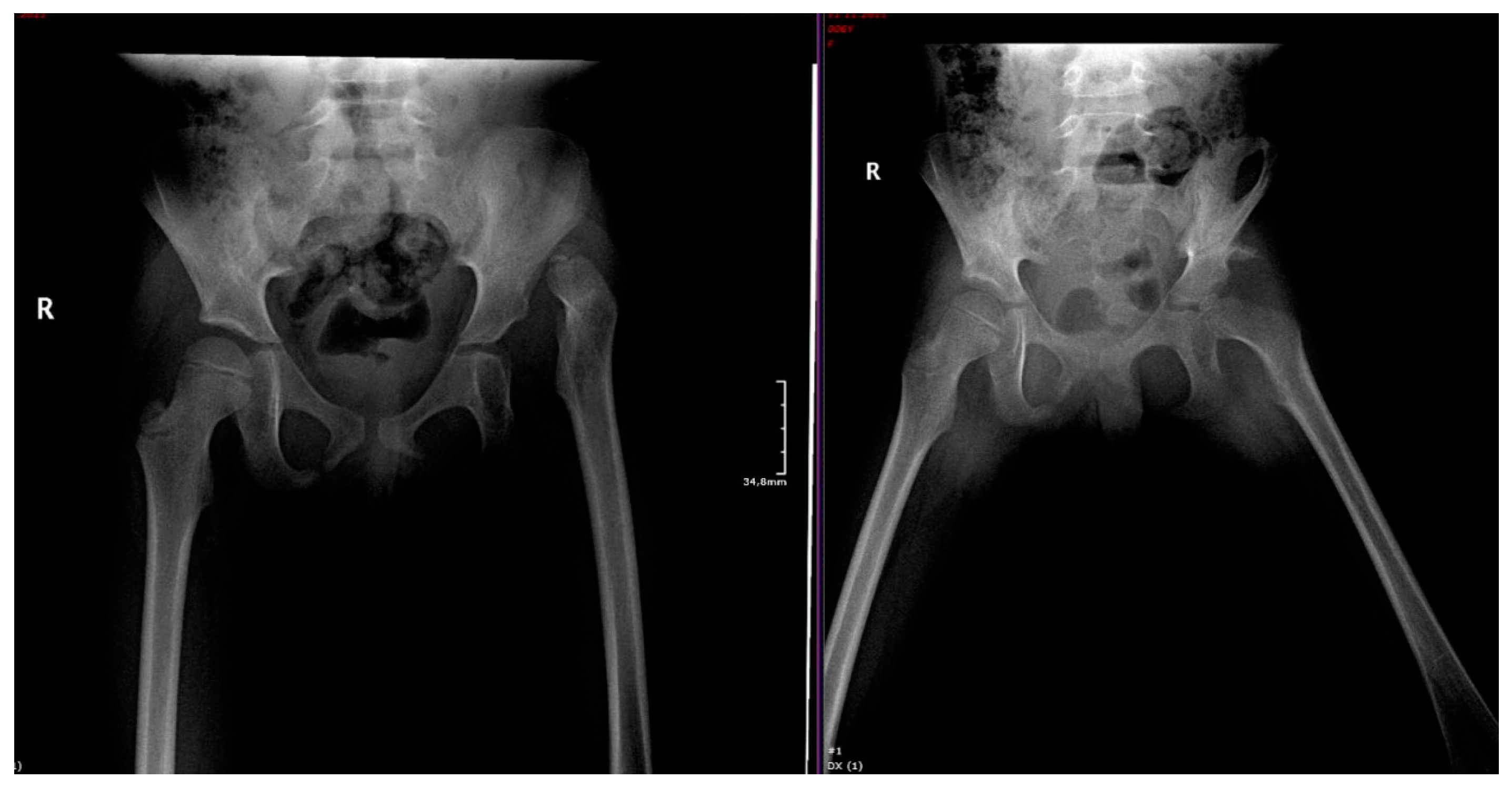
3. Hip Dysplasia at Birth: From Diagnosis to Appropriate Treatment
4. Management of Pregnancy Associated with Maternal DDH
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manaster, B.J. Adult Chronic Hip Pain: Radiographic Evaluation. Radiographics 2000, 20. [Google Scholar] [CrossRef][Green Version]
- Bracken, J.; Tran, T.; Ditchfield, M. Developmental dysplasia of the hip: Controversies and current concepts. J. Paediatr. Child Health 2012, 48, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Gosvig, K.K.; Jacobsen, S.; Sonne-Holm, S.; Palm, H.; Troelsen, A. Prevalence of Malformations of the Hip Joint and Their Relationship to Sex, Groin Pain, and Risk of Osteoarthritis. J. Bone Jt. Surg. Am. 2010, 92, 1162–1169. [Google Scholar] [CrossRef]
- Jacobsen, S.; Sonne-Holm, S. Hip dysplasia: A significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology 2005, 44, 211–218. [Google Scholar] [CrossRef]
- Hines, A.C.; Neal, D.; Beckwith, T.; Jo, C.; Kim, H.K. A Comparison of Pavlik Harness Treatment Regimens for Dislocated But Reducible (Ortolani+) Hips in Infantile Developmental Dysplasia of the Hip. J. Pediatr. Orthop. 2019, 39, 505–509. [Google Scholar] [CrossRef] [PubMed]
- LeBa, T.-B.; Carmichael, K.D.; Patton, A.G.; Morris, R.P.; Swischuk, L.E. Ultrasound for Infants at Risk for Developmental Dysplasia of the Hip. Orthopedics 2015, 38, e722–e726. [Google Scholar] [CrossRef] [PubMed]
- Õmeroğlu, H. Use of ultrasonography in developmental dysplasia of the hip. J. Child. Orthop. 2014, 8, 105–113. [Google Scholar] [CrossRef]
- Wynne-Davies, R. Acetabular dysplasia and familial joint laxity: Two etiological factors in congenital dislocation of the hip. A review of 589 patients and their families. J. Bone Jt. Surg. Br. 1970, 52, 704–716. [Google Scholar] [CrossRef]
- Yoshimura, N.; Campbell, L.; Hashimoto, T.; Kinoshita, H.; Okayasu, T.; Wilman, C.; Coggon, D.; Croft, P.; Cooper, C. Acetabular dysplasia and hip osteoarthritis in Britain and Japan. Rheumatology 1998, 37, 1193–1197. [Google Scholar] [CrossRef]
- Gala, L.; Clohisy, J.C.; Beaulé, P.E. Hip Dysplasia in the Young Adult. J. Bone Jt. Surg. Am. 2016, 98, 63–73. [Google Scholar] [CrossRef]
- Wedge, J.; Wasylenko, M. The natural history of congenital disease of the hip. J. Bone Jt. Surg. Br. 1979, 61, 334–338. [Google Scholar] [CrossRef]
