Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19
Abstract
1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
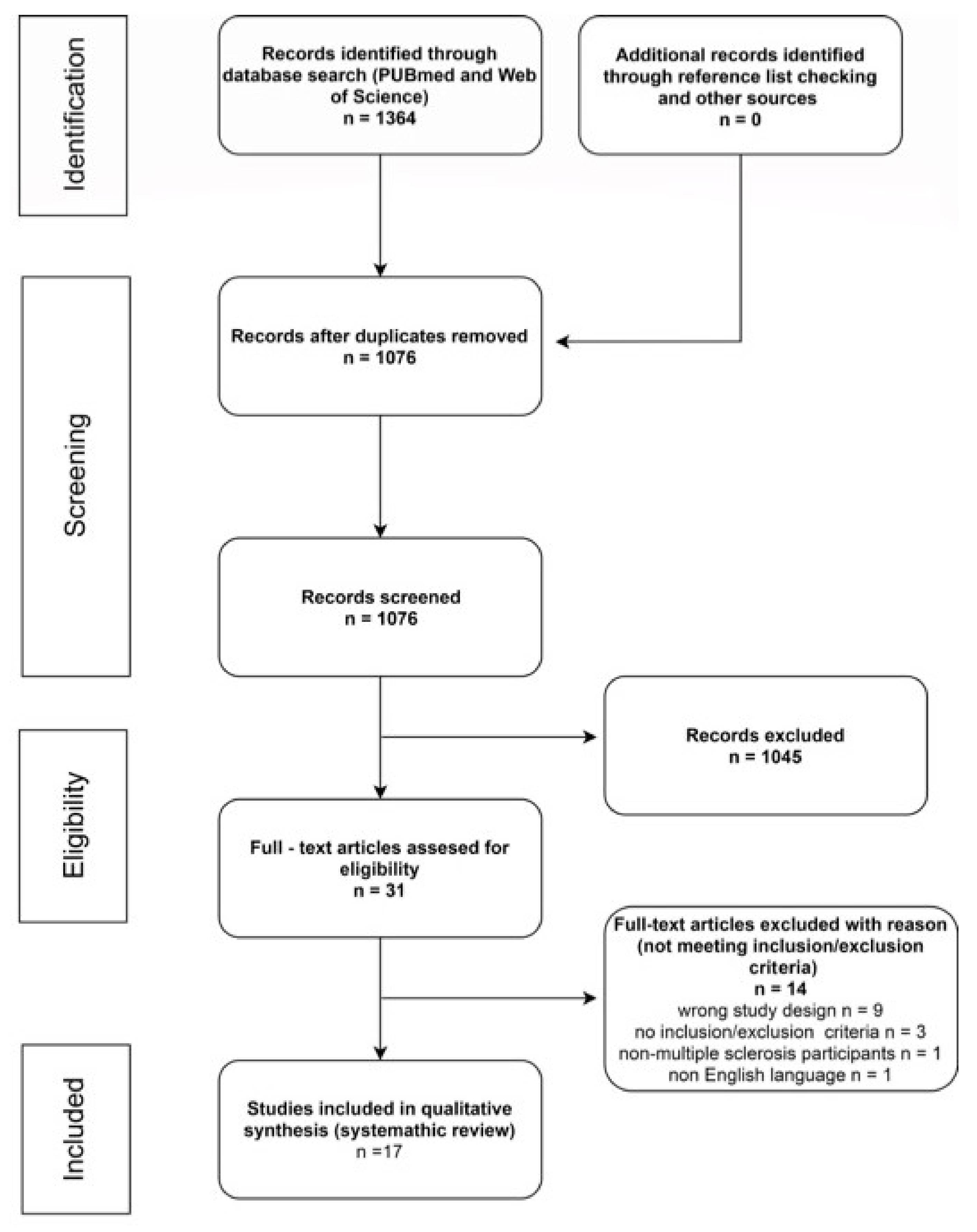
3. Results
3.1. Evaluation of the Study
3.2. Characteristics of Research Participants and Study Criteria
3.3. The Impact of Applying of New Technologies on Balance and Gait Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, E. Multiple sclerosis. Adv. Exp. Med. Biol. 2012, 724, 222–238. [Google Scholar] [CrossRef]
- Di Cara, M.; Lo Buono, V.; Corallo, F.; Cannistraci, C.; Rifici, C.; Sessa, E.; D’Aleo, G.; Bramanti, P.; Marino, S. Body image in multiple sclerosis patients: A descriptive review. Neurol. Sci. 2019, 40, 923–928. [Google Scholar] [CrossRef]
- Macías Islas, M.Á.; Ciampi, E. Assessment and Impact of Cognitive Impairment in Multiple Sclerosis: An Overview. Biomedicines 2019, 7, 22. [Google Scholar] [CrossRef]
- Cattaneo, D.; Jonsdottir, J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult. Scler. 2009, 15, 59–67. [Google Scholar] [CrossRef]
- Multiple Sclerosis International Federation; World Health Organization. Atlas: Multiple Sclerosis Resources in the World Health Organization; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Matsuda, P.N.; Shumway-Cook, A.; Bamer, A.M.; Johnson, S.L.; Amtmann, D.; Kraft, G.H. Falls in multiple sclerosis. PMR 2011, 3, 624–632. [Google Scholar] [CrossRef]
- Campbell, E.; Coulter, E.H.; Mattison, P.G.; Miller, L.; McFadyen, A.; Paul, L. Physiotherapy Rehabilitation for People With Progressive Multiple Sclerosis: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 141–151. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Pilutti, L.A. The benefits of exercise training in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 487–497. [Google Scholar] [CrossRef]
- Sá, M.J. Exercise therapy and multiple sclerosis: A systematic review. J. Neurol. 2014, 261, 1651–1661. [Google Scholar] [CrossRef]
- Mares, J.; Hartung, H.-P. Multiple sclerosis and COVID-19. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2020, 164, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cano de la Cuerda, R.; Muñoz-Hellín, E.; Alguacil-Diego, I.M.; Molina-Rueda, F. Telerrehabilitación y neurología. Rev. Neurol. 2010, 51, 49–56. [Google Scholar] [CrossRef]
- Maggio, M.G.; Maresca, G.; de Luca, R.; Stagnitti, M.C.; Porcari, B.; Ferrera, M.C.; Galletti, F.; Casella, C.; Manuli, A.; Calabrò, R.S. The Growing Use of Virtual Reality in Cognitive Rehabilitation: Fact, Fake or Vision? A Scoping Review. J. Natl. Med. Assoc. 2019, 111, 457–463. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; de Luca, R.; Calabrò, R.S. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]
- Kalron, A.; Fonkatz, I.; Frid, L.; Baransi, H.; Achiron, A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 13. [Google Scholar] [CrossRef]
- Russo, M.; Dattola, V.; de Cola, M.C.; Logiudice, A.L.; Porcari, B.; Cannavò, A.; Sciarrone, F.; de Luca, R.; Molonia, F.; Sessa, E.; et al. The role of robotic gait training coupled with virtual reality in boosting the rehabilitative outcomes in patients with multiple sclerosis. Int. J. Rehabil. Res. 2018, 41, 166–172. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- National Collaborating Centre for Methods and Tools. National Collaborating Centre for Methods and Tools: Quality Assessment Tool for Quantitative Studies, Updated 2017; McMaster University: Hamilton, ON, Canada, 2008. [Google Scholar]
- Ortiz-Gutiérrez, R.; Del Galán Río, F.; La Cano de Cuerda, R.; Alguacil Diego, I.M.; González, R.A.; Page, J.C.M. A telerehabilitation program by virtual reality-video games improves balance and postural control in multiple sclerosis patients. NeuroRehabilitation 2013, 33, 545–554. [Google Scholar] [CrossRef]
- Ortiz-Gutiérrez, R.; Cano-de-la-Cuerda, R.; Galán-del-Río, F.; Alguacil-Diego, I.M.; Palacios-Ceña, D.; Miangolarra-Page, J.C. A telerehabilitation program improves postural control in multiple sclerosis patients: A Spanish preliminary study. Int. J. Environ. Res. Public Health 2013, 10, 5697–5710. [Google Scholar] [CrossRef]