- Price, C.T.; Ramo, B.A. Prevention of Hip Dysplasia in Children and Adults. Orthop. Clin. N. Am. 2012, 43, 269–279. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Mubarak, S.J.; Wenger, D.R. Developmental hip dysplasia and dislocation: Part I. Instr. Course Lect. 2004, 53, 523–530. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Mubarak, S.J.; Wenger, D.R. Developmental hip dysplasia and dislocation: Part II. Instr. Course Lect. 2004, 53, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Mata-Fink, A.; Millis, M.B.; Kim, Y.-J. Demographic Differences in Adolescent-Diagnosed and Adult-Diagnosed Acetabular Dysplasia Compared with Infantile Developmental Dysplasia of the Hip. J. Pediatr. Orthop. 2013, 33, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Neira, C.L.; Paolucci, E.O.; Donnon, T. A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur. J. Radiol. 2012, 81, e344–e351. [Google Scholar] [CrossRef] [PubMed]
- Loder, R.T.; Skopelja, E.N. The Epidemiology and Demographics of Hip Dysplasia. ISRN Orthop. 2011, 2011, 1–46. [Google Scholar] [CrossRef]
- Woodacre, T.; Ball, T.; Cox, P. Epidemiology of developmental dysplasia of the hip within the UK: Refining the risk factors. J. Child. Orthop. 2016, 10, 633–642. [Google Scholar] [CrossRef]
- Aronsson, D.D.; Goldberg, M.J.; Kling, T.F., Jr.; Roy, D.R. Developmental dysplasia of the hip. Pediatrics 1994, 94, 201–208. [Google Scholar]
- Uhorchak, J.M.; Scoville, C.R.; Williams, G.N.; Arciero, R.A.; Pierre, P.S.; Taylor, D.C. Risk factors associated with noncontact injury of the anterior cruciate ligament: A prospective four-year evaluation of 859 West Point cadets. Am. J. Sports Med. 2003, 31, 831–842. [Google Scholar] [CrossRef]
- Zazulak, B.T.; Ponce, P.L.; Straub, S.J.; Medvecky, M.J.; Avedisian, L.; Hewett, T.E. Gender Comparison of Hip Muscle Activity During Single-Leg Landing. J. Orthop. Sports Phys. Ther. 2005, 35, 292–299. [Google Scholar] [CrossRef]
- Zeller, B.L.; McCrory, J.L.; Ben Kibler, W.; Uhl, T.L. Differences in Kinematics and Electromyographic Activity between Men and Women during the Single-Legged Squat. Am. J. Sports Med. 2003, 31, 449–456. [Google Scholar] [CrossRef]
- Lange, A.E.; Lange, J.; Ittermann, T.; Napp, M.; Krueger, P.-C.; Bahlmann, H.; Kasch, R.; Heckmann, M. Population-based study of the incidence of congenital hip dysplasia in preterm infants from the Survey of Neonates in Pomerania (SNiP). BMC Pediatr. 2017, 17, 78. [Google Scholar] [CrossRef]
- Hinderaker, T.; Daltveit, A.K.; Irgens, L.M.; Udén, A.; Reikeräs, O. The impact of intra-uterine factors on neonatal hip instability: An analysis of 1,059,479 children in Norway. Acta Orthop. Scand. 1994, 65, 239–242. [Google Scholar] [CrossRef]
- Chan, A.; McCaul, K.A.; Cundy, P.J.; Haan, E.A.; Byron-Scott, R. Perinatal risk factors for developmental dysplasia of the hip. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 76, F94–F100. [Google Scholar] [CrossRef]
- Dunn, P.M. Perinatal observations on the etiology of congenital dislocation of the hip. Clin. Orthop. Relat. Res. 1976, 119, 11–22. [Google Scholar] [CrossRef]
- Kojima, S.; Kobayashi, S.; Saito, N.; Nawata, M.; Horiuchi, H.; Takaoka, K. Morphological characteristics of the bony birth canal in patients with developmental dysplasia of the hip (DDH): Investigation by three-dimensional CT. J. Orthop. Sci. 2001, 6, 217–222. [Google Scholar] [CrossRef]
- Kojima, S.; Kobayashi, S.; Saito, N.; Nawata, M.; Horiuchi, H.; Takaoka, K. Three-dimensional computed tomography evaluation of bony birth canal morphologic deformity (small pelvic cavity) after dome pelvic osteotomy for developmental dysplasia of the hip. Am. J. Obstet. Gynecol. 2002, 187, 1591–1595. [Google Scholar] [CrossRef]
- Savulescu, D. Labour, delivery and Pelvic bone. In Obstetrics, 1st ed.; Editura Medicala: Bucharest, Romania, 1956; pp. 161–167. [Google Scholar]
- Longo, L.D. Classic pages in obstetrics and gynecology. Anatomical variations in the female pelvis and their effect in labor with a suggested classification. William Edgar Caldwell and Howard Carmen Moloy. American Journal of Obstetrics and Gynecology, vol. 26, pp. 479–505, 1933. Am. J. Obstet. Gynecol. 1977, 127, 798. [Google Scholar]
- Maharaj, D. Assessing Cephalopelvic Disproportion: Back to the Basics. Obstet. Gynecol. Surv. 2010, 65, 387–395. [Google Scholar] [CrossRef]
- Winkelmann, W. The narrowing of the bony pelvic cavity (birth canal) by the different osteotomies of the pelvis. Arch. Orthop. Trauma Surg. 1984, 102, 159–162. [Google Scholar] [CrossRef]
- Pemberton, P.A. Pericapsular Osteotomy of the Ilium for Treatment of Congenital Subluxation and Dislocation of the Hip. J. Bone Jt. Surg. Am. 1965, 47, 65–86. [Google Scholar] [CrossRef]
- Ganz, R.; Klaue, K.; Vinh, T.; Mast, J.W. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin. Orthop. Relat. Res. 1988, 232, 26–36. [Google Scholar] [CrossRef]
- Trousdale, R.T.; Ekkernkamp, A.; Ganz, R.; Wallrichs, S.L. Periacetabular and intertrochanteric osteotomy for the treatment of osteoarthrosis in dysplastic hips. J. Bone Jt. Surg. Am. 1995, 77, 73–85. [Google Scholar] [CrossRef]
- Murphy, S.B.; Kijewski, P.K.; Millis, M.B.; Harless, A. Acetabular dysplasia in the adolescent and young adult. Clin. Orthop. Relat. Res. 1990, 261, 214–223. [Google Scholar] [CrossRef]
- Trumble, S.J.; Mayo, K.A.; Mast, J.W. The periacetabular osteotomy. Minimum 2 year followup in more than 100 hips. Clin. Orthop. Relat. Res. 1999, 54–63. [Google Scholar]
- Loder, R.T.; Karol, L.A.; Johnson, S. Influence of pelvic osteotomy on birth canal size. Arch. Orthop. Trauma Surg. 1993, 112, 210–214. [Google Scholar] [CrossRef]
- Trousdale, R.T.; Cabanela, M.E.; Berry, D.J.; Wenger, D.E. Magnetic resonance imaging pelvimetry before and after a periacetabular osteotomy. J. Bone Jt. Surg. Am. 2002, 84, 552–556. [Google Scholar] [CrossRef]