- Charvet, L.E.; Yang, J.; Shaw, M.T.; Sherman, K.; Haider, L.; Xu, J.; Krupp, L.B. Cognitive function in multiple sclerosis improves with telerehabilitation: Results from a randomized controlled trial. PLoS ONE 2017, 12, e0177177. [Google Scholar] [CrossRef]
- Conroy, S.S.; Zhan, M.; Culpepper, W.J.; Royal, W.; Wallin, M.T. Self-directed exercise in multiple sclerosis: Evaluation of a home automated tele-management system. J. Telemed. Telecare 2018, 24, 410–419. [Google Scholar] [CrossRef]
- Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Oña-Simbaña, E.D.; Martínez-Medina, A.; Ortiz-Comino, C.; Balaguer-Bernaldo-de-Quirós, C.; Jardón-Huete, A.; Cano-de-la-Cuerda, R. Effects of virtual reality associated with serious games for upper limb rehabilitation inpatients with multiple sclerosis: Randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 90. [Google Scholar] [CrossRef]
- Maggio, M.G.; de Luca, R.; Manuli, A.; Buda, A.; Foti Cuzzola, M.; Leonardi, S.; D’Aleo, G.; Bramanti, P.; Russo, M.; Calabrò, R.S. Do patients with multiple sclerosis benefit from semi-immersive virtual reality? A randomized clinical trial on cognitive and motor outcomes. Appl. Neuropsychol. Adult 2020, 1–7. [Google Scholar] [CrossRef]
- Molhemi, F.; Monjezi, S.; Mehravar, M.; Shaterzadeh-Yazdi, M.-J.; Salehi, R.; Hesam, S.; Mohammadianinejad, E. Effects of Virtual Reality vs Conventional Balance Training on Balance and Falls in People with Multiple Sclerosis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 290–299. [Google Scholar] [CrossRef]
- Novotna, K.; Janatova, M.; Hana, K.; Svestkova, O.; Preiningerova Lizrova, J.; Kubala Havrdova, E. Biofeedback Based Home Balance Training can Improve Balance but Not Gait in People with Multiple Sclerosis. Mult. Scler. Int. 2019, 2019, 2854130. [Google Scholar] [CrossRef]
- Ozdogar, A.T.; Ertekin, O.; Kahraman, T.; Yigit, P.; Ozakbas, S. Effect of video-based exergaming on arm and cognitive function in persons with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 40, 101966. [Google Scholar] [CrossRef]
- Pawlukowska, W.; Dobrowolska, N.; Szylinska, A.; Koziarska, D.; Meller, A.; Rotter, I.; Nowacki, P. Influence of RehaCom Therapy on the Improvement of Manual Skills in Multiple Sclerosis Subjects. Ann. Rehabil. Med. 2020, 44, 142–150. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Lozano-Quilis, J.-A.; Gil-Gómez, H.; Gil-Gómez, J.-A.; Albiol-Pérez, S.; Palacios-Navarro, G.; Fardoun, H.M.; Mashat, A.S. Virtual rehabilitation for multiple sclerosis using a kinect-based system: Randomized controlled trial. JMIR Serious Games 2014, 2, e12. [Google Scholar] [CrossRef]
- Norouzi, E.; Gerber, M.; Pühse, U.; Vaezmosavi, M.; Brand, S. Combined virtual reality and physical training improved the bimanual coordination of women with multiple sclerosis. Neuropsychol. Rehabil. 2020, 1–18. [Google Scholar] [CrossRef]
- Peruzzi, A.; Zarbo, I.R.; Cereatti, A.; Della Croce, U.; Mirelman, A. An innovative training program based on virtual reality and treadmill: Effects on gait of persons with multiple sclerosis. Disabil. Rehabil. 2017, 39, 1557–1563. [Google Scholar] [CrossRef]
- Robinson, J.; Dixon, J.; Macsween, A.; van Schaik, P.; Martin, D. The effects of exergaming on balance, gait, technology acceptance and flow experience in people with multiple sclerosis: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Tallner, A.; Streber, R.; Hentschke, C.; Morgott, M.; Geidl, W.; Mäurer, M.; Pfeifer, K. Internet-Supported Physical Exercise Training for Persons with Multiple Sclerosis-A Randomised, Controlled Study. Int. J. Mol. Sci. 2016, 17, 1667. [Google Scholar] [CrossRef] [PubMed]
- Waliño-Paniagua, C.N.; Gómez-Calero, C.; Jiménez-Trujillo, M.I.; Aguirre-Tejedor, L.; Bermejo-Franco, A.; Ortiz-Gutiérrez, R.M.; Cano-de-la-Cuerda, R. Effects of a Game-Based Virtual Reality Video Capture Training Program Plus Occupational Therapy on Manual Dexterity in Patients with Multiple Sclerosis: A Randomized Controlled Trial. J. Healthc. Eng. 2019, 2019, 9780587. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, Y.Z.; Tarakci, E.; Tarakci, D.; Ozdincler, A.R.; Kurtuncu, M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2019, 39, 101902. [Google Scholar] [CrossRef]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 3 February 2021).