- Varner, M.W.; Cruikshank, D.P.; Laube, D.W. X-ray pelvimetry in clinical obstetrics. Obstet. Gynecol. 1980, 56, 296–299. [Google Scholar]
- Flückiger, G.; Eggli, S.; Kosina, J.; Ganz, R. Birth after bernese periacetabular osteotomy. Der Orthopäde 2000, 29, 0063–0067. [Google Scholar] [CrossRef]
- Paton, R.W. Screening in Developmental Dysplasia of the Hip (DDH). Surgery 2017, 15, 290–296. [Google Scholar] [CrossRef]
- Vaquero-Picado, A.; González-Morán, G.; Gil Garay, E.; Moraleda, L. Developmental dysplasia of the hip: Update of management. EFORT Open Rev. 2019, 4, 548–556. [Google Scholar] [CrossRef]
- Woodacre, T.; Dhadwal, A.; Ball, T.; Edwards, C.; Cox, P.J.A. The costs of late detection of developmental dysplasia of the hip. J. Child. Orthop. 2014, 8, 325–332. [Google Scholar] [CrossRef]
- Stevenson, D.A.; Mineau, G.; Kerber, R.A.; Viskochil, D.H.; Schaefer, C.; Roach, J.W. Familial Predisposition to Developmental Dysplasia of the Hip. J. Pediatr. Orthop. 2009, 29, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.L. Natural history of congenital hip dislocation (CDH) and hip dysplasia. Clin. Orthop. Relat. Res. 1987, 225, 62–76. [Google Scholar] [CrossRef]
- Akman, A.; Korkmaz, A.; Aksoy, M.C.; Yazici, M.; Yurdakök, M.; Tekinalp, G. Evaluation of risk factors in developmental dysplasia of the hip: Results of infantile hip ultrasonography. Turk. J. Pediatr. 2007, 49, 290–294. [Google Scholar] [PubMed]
- Bache, C.; Clegg, J.; Herron, M. Risk factors for developmental dysplasia of the hip: Ultrasonographic findings in the neonatal period. J. Pediatr. Orthop. 2002, 11, 212–218. [Google Scholar]
- Hadlow, V. Neonatal detection of developmental dysplasia of the hip (DDH). J. Bone Jt. Surg. Br. 1999, 81, 744. [Google Scholar] [CrossRef]
- Rosendahl, K.; Markestad, T.; Lie, R.T. Ultrasound screening for developmental dysplasia of the hip in the neonate: The effect on treatment rate and prevalence of late cases. Pediatrics 1994, 94, 47–52. [Google Scholar]
- Scrutton, D.; Baird, G.; Smeeton, N. Hip dysplasia in bilateral cerebral palsy: Incidence and natural history in children aged 18 months to 5 years. Dev. Med. Child Neurol. 2001, 43, 586–600. [Google Scholar] [CrossRef]
- Shipman, S.A.; Helfand, M.; Moyer, V.A.; Yawn, B.P. Screening for Developmental Dysplasia of the Hip: A Systematic Literature Review for the US Preventive Services Task Force. Pediatrics 2006, 117, e557–e576. [Google Scholar] [CrossRef]
- Gkiatas, I.; Boptsi, A.; Tserga, D.; Gelalis, I.; Kosmas, D.; Pakos, E. Developmental dysplasia of the hip: A systematic literature review of the genes related with its occurrence. EFORT Open Rev. 2019, 4, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Čengić, T.; Trkulja, V.; Pavelic, S.K.; Ratkaj, I.; Markova-Car, E.; Mikolaučić, M.; Kolundzic, R. Association of TGFB1 29C/T and IL6 -572G/C polymorphisms with developmental hip dysplasia: A case–control study in adults with severe osteoarthritis. Int. Orthop. 2015, 39, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.J.; Peters, C.L.; Erickson, J.A.; Hozack, B.A.; Jaraha, R.; Parvizi, J. Variable Expression and Incomplete Penetrance of Developmental Dysplasia of the Hip: Clinical Challenge in a 71-Member Multigeneration Family. J. Arthroplast. 2012, 27, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.; Dalsey, C.; Fertala, K.; Azimi, D.; Fortina, P.; Devoto, M.; Pacifici, M.; Parvizi, J. The Otto Aufranc Award: Identification of a 4 Mb Region on Chromosome 17q21 Linked to Developmental Dysplasia of the Hip in One 18-member, Multigeneration Family. Clin. Orthop. Relat. Res. 2010, 468, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, H.W.; Lu, Y.; Wang, Y.P.; Wang, Y.; Li, Q.W.; Ji, S.J. Transmission disequilibrium test for congenital dislocation of the hip and HOXB9 gene or COL1AI gene. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2003, 20, 193–195. (In Chinese) [Google Scholar] [PubMed]
- Harsanyi, S.; Zamborsky, R.; Krajciova, L.; Kokavec, M.; Danisovic, L. Developmental Dysplasia of the Hip: A Review of Etiopathogenesis, Risk Factors, and Genetic Aspects. Medicines 2020, 56, 153. [Google Scholar] [CrossRef]
- Jia, J.; Li, L.; Zhao, Q.; Zhang, L.; Ru, J.; Liu, X.; Li, Q.; Shi, L. Association of a single nucleotide polymorphism in pregnancy-associated plasma protein-A2 with developmental dysplasia of the hip: A case–control study. Osteoarthr. Cartil. 2012, 20, 60–63. [Google Scholar] [CrossRef]
- Harsanyi, S.; Zamborsky, R.; Kokavec, M.; Danisovic, L. Genetics of developmental dysplasia of the hip. Eur. J. Med. Genet. 2020, 63, 103990. [Google Scholar] [CrossRef]
- Rouault, K.; Scotet, V.; Autret, S.; Gaucher, F.; Dubrana, F.; Tanguy, D.; El Rassi, C.Y.; Fenoll, B.; Férec, C. Do HOXB9 and COL1A1 genes play a role in congenital dislocation of the hip? Study in a Caucasian population. Osteoarthr. Cartil. 2009, 17, 1099–1105. [Google Scholar] [CrossRef]
- Kenanidis, E.; Gkekas, N.; Karasmani, A.; Anagnostis, P.; Christofilopoulos, P.; Tsiridis, E. Genetic Predisposition to Developmental Dysplasia of the Hip. J. Arthroplast. 2020, 35, 291–300.e1. [Google Scholar] [CrossRef]
- Landa, J.; Benke, M.; Feldman, D.S. The Limbus and the Neolimbus in Developmental Dysplasia of the Hip. Clin. Orthop. Relat. Res. 2008, 466, 776–781. [Google Scholar] [CrossRef]