- European Centre for Disease Prevention and Control. Situation Updates on COVID-19. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 4 February 2021).
- Centers for Disease Control and Prevention. Public Health Recommendations for Community-Related Exposure. Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html (accessed on 5 February 2021).
- Bloem, B.R.; Dorsey, E.R.; Okun, M.S. The Coronavirus Disease 2019 Crisis as Catalyst for Telemedicine for Chronic Neurological Disorders. JAMA Neurol. 2020, 77, 927–928. [Google Scholar] [CrossRef]
- Henry, K.D.; Rosemond, C.; Eckert, L.B. Effect of number of home exercises on compliance and performance in adults over 65 years of age. Phys. Ther. 1999, 79, 270–277. [Google Scholar] [CrossRef]
- Hayden, J.A.; van Tulder, M.W.; Tomlinson, G. Systematic review: Strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann. Intern. Med. 2005, 142, 776–785. [Google Scholar] [CrossRef]
- Vermeire, E.; Hearnshaw, H.; van Royen, P.; Denekens, J. Patient adherence to treatment: Three decades of research. A comprehensive review. J. Clin. Pharm. Ther. 2001, 26, 331–342. [Google Scholar] [CrossRef]
- Spink, M.J.; Fotoohabadi, M.R.; Wee, E.; Landorf, K.B.; Hill, K.D.; Lord, S.R.; Menz, H.B. Predictors of adherence to a multifaceted podiatry intervention for the prevention of falls in older people. BMC Geriatr. 2011, 11, 51. [Google Scholar] [CrossRef]
- Jurkiewicz, M.T.; Marzolini, S.; Oh, P. Adherence to a home-based exercise program for individuals after stroke. Top. Stroke Rehabil. 2011, 18, 277–284. [Google Scholar] [CrossRef]
- Mayoux-Benhamou, M.A.; Roux, C.; Perraud, A.; Fermanian, J.; Rahali-Kachlouf, H.; Revel, M. Predictors of compliance with a home-based exercise program added to usual medical care in preventing postmenopausal osteoporosis: An 18-month prospective study. Osteoporos. Int. 2005, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Donovan-Hall, M. Predicting adherence to exercise-based therapy in rehabilitation. Rehabil. Psychol. 2007, 52, 56–64. [Google Scholar] [CrossRef]
- Medina-Mirapeix, F.; Escolar-Reina, P.; Gascón-Cánovas, J.J.; Montilla-Herrador, J.; Jimeno-Serrano, F.J.; Collins, S.M. Predictive factors of adherence to frequency and duration components in home exercise programs for neck and low back pain: An observational study. BMC Musculoskelet. Disord. 2009, 10, 155. [Google Scholar] [CrossRef]
- Kirby, S.; Donovan-Hall, M.; Yardley, L. Measuring barriers to adherence: Validation of the Problematic Experiences of Therapy Scale. Disabil. Rehabil. 2014, 36, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, W.J.; Brawley, L.R.; Ettinger, W.; Morgan, T.; Thompson, C. Compliance to exercise therapy in older participants with knee osteoarthritis: Implications for treating disability. Med. Sci. Sports Exerc. 1997, 29, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Essery, R.; Geraghty, A.W.A.; Kirby, S.; Yardley, L. Predictors of adherence to home-based physical therapies: A systematic review. Disabil. Rehabil. 2017, 39, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Dlugonski, D.; Wójcicki, T.R.; McAuley, E.; Mohr, D.C. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult. Scler. 2011, 17, 116–128. [Google Scholar] [CrossRef]
- Dlugonski, D.; Motl, R.W.; Mohr, D.C.; Sandroff, B.M. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: Sustainability and secondary outcomes. Psychol. Health Med. 2012, 17, 636–651. [Google Scholar] [CrossRef]

| Selection Bias | Design | Confounders | Blinding | Data Collection Methods | Withdrawals and Dropouts | Summary | ||
|---|---|---|---|---|---|---|---|---|
| Chervet 2017 [21] | + | + | + | + | + | + | + | |
| Conroy 2017 [22] | ? | + | + | ? | + | - | ? | |
| Cuesta-Gomez 2020 [23] | ? | + | + | ? | + | + | + | |
| Lozano-Quilis 2014 [31] | - | + | + | ? | + | + | ? | - |
| Maggio 2020 [24] | ? | + | + | ? | + | + | + | Weak |
| Molhemi 2020 [25] | ? | + | + | ? | + | + | + | |
| Norouzi 2020 [32] | + | + | + | ? | + | + | + | |
| Novotna 2019 [26] | ? | + | ? | ? | + | + | + | |
| Ortiz-Gutierrez 2013 (a) [19] | ? | + | + | ? | + | + | + | ? |
| Ortiz-Gutierrez 2013 (b) [20] | ? | + | + | ? | + | + | + | Moderate |
| Ozdogar 2020 [27] | ? | + | + | ? | + | + | + | |
| Pawlukowska 2020 [28] | - | + | + | + | + | - | - | |
| Peruzzi 2017 [33] | ? | + | + | ? | + | ? | + | + |
| Robinson 2015 [34] | ? | + | + | - | + | ? | ? | Strong |
| Tallner 2016 [35] | + | + | + | ? | - | ? | ? | |
| Waliño-Peniagua 2019 [36] | - | + | + | + | + | ? | ? | |
| Yazgan 2020 [37] | ? | + | + | ? | + | ? | + |
| Study (Author and Year) | Number of Participants | Age (Years as Mean (SD)) | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Conroy 2017 [22] | N = 51 (24 completed the study), IG = 26(16 completed the study); CG = 25 (8 completed the study) | All = 51 (8.1) IG = 50.4 (8.1) CG = 54.3 (5.9) | Age 18–65 years, confirmed MS diagnosis using McDonald MS diagnostic criteria, The Patient Determined Disease Steps score range of 2–6; and ability to use the “MS HAT” platform with modifications as needed; 25fW ≤ 3 min; ability to perform exercise independently or have an identified caregiver provided assistance; access to a working telephone line | MS exacerbation within 3 months of enrolment, a corticosteroid course within 60 days of screening, any medical condition or cognitive impairment that would interfere with exercise performance or understanding |
| Maggio 2020 [24] | N = 60; IG = 30; CG = 30 | All = 50.0 (11.4) IG = 51.9 (9.9) CG = 48.2 (12.2) | MS diagnosis according to the McDonald criteria, stable in therapy for at least 6 months before the study entry; mild/moderate cognitive impairment (Montreal Cognitive Assessment > 18); absence of severe medical and psychiatric illness potentially interfering with the VR training; absence of disabling sensory alterations | Age >75 or <18 year; severe medical and psychiatric illness according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition and International Classification of Disease; MS clinical and/or neuroradiological relapse in the 6 months before enrolment; EDSS > 7 |