- Mulpuri, K.; Song, K.M.; Gross, R.H.; Tebor, G.B.; Otsuka, N.Y.; Lubicky, J.P.; Szalay, E.A.; Harcke, H.T.; Zehr, B.; Spooner, A.; et al. The American Academy of Orthopaedic Surgeons Evidence-Based Guideline on Detection and Nonoperative Management of Pediatric Developmental Dysplasia of the Hip in Infants up to Six Months of Age. J. Bone Jt. Surg. Am. 2015, 97, 1717–1718. [Google Scholar] [CrossRef]
- Bond, C.D.; Hennrikus, W.L.; DellaMaggiore, E.D. Prospective evaluation of newborn soft-tissue hip “clicks” with ultrasound. J. Pediatr. Orthop. 1997, 17, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Samora, J.; Quinn, R.H.; Murray, J.; Pezold, R.; Hall, Q. Management of Developmental Dysplasia of the Hip in Infants up to Six Months of Age. J. Am. Acad. Orthop. Surg. 2019, 27, e360–e363. [Google Scholar] [CrossRef] [PubMed]
- Shorter, D.; Hong, T.; Osborn, D.A. Cochrane Review: Screening programmes for developmental dysplasia of the hip in newborn infants. Evid.-Based Child Health A Cochrane Rev. J. 2013, 8, 11–54. [Google Scholar] [CrossRef]
- Atalar, H.; Sayli, U.; Yavuz, O.Y.; Uras, I.; Dogruel, H. Indicators of successful use of the Pavlik harness in infants with developmental dysplasia of the hip. Int. Orthop. 2006, 31, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Laborie, L.B.; Lehmann, T.G.; Rosendahl, K.; Engesæter, I.Ø.; Eastwood, D.M. Frcs Screening Strategies for Hip Dysplasia: Long-term Outcome of a Randomized Controlled Trial. Pediatrics 2013, 132, 492–501. [Google Scholar] [CrossRef]
- Engesæter, I.Ø.; Laborie, L.B.; Lehmann, T.G.; Fevang, J.M.; Lie, S.A.; Engesæter, L.B.; Rosendahl, K. Prevalence of radiographic findings associated with hip dysplasia in a population-based cohort of 2081 19-year-old Norwegians. Bone Jt. J. 2013, 95, 279–285. [Google Scholar] [CrossRef]
- Zamborsky, R.; Kokavec, M.; Harsanyi, S.; Attia, D.; Danisovic, L. Developmental Dysplasia of Hip: Perspectives in Genetic Screening. Med. Sci. 2019, 7, 59. [Google Scholar] [CrossRef]
- Roser, W. Uber angeborene Huftverrenkung. Langenbecks Arch. Klin. Chir. 1879, 24, 309–313. [Google Scholar]
- Putti, V. Early Treatment of Congenital Dislocation of the Hip. J. Bone Jt. Surg. 1929, 11, 789–809. [Google Scholar]
- Murphy, R.F.; Kim, Y.-J. Surgical Management of Pediatric Developmental Dysplasia of the Hip. J. Am. Acad. Orthop. Surg. 2016, 24, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wenger, D.R. Surgical treatment of developmental dysplasia of the hip. Instr. Course Lect. 2014, 63, 313–323. [Google Scholar] [PubMed]
- Tibrewal, S.; Gulati, V.; Ramachandran, M. The Pavlik method. J. Pediatr. Orthop. B 2013, 22, 516–520. [Google Scholar] [CrossRef]
- Roof, A.C.; Jinguji, T.M.; White, K.K. Musculoskeletal Screening: Developmental Dysplasia of the Hip. Pediatr. Ann. 2013, 42, e238–e244. [Google Scholar] [CrossRef]