| Molhemi 2020 [25] | N = 39 (35 completed the study); IG = 19 (17 completed the study) CG = 20 (18 completed the study) | All = n/d IG = 36.8 (8.4) CG = 41.6 (8.4) | Confirmed diagnosis of relapsing-remitting or secondary progressive MS according to the McDonald criteria by a neurologist specialized in treating MS, aged 18–64 years, EDSS < 6, and BBS < 53 | Exacerbation of symptoms in the past 3 months, MMSE < 24, neurologic or musculoskeletal diagnosis except MS that negatively affected their gait and balance, uncorrected visual or auditory impairments, pregnancy |
| Nowotna 2019 [26] | N = 39; IG = 23; CG = 16 | All = 40.69 (10.2) IG = 39.39 (9.68) CG = 42.56 (10.63) | Clinically stable MS, without relapse or worsening in the previous three months; aged 18–60 years; ability to walk with or without a walking aid for at least 5 m; and ability to maintain a standing position for at least 10 min, ability to perform exercise (assessed by physiotherapist) | Inpatient rehabilitation program during the previous 3 months; orthopedic problems or other conditions affecting balance and gait performance; blurred vision; severe cognitive impairment or psychiatric disorders; pregnancy; weight over 150 kg |
| Ortiz-Gutierrez 2013(a) [19] | N = 50 (47 completed the study); IG = 25 (24 completed the study); CG = 25 (23 completed the study) | All = n/d IG = 39.69 (8.13) CG = 42.78 (7.38) | Age between 20 and 60 years; confirmed diagnosis of MS for over 2 years based on the McDonald criteria; medically stable during the 6 months prior to baseline; impaired balance with demyelinated lesions in the cerebellum and its connections demonstrated by Magnetic Resonance Imaging; EDSS score ranging from 3 to 5; Hauser ambulatory index value > 4; MMSE ≥ 24; no visual deficits; internet connection at home | Diagnosed with another disease or pathological condition that affects balance; had a relapse in the month prior to baseline or during the intervention process, received steroid cycle prior to beginning the evaluation protocol and within the 4 months duration of the project intervention |
| Ortiz-Gutiérrez 2013 (b) [20] | N = 50 (47 completed the study); IG = 25 (24 completed the study); CG = 25 (23 completed the study) | All = n/d IG = 39.69 (8.13) CG = 42.78 (7.38) | Age between 20 and 60 years; confirmed diagnosis of MS for over 2 years based on the McDonald criteria; medically stable during the 6 months prior to baseline; impaired balance associated with demyelinated lesions in the cerebellum and its connections; EDSS from 3 to 5; Hauser ambulatory index value > 4, absence of cognitive impairment according to the MMSE, no visual deficits, internet connection at home | Diagnosis of another disease or pathological condition that affects balance; an attack in the month prior to baseline or during the intervention process; receiving a cycle of steroids 6 months prior to beginning the protocol and within the 4 months duration of the project intervention |
| Peruzzi 2017 [33] | N = 31 (25 completed the study); IG = 16 (14 completed the study); CG = 15 (11 completed the study) | All = n/d IG = 43.6 (10.2) CG = 42.0 (12.0) | Diagnosis of relapsing-remitting MS according to the McDonald criteria, an expanded disability status scale between 3 and 5.5 and, a MMSE ≥ 26, no relapses within the six months prior to the study | Chronic medical illnesses, severe visual deficits, severe ataxia or severe depression, botulinum toxin inj. within the past 4 months or functional surgery in the past 6 months. |
| Robinson 2015 [34] | N = 56; IG1 = 20, IG2 = 18(15 completed the study), CG = 18 (11 completed the study) | All = 52 (5.8) IG1 = 52.6 (6.1) IG2 = 53.9 (6.5) CG = 51.9 (4.7) | Male or female, aged 18–65 years, clinical diagnosis of MS, self-reported ability to walk 100 m with or without resting with the use of one stick or crutch (EDSS score of 6), able to read and comprehend written and spoken English | Acute exacerbation and/or relapse of MS symptoms within the last three months, diagnoses of any other condition affecting CNS, any musculoskeletal injury, or receiving physical therapy |
| Lozano-Quilis 2014 [31] | N = 11; IG = 6; CG = 5 | All = n/d IG = 48.33 (10.82) CG = 40.60 (9.24) | Age 18–65 years, relapsing-remitting and secondary progressive MS, minimum score of 6 on all items of the domain of the Functional Independence Measure, do not need assistive devices for ambulation or at most a cane, do not have cognitive impairments | Flare-up symptoms, cannot physically complete all rehabilitation sessions |
| Yazgan 2020 [37] | N = 47 (42 completed the study); IG1 = 16 (15 completed the study); IG2 = 16 (12 completed the study); CG = 15 | All = n/d IG1 = 47.46 (10.53) IG2 = 43.08 (8.74) CG = 40.66 (8.82) | MS patients followed up regularly at the MS Outpatient Clinic of our Neurology Department; ambulatory and volunteered to participate in the study; stable phase of the disease, without relapses or worsening in the last 3 months; EDSS between 2.5 and 6; age between 25 and 60 years | Diagnosis of any other disorder affecting CNS, musculoskeletal disorder, pregnancy, blurred vision, psychiatric problems or severe cognitive impairment |
| Study (Author and Year) | Number of Participants | Age (Years as Mean(SD)) | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Cuesta-Gomez 2020 [23] | N = 30; IG = 16; CG = 14 | All = 46.66 (2.04) IG = 49.86 (2.46) CG = 42.66 (3.14) | Diagnosis of MS according to the McDonald criteria with over 2 years evolution; a score of between 3.5 and 7.5 on the EDSS; with stable medical treatment during at least the 6 months prior to the intervention; muscle tone in the upper limbs ≤2 on the modified Ashworth Scale; ≤4 in the “Pyramidal Function” section of the EDSS functional scale; absence of cognitive decline (≥24 in the MMSE; and ≤2 in the “Mental Functions” section of the EDSS) | Diagnosis of another neurological illness or musculoskeletal disorder different to MS; the diagnosis of a cardiovascular, respiratory, or metabolic illness or other conditions which may interfere with the study; suffering a flare-up or hospitalization in the last 3 months. prior to commencement of the assessment protocol or during the process of the therapeutic intervention; receiving a cycle of steroids 6 months. prior to the commencement of the assessment protocol and within the study period of intervention; receiving treatment with botulinum toxin in the 6 months. prior to the beginning of the study; visual disorders non-corrected by optical devices |
| Ozdogar 2020 [27] | N = 60 (57 completed the study); IG1 (video-based exergaming group) = 21 (20 completed the study); IG2 (conventional rehabilitation) = 19 (17 completed the study); CG = 20 | All = 40.1 (10.7) IG1 = 39.2 (8.6) IG2 = 43.6 (10.5) CG = 37.9 (12.4) | Relapsing-remitting or secondary progressive type of MS, being able to walk at least 100 m without resting, being able to stably stand for half an hour, relapse-free period of 3 months, willing to participate in the study | Another neurological disorder, relapse during the study period, orthopedic surgery history covering the ankle-foot, knee, hip, or spine, affecting balance, and diagnosis of severe cognitive and/or psychiatric impairment |
| Pawlukowska 2020 [28] | N = 40 (30 completed the study) IG = 20 (10 completed the study); CG = 20 | All = n/d IG = 53.9 (n/d) CG = 49.6 (n/d) | Between 18 and 65 years, MS clinically diagnosed based on the McDonald criteria, EDSS range from 1.5–4 points, impairment of the upper limb, NHPT score <2 standard deviations (SDs) from the norm for their age and sex | MMSE score <26, alcoholism, and severe vision disorders including diplopia and coinciding upper limb therapy |
| Waliño-Paniagua 2019 [36] | N = 16; IG = 8; CG = 8 | All = 46.44 (9.09) SG = 46.13 (9.49) CG = 46.75 (9.31) | A diagnosis of MS according to the McDonald criteria with over two years evolution; a score of between 3.5 and 6 on the EDSS (as well as a score ≤ 4 in the “Pyramidal Function” section of the EDSS functional scale, or score ≤ 2 in the “Mental Functions” section of the EDSS); stable medical treatment during at least the six months prior to the intervention; muscle tone in the upper limbs not greater than two points on the modified Ashworth Scale; absence of cognitive decline; ability to understand instructions and a score ≥ 24 in MMSE | Diagnosis of another neurological illness or musculoskeletal disorder different to MS; the diagnosis of a cardiovascular, respiratory, or metabolic illness or other conditions which may interfere with the study; suffering a flare-up or hospitalization in the last 3 months. prior to commencement of the assessment protocol or during the process of the therapeutic intervention; receiving a cycle of steroids 6 months. prior to the commencement of the assessment protocol and within the study period of intervention; receiving treatment with botulinum toxin in 6 mth. prior to the beginning of the study; presence of visual disorders non-corrected by optical devices |
| Norouzi 2020 [32] | N = 45; IG1 = 15 (VR); IG2(VR+ Conventional Physical Training) = 15; CG (Conventional Physical Training) = 15 | All = 26.39 (3.45) IG1 = n/d IG2 = n/d CG = n/d | Female with MS; age 20–30 years; right handed; a diagnosis of poor fine manual dexterity (according to the NHPT criteria); signed written informed consent; normal vision based on the Snellen Chart Test; self-reported normal audition | Psychiatric issues (ascertained by a brief psychiatric interview—Mini International Neuropsychiatric Interview); intake of mood- and arousal-medications or substances; orthopedic problems; pregnancy; and somatic diseases such as diabetes |
| Study (Author and Year) | Number of Participants | Age (Years as Mean(SD)) | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| Tallner 2016 [35] | N = 126 (78 completed the study); IG = 59 (36 completed the study), CG = 67 (41 completed the study) | All = 40.8 (9.9) IG = 40.9 (10.4) CG = 40.7 (9.5) | Diagnosed multiple sclerosis, an EDSS ≤ 4.0, not less than four weeks of clinical stability prior to inclusion in the study, access to the Internet | Primary progressive multiple sclerosis and clinically relevant cardiological, internal, or orthopedic contraindications to exercise, which were assessed by the patients’ attending physicians |
| Charvet 2017 [21] | N = 135; IG = 74; CG = 61 | All = 50 (12) IG = 52 (11) CG = 48 (13) | Meeting diagnostic criteria for MS (McDonald criteria), scoring one or more standard deviations below published normative data on the Symbol Digit Modalities Test; reading recognition standard score of 85 or above (Wide Range Cognitive Achievement Test Third Edition); learned English by age 12 years; adequate visual, auditory, and motor capacity to operate computer software; no anticipated medication changes during the course of the three-month study period, and no relapses or steroids in the previous month | History of any developmental disorders, conditions other than MS associated with cognitive impairment, a primary psychiatric disorder, any serious medical conditions, alcohol or substance use disorder, history of use of computer-based CT developed by Posit Science (the developer of the study program) |
| Study (First Author and Year) | Study Design | Type of Therapeutic Intervention | Intervention Description | Frequency and Duration of Sessions | Period of Therapeutic Intervention (Number of Sessions) | Measured Domains | Measurements | Key Results |
|---|---|---|---|---|---|---|---|---|