- Marnach, M.L.; Ramin, K.D.; Ramsey, P.S.; Song, S.W.; Stensland, J.J.; An, K.N. Characterization of the relationship between joint laxity and maternal hormones in pregnancy. Obstet. Gynecol. 2003, 101, 331–335. [Google Scholar] [CrossRef]
- Smith, M.W.; Marcus, P.S.; Wurtz, L.D. Orthopedic Issues in Pregnancy. Obstet. Gynecol. Surv. 2008, 63, 103–111. [Google Scholar] [CrossRef]
- Ng, G.; Jeffers, J.R.; Beaulé, P.E. Hip Joint Capsular Anatomy, Mechanics, and Surgical Management. J. Bone Jt. Surg. Am. 2019, 101, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Hooke, A.W.; An, K.-N.; Trousdale, R.T.; Sierra, R.J. Contribution of the Ligamentum Teres to Hip Stability in the Presence of an Intact Capsule: A Cadaveric Study. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1480–1487. [Google Scholar] [CrossRef]
- Ito, H.; Song, Y.; Lindsey, D.P.; Safran, M.R.; Giori, N.J. The proximal hip joint capsule and the zona orbicularis contribute to hip joint stability in distraction. J. Orthop. Res. 2009, 27, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Olausson, H.; Laskey, M.A.; Goldberg, G.R.; Prentice, A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Am. J. Clin. Nutr. 2008, 88, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Angeletti, M.; Luziatelli, D.; Luziatelli, S.; Venturella, R.; Di Carlo, C.; Bernardo, S. Pregnancy associated transient osteoporosis of the hip (PR-TOH): A non–obstetric indication to caesarean section. A case report with literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vleeming, A.; Albert, H.; Östgaard, H.; Stuge, B.; Sturesson, B. European guidelines on the diagnosis and treatment of pelvic girdle pain. Mov. Stab. Lumbopelvic Pain 2007, 17, 465–470. [Google Scholar] [CrossRef]
- Ji, X.; Morino, S.; Iijima, H.; Ishihara, M.; Kawagoe, M.; Umezaki, F.; Hatanaka, Y.; Yamashita, M.; Tsuboyama, T.; Aoyama, T. The Association of Variations in Hip and Pelvic Geometry With Pregnancy-Related Sacroiliac Joint Pain Based on a Longitudinal Analysis. Spine 2019, 44, E67–E73. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Garabekyan, T.; Pascual-Garrido, C.; Mei-Dan, O. Hip instability: A review of hip dysplasia and other con-tributing factors. Muscles Ligaments Tendons J. 2016, 6, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Safran, M.R.; Scillia, A.J.; Ayeni, O.R.; Garabekyan, T.; Mei-Dan, O. A Contemporary Look at the Evaluation and Treatment of Adult Borderline and Frank Hip Dysplasia. Am. J. Sports Med. 2020, 48, 2314–2323. [Google Scholar] [CrossRef]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 2–4. [Google Scholar]