| Conroy 2017 [22] | single-blinded, randomized controlled trial | Internet-supported exercise | CG—individualized exercise prescriptions in paper hand-out form common for physiotherapy home exercise programs. IG—the baseline written exercises and access to asynchronous text messaging for exercise updates from the therapist via the “MS HAT” platform. No live on-line exercise supervision | n/d | 6 months (n/d) | Balance, gait | 25fW, 6MW, BBS, MSWS12 | No improvements in regard to walking ability and balance in IG and CG |
| Maggio 2020 [24] | single-blinded, randomized controlled trial | VR-based, semi-immersive motor and cognitive rehabilitation | All participants underwent a standard physical treatment consisting of general conditioning exercises and cognitive rehabilitation. IG—cognitive training was performed using VR, CG—conventional cognitive training | Cognitive training 60 min 3 x/wk. General conditioning training 30 min—no data regarding frequency | 8 wks (24) | The neuropsychological battery test markers, i.e.,: depression, recall, quality of life, balance | Montreal Cognitive Assessment; BDI; Rey-Osterrieth complex figure test; Multiple Sclerosis Quality of Life-54; Paced auditory serial addition task for two seconds; Spatial recall test; TUG; Tinetti scale | Improvements in Tinetti scale, Rey–Osterrieth complex figure test; Multiple Sclerosis Quality of Life-54 and BDI were observed in both groups. Significant increase in visual perception, visuospatial abilities, short term visual memory, working memory and executive functions, speed of information processing, sustained attention and TUG test score was observed only in IG |
| Molhemi 2020 [25] | prospective randomized controlled trial | VR-based balance training | Participants in both groups received exercises including standing, walking, and weight-shifting. CG—standing exercise included multidirectional stepping, single and double-leg standing; walking exercise involved forward, backward, and side walking and weight-shifting, half-squat, leaning, and reaching. In the IG, progressive balance exercises were employed using the Xbox360 with Microsoft’s Kinect® with “Light Race”, “Stack’em up”, and “20,000 leaks” exergames | 35 min (5 min—warm-up; 30 min exercises) 3 x/wk | 6 wks (18) | Balance in static and dynamic conditions | Single- and dual-task TUG, single- and dual-task 10 MWT, Dual Task Costs, BBS, MSWS12, Fall Efficacy Scale-international, Activities-specific Balance Confidence scale | At the follow-up, reaction time and the number of falls demonstrated significant differences favoring IG. At the follow-up, there were no significant between-group differences in regard to TUG, BBS, MSWS12, Fall Efficacy Scale-international, Activities-specific Balance Confidence scale |
| Novotna 2019 [26] | wait list randomized controlled study | Balance training with audio-visual biofeedback | IG—individually tailored home-based balance exercise training using Homebalance® (therapeutic games where the therapeutic task can be set to different positions/directions, or the therapeutic task was to increase the limits of stability combined with cognitive training). CG—waiting list (no intervention) | At least 15 min x7/wk | 4 weeks (28 (approx. 7 hrs in total)) | Balance, gait parameters, falls | BBS; Mini-BESTest; TUG; assessment of the spatio-temporal gait parameters (by GAITRite walkway system). Falls Efficacy Scale, Activities-specific Balance Confidence Scale, MSWS12. | Statistically significant improvement in the mean BBS and in the Mini-BESTest. No improvement among other outcomes measured |
| Ortiz-Gutierrez 2013 (a) [19] | Non-blinded, non-randomized controlled trial | VR video games training | The CG—physiotherapy treatment (low-loads strength exercises, proprioception exercises on unstable surfaces and gait facilitation exercises, and muscle-tendon stretching). IG—individual TR treatments using the Xbox360® console with Microsoft® Kinect. The protocol proposed tasks such as throwing and hitting objects with one’s hands and feet, hitting and receiving balls with different body parts, dodging objects, overcoming obstacles, imitating postures, or managing virtual elements | SG 20 min 4 x/wk; CG 40 min x2/wk | 10 wks (SG—40 (up to 800 min)in total, CG—20 (up to 800 min) in total) | Posturography parameters, balance | BBS; Tinetti scale; Computerized Dynamic Posturography (The Sensory Organization Test and the Motor Control Test) | BBS and Tinetti scale scores revealed significant between-group differences in the IG, achieving higher values. Composite Equilibrium Score (part of The Sensory Organization Test) was significantly higher in IG in comparison with CG at the post intervention assessment |
| Ortiz-Gutiérrez 2013 (b) [20] | Non-blinded, controlled trial | VR-based balance training | IG received individual treatments using the Xbox 360 TM console with MicrosoftTM Kinect following a protocol consisting of three games (Kinect SportsTM, Kinect Joy RideTM, and Kinect AdventuresTM). CG received physiotherapy treatment based on low-loads strength exercises, proprioception exercises on unstable surfaces and gait facilitation exercises, muscle-tendon stretching | SG 20 min 4 x/wk; CG 40 min x2/wk | 10 weeks (SG—40 (up to 800 min) in total, CG—20 (up to 800 min) in total) | Posturography parameters, postural control | Computerized dynamic posturography (by Smart EquitestTM Version 8.2 CDP device); The Sensory Organization Test | Statistically significant improvement in composite equilibrium score in IG, non-significant improvements in CG |
| Peruzzi 2017 [33] | single-blinded, randomized controlled trial | VR-based treadmill training | A medical treadmill with a harness was used to administer the training programs in both groups. The IG—were walking on the treadmill while watching a virtual tree-lined trail (passing the obstacles appearing on the trail and following a road map, which was shown to them at the beginning of each walking bout). CG -only treadmill training with no VR | 45 min 3 x/wk | 6 wks (18) | Gait parameters, walking endurance and speed, mobility, balance, obstacle negotiation, disability | Gait analysis (gait data were collected using a six-camera stereophotogrammetric system with two force platforms, gait analysis was carried using the Motion Capture software (Vicon Nexus 2.0, Plug-in Gait), 6 MWT,10 MWT, TUG, BBS, four square step test, timed test consisting of stepping over an obstacle, Expanded Disability Status Scale) | Both the IG and CG significantly improved gait speed, cadence and stride length. Significantly larger improvements in kinematics and kinetics of gait in IG (knee range of motion p < 0.013, hip range of motion p < 0.001) |
| Robinson 2015 [34] | prospective, randomized controlled three-arm trial | Video-based exergaming balance training | IG 1 received exergaming with Wii Fit™; IG2 received traditional balance training, and CG received no intervention | 40–60 min 2 x/wk | 4 wks (8) | Postural sway, gait, walking ability, perceived activity and participation restrictions | Force plate, GAITRite™ walkway, MSWS12, 12-item World Health Organization Disability Assessment Schedule 2.0 questionnaire | Greater improvement in balance scores in all three of measures of postural sway in the IG1 group when compared to CG, and in postural sway antero-posterior and medio-lateral range in IG2 when compared to the CG group. No significant differences were found between IG1 and IG2 in outcome measured |
| Lozano-Quilis 2014 [31] | single-blinded, randomized controlled trial | VR-based balance training | In each session, CG—standard balance and gait rehabilitation exercises. IG—45 min performing the same (as in CG) exercises, and 15 min of the virtual rehabilitation exercises | 60 min 1 x/week; SG—45 min of exercises and 15 min of VR; CG—60 min of exercises | 10 weeks (10) | Balance in static and dynamic conditions | TBB; Tinetti; the Single Leg Balance test; 10 MWT; TUG | Significant group-by-time interaction was detected in the scores for the BBS (p = 0.030) and Single Leg Balance test of right foot (p = 0.033) |
| Yazgan 2020 [37] | single-blinded, randomized controlled, three-arm trial | Exergaming program | IG1—exergaming program (Nintendo Wii Fit) based on exergames selected from the Wii Fit Plus balance games section. IG2—exergaming program (Balance Trainer) CG participants were placed on a waiting list and invited to start exercising using Nintendo Wii Fit or Balance Trainer after the end of the study period | 60 min 2 x/wk | 8 wk (16) | Balance, gait, mobility, fatigue, quality of life | BBS, TUG, 6 MWT, Fatigue Severity Scale, Multiple Sclerosis International QoL Questionnaire | Statistically significant improvement in IG1 and IG2. IG1 noted better improvements than CG in balance tests, walking efficiency, fatigue and quality of life. IG2 was superior to CG in regard to balance, fatigue and QoL improvements |
| Study (First Author and Year) | Study Design | Type of Therapeutic Intervention | Intervention Description | Frequency and Duration of Sessions | Period of Therapeutic Intervention (Number of Sessions) | Measured Domains | Measurements | Key Results |
|---|---|---|---|---|---|---|---|---|
| Cuesta-Gomez 2020 [23] | single-blinded, randomized controlled trial | Leap Motion Controller (LMC) System | CG—a specific upper limb conventional motor rehabilitation therapy (60 min) (joints mobilization, muscles strengthening, functional task practice). IG—the same conventional motor rehabilitation therapy (45 min) plus VR (Leap Motion Controller) (15 min). Six serious games were performed first unilaterally and then bilaterally | 60 min 2 x/wk | 10 wks (20) | Grip Strength; gross manual dexterity on both sides; speed and motor dexterity of each hand; handfunction; fatigue; physical and psychological well-being | Grip strength (dynamometry); The Box and Blocks Test; TPPT; NHPT; Fatigue Severity Scale; Multiple Sclerosis Impact Scale | Significant improvements in IG in comparison with CG were found for the TPPT on the more affected side, both hands, assembly, and the Box and Blocks Test on the more affected side. For the follow-up measurements, significant improvements were found for The Box and Blocks Test on the more affected side and the NHPT on the more affected side |
| Ozdogar 2020 [27] | three-arm, non-blinded randomized controlled trial | Video-based exergaming therapy of arm and cognitive function | The video-based exergaming (The Kinect Sports Rivals game) was applied in IG. In CG patient-specific rehabilitation program (included balance, arm, and core stability exercises) was applied to the participants | 45 min 1 x/wk | 8 wks (8) | Hand dexterity, unilateral and bilateral activities of daily living, cognitive functioning, lower limb and trunk strength and endurance, gait, balance, depression, fatigue, QoL | NHPT; The Manual Ability Measurement-36; The Brief International Cognitive Assessment in MS; The Activities-specific Balance Confidence; Sit-to-stand test; The curl-up; 25 fW; MSWS12; The Six Spot Step Test; BDI; The Modified Fatigue Impact Scale; The Multiple Sclerosis International QoL | Significant improvements in the arm functions and in the most cognitive function, leg function and balance-related outcome measures were observed in the IG1 and IG2. No significant difference was observed in the changes from baseline at 8 weeks in the study outcomes between the IG1 and IG2 while several significant differences were observed in the changes of the CG compared to the IG1 and IG2 |