- Valenzuela, R.G.; Cabanela, M.E.; Trousdale, R.T. Sexual Activity, Pregnancy, and Childbirth After Periacetabular Osteotomy. Clin. Orthop. Relat. Res. 2004, 418, 146–152. [Google Scholar] [CrossRef]
- Sierra, R.J.; Trousdale, R.T.; Cabanela, M.E. Pregnancy and childbirth after total hip arthroplasty. J. Bone Jt. Surg. Br. 2005, 87, 21–24. [Google Scholar] [CrossRef]
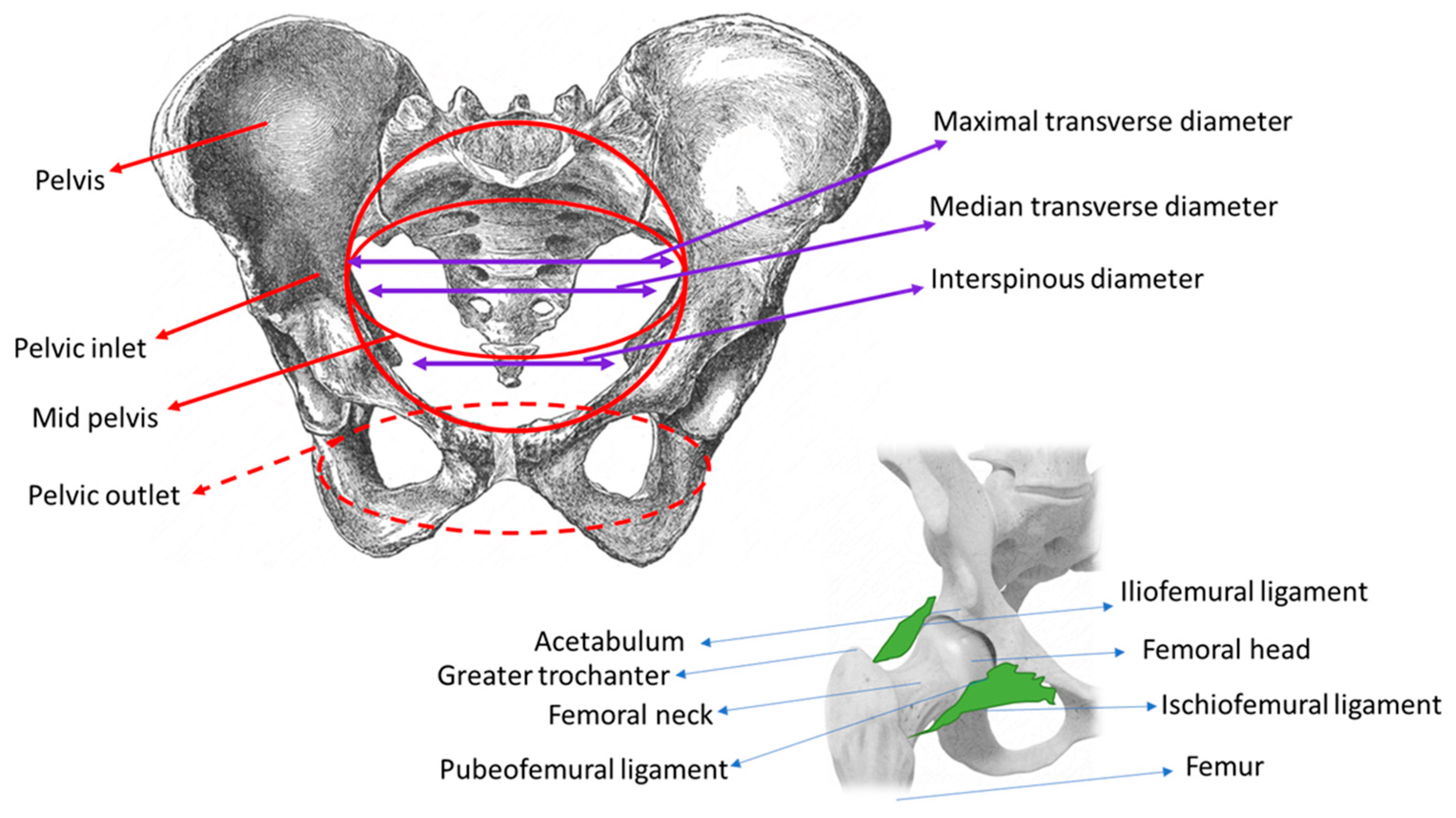
| Antero-Posterior Diameters | Transverse Diameters | Oblique Diameters |
|---|---|---|
| Pelvic Inlet Promonto-suprapubic diameter (true conjugate, conjugate vera, anatomic conjugate): 11.5 cm Promonto-pubic diameter (obstetric conjugate): 10.8–11 cm | Maximal transverse diameter, at the widest point between the unnominate lines: 13.5 cm Median transverse diameter: 13 cm | Oblique diameters right and left: 12 cm |
| Mid-Pelvis Antero-posterior diameter, from S4 to S5 to the lower border of the pubic symphysis: 11.5 cm | Bispinous: 10.5 cm | Oblique diameters right and left: 11 cm |
| Pelvic Outlet Antero-posterior diameter from the coccyx to the subpubic area—during the second stage of labor, this diameter is 9.5 cm; by mobilizing the coccyx posteriorly, the diameter reaches 12 cm | Transverse diameter between ischiatic tuberosities: 11 cm | Oblique diameters right and left: 11 cm |
| Pelvic inlet | obstetric conjugate > 10.5 cm transverse diameter > 13 cm posterior sagittal diameter > 4.5 cm |
| Mid-pelvis | Plane of greatest diameter: anteroposterior diameter > 12.5 cm transverse diameter > 12.5 cm, posterior sagittal diameter > 4.5 cm Plane of least diameter: anteroposterior diameter > 12 cm interspinous diameter > 10.5 cm posterior sagittal diameter > 4.5 cm |
| Outlet pelvis | anteroposterior diameter > 11 cm intertuberous diameter > 11 cm posterior sagittal diameter > 4 cm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionescu, A.A.; Cirstoiu, M.M.; Cirstoiu, C.; Stanescu, A.M.A.; Crețu, B. Current Evidence about Developmental Dysplasia of the Hip in Pregnancy. Medicina 2021, 57, 655. https://doi.org/10.3390/medicina57070655
Simionescu AA, Cirstoiu MM, Cirstoiu C, Stanescu AMA, Crețu B. Current Evidence about Developmental Dysplasia of the Hip in Pregnancy. Medicina. 2021; 57(7):655. https://doi.org/10.3390/medicina57070655
Chicago/Turabian StyleSimionescu, Anca Angela, Monica Mihaela Cirstoiu, Catalin Cirstoiu, Ana Maria Alexandra Stanescu, and Bogdan Crețu. 2021. "Current Evidence about Developmental Dysplasia of the Hip in Pregnancy" Medicina 57, no. 7: 655. https://doi.org/10.3390/medicina57070655
APA StyleSimionescu, A. A., Cirstoiu, M. M., Cirstoiu, C., Stanescu, A. M. A., & Crețu, B. (2021). Current Evidence about Developmental Dysplasia of the Hip in Pregnancy. Medicina, 57(7), 655. https://doi.org/10.3390/medicina57070655