| Pawlukowska 2020 [28] | double-blinded, randomized controlled trial | Computer-assisted hand therapy | Participants in each group received progressive hand therapy treatments. IG—upper limb treatment with the RehaCom cognitive function platform (object moving along a pre-defined track and a cursor controlled by a joystick). CG—only progressive hand therapy treatments without RehaCom hand therapy | SG 20 min 3 x/wk; CG = n/d | SG up to 3 mth; CG n/d IG (540–640 min. in total mean—586 min.); (CG n/d) | Hand dexterity | NHPT | Improvement in time-to-complete NHPT in IG in regard to dominant (p = 0.007) and non-dominant hand (p = 0.037). No significant improvements in CG |
| Waliño-Paniagua 2019 [36] | single-blinded, randomized controlled trial | Hand dexterity—Game-Based VR Video Capture Training Program | Both groups received conventional occupational therapy treatment. IG additionally received VR treatment sessions via the online and free website motiongamingconsole.com, during which they performed exercises with video capture of the upper limb movements | SG = 30 min of occupational therapy 2 x/week + 20 min of VR treatment 2 x/week; CG 30 min of occupational therapy 2 x/week | 10 weeks (SG = 40 (20 sessions of occupational therapy + 20 sessions of VR therapy in total); CG = 20 (only occupational therapy)) | Manual dexterity and coordination, hand functional capacity | TPPT, Jebsen-Taylor Hand Function Test, Grooved Pegboard Test | No significant differences between outcome measures among IG and CG. Statistically significant differences were found in picking up small common objects in both groups |
| Norouzi 2020 [32] | three-arm controlled study | VR bimanual coordination training | In IG1 (VR group)—coordination of the movement of both hands with the movements of a visual stimulus (Kinect captured the hand movements of the participants). CG (Conventional Physical Training)—performing a complete cycle of in–out-in handle displacements in time with the beat of metronome. The metronome begun to pace at slow frequency (58 bpm) for 20 s. The same coordination task was paced at a medium metronome frequency and at a fast metronome frequency (152 bpm). IG2 (VR + Conventional Physical Training)—combined therapy | 30 min 2 x/wk | 8 wks (16) | Bimanual coordination | Bimanual coordination assessing procedure: Participants received a general orientation to the task. The task required them to grasp two handles attached to the moving slides and displace them horizontally in the left-right dimension (wrist extension and flexion). While grasping the two handles, participants produced a 180° relative phase (anti-phase) pattern. Potentiometers encoded the displacement of the handles over a 20 s trial | Bimanual coordination accuracy and consistency improved over time from baseline to study completion and to follow-up, butmore so in IG2 than CG or IG1. Improvements were greater in IG1 compared to CG |
| Study (First Author and Year) | Study Design | Type of Therapeutic Intervention | Intervention Description | Frequency and Duration of Sessions | Period of Therapeutic Intervention (Number of Sessions) | Measured Domains | Measurements | Key Results |
|---|---|---|---|---|---|---|---|---|
| Tallner 2016 [35] | randomized, controlled trial with a wait list control group | Internet-Supported Physical Exercise Training | In IG the aerobic and endurance exercise training was home-based and supervised via the Internet. CG participants were instructed to maintain their previous physical activity behavior. After waiting three months, they received the same e-training intervention as the intervention group had received from the start | 2 x/wk. of aerobic training and 1 x/wk. endurance training | 6 months. in SG; 3 months. waiting and 3 months. of exercises in CG | Health-related QoL, fatigue, maximum isometric muscle strength of lower limbs, lung function, habitual physical activity | Hamburg QoL Questionnaire for MS, Würzburg Fatigue Scale for MS, M3 Diagnos machine (for lower limb strength test), Forced vital capacity and peak expiratory flow (by Master Screen CPX System), Baecke Questionnaire (German version) | No improvement in health-related QoL and fatigue, IG recorded significant increases in strength of the lower extremities, lung function (peak expiratory flow) and physical activity. Improvement was significant in comparison to CG in regard to muscle strength and physical activity |
| Charvet 2017 [21] | double-blinded, randomized, active-placebo-controlled trial | Computer-based adaptive cognitive training program | IG—online adaptive cognitive training program (with a set of 15 exercises targeting speed, attention, working memory, and executive function through the visual and auditory domains). CG—an intervention based on a software gaming suite developed by Hoyle Puzzle and Board Games (2008 version) | 60 min 5 x/wk. | 12 wks. (60) | MS- related cognitive impairment, change in cognitive functioning | Neuropsychological Composite Score, Self-Reported Change in Cognitive Functioning (participants rated whether their cognition stayed the same (0), improved (1) or declined (−1) from baseline to study end) | IG had a significantly higher change in the neuropsychological composite from baseline to study end than CG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasadzka, E.; Trzmiel, T.; Pieczyńska, A.; Hojan, K. Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19. Medicina 2021, 57, 549. https://doi.org/10.3390/medicina57060549
Zasadzka E, Trzmiel T, Pieczyńska A, Hojan K. Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19. Medicina. 2021; 57(6):549. https://doi.org/10.3390/medicina57060549
Chicago/Turabian StyleZasadzka, Ewa, Tomasz Trzmiel, Anna Pieczyńska, and Katarzyna Hojan. 2021. "Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19" Medicina 57, no. 6: 549. https://doi.org/10.3390/medicina57060549
APA StyleZasadzka, E., Trzmiel, T., Pieczyńska, A., & Hojan, K. (2021). Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19. Medicina, 57(6), 549. https://doi.org/10.3390/medicina57060549







