A Simulation Case Study of Knee Joint Compressive Stress during the Stance Phase in Severe Knee Osteoarthritis Using Finite Element Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Gait Analysis
2.3. Musculoskeletal Modelling
2.4. Computed Tomography-Based Finite Element Method (CT-FEM)
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shelburne, K.B.; Torry, M.R.; Pandy, M.G. Contributions of muscles, ligaments, and the ground—reaction force to tibiofemoral joint loading during normal gait. J. Orthop. Res. 2006, 24, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Oatis, C.A.; Wolff, E.F.; Lockard, M.A.; Michener, L.A.; Robbins, S.J. Correlations among measures of knee stiffness, gait performance and complaints in individuals with knee osteoarthritis. Clin. Biomech. 2013, 28, 306–311. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.C.; Jones, A.; Muir, K.R.; Doherty, M. Quadriceps weakness in knee osteoarthritis: The effect on pain and disability. Ann. Rheum. Dis. 1998, 57, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Banks, S.A.; Mitchell, K.H.; D’Lima, D.D.; Colwell, C.W., Jr.; Fregly, B.J. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J. Orthop. Res. 2007, 25, 789–797. [Google Scholar] [CrossRef]
- Sharma, L.; Hurwitz, D.E.; Thonar, E.J.; Sum, J.A.; Lenz, M.E.; Dunlop, D.D.; Schnitzer, T.J.; Kirwan-Mellis, G.; Andriacchi, T.P. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis. Rheum. 1998, 41, 1233–1240. [Google Scholar] [CrossRef]
- Creaby, M.W.; Wang, Y.; Bennell, K.L.; Hinman, R.S.; Metcalf, B.R.; Bowles, K.A.; Cicuttini, F.M. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1380–1385. [Google Scholar] [CrossRef]
- Chang, A.; Hochberg, M.; Song, J.; Dunlop, D.; Chmiel, J.S.; Nevitt, M.; Hayes, K.; Eaton, C.; Bathon, J.; Jackson, R.; et al. Frequency of varus and valgus thrust, and factors associated with thrust presence in persons with or at higher risk of developing knee osteoarthritis. Arthritis. Rheumatol. 2010, 62, 1403–1411. [Google Scholar] [CrossRef]
- Dixon, P.C.; Gomes, S.; Preuss, R.A.; Robbins, S.M. Muscular co-contraction is related to varus thrust in patients with knee osteoarthritis. Clin. Biomech. 2018, 60, 164–169. [Google Scholar] [CrossRef]
- Lo, G.H.; Harvey, W.F.; McAlindon, T.E. Associations of varus thrust and alignment with pain in knee osteoarthritis. Arthritis. Rheum. 2012, 64, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Baliunas, A.J.; Hurwitz, D.E.; Ryals, A.B.; Karrar, A.; Case, J.P.; Block, J.A.; Andriacchi, T.P. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthr. Cartil. 2002, 10, 573–579. [Google Scholar] [CrossRef]
- Watanabe, K.; Mutsuzaki, H.; Fukaya, T.; Aoyama, T.; Nakajima, S.; Sekine, N.; Mori, K. Development of a knee joint CT-FEM model in load response of the stance phase during walking using muscle exertion, motion analysis, and ground reaction force data. Medicina 2020, 56, 56. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.A.; Charlton, J.M.; Krowchuk, N.M.; Tse, C.T.F.; Hatfield, G.L. Clinical and biomechanical changes following a 4-month toe-out gait modification program for people with medial knee osteoarthritis: A randomized controlled trial. Osteoarthr. Cartil. 2018, 26, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Burnfield, J. Gait Analysis: Normal and Pathological Function; SLACK Incorporated: Thorofare, NJ, USA, 1992; pp. 89–108. [Google Scholar]
- Damsgaard, M.; Rasmussen, J.; Soren, T.C.; Surma, E.; de Zee, M. Analysis of musculoskeletal systems in the AnyBody modeling system. Simul. Model. Pract. Theory 2006, 14, 1100–1111. [Google Scholar] [CrossRef]
- Lund, M.E.; Andersen, M.S.; de Zee, M.; Rasmussen, J. Scaling of musculoskeletal models from static and dynamic trials. Int. Biomech. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Imai, K.; Ohnishi, I.; Yamamoto, S.; Nakamura, K. In vivo assessment of lumbar vertebral strength in elderly women using computed tomography-based nonlinear finite element model. Spine 2008, 33, 27–32. [Google Scholar] [CrossRef]
- Silva, M.J.; Keaveny, T.M.; Hayes, W.C. Load sharing between the shell and centrum in the lumbar vertebral body. Spine 1997, 22, 140–150. [Google Scholar] [CrossRef]
- Keyak, J.H.; Rossi, S.A.; Jones, K.A.; Skinner, H.B. Prediction of femoral fracture load using automated finite element modelling. J. Biomech. 1998, 31, 125–133. [Google Scholar] [CrossRef]
- Keyak, J.H.; Sigurdsson, S.; Karlsdottir, G.; Oskarsdottir, D.; Sigmarsdottir, A.; Zhao, S.; Kornak, J.; Harris, T.B.; Sigurdsson, G.; Jonsson, B.Y.; et al. Male-female differences in the association between incident hip fracture and proximal femoral strength: A finite element analysis study. Bone 2011, 48, 1239–1245. [Google Scholar] [CrossRef]
- Kawabata, Y.; Matsuo, K.; Nezu, Y.; Kamiishi, T.; Inaba, Y.; Saito, T. The risk assessment of pathological fracture in the proximal femur using a CT-based finite element method. J. Orthop. Sci. 2017, 22, 931–937. [Google Scholar] [CrossRef]
- Takano, H.; Yonezawa, I.; Todo, M.; Mazlan, M.H.; Sato, T.; Kaneko, K. Biomechanical study of vertebral compression fracture using finite element analysis. J. Appl. Math. Phys. 2017, 5, 953–965. [Google Scholar] [CrossRef]
- Matsuura, Y.; Giambini, H.; Ogawa, Y.; Fang, Z.; Thoreson, A.R.; Yaszemski, M.J.; Lu, L.; An, K.N. Specimen-specific nonlinear finite element modeling to predict vertebrae fracture loads after vertebroplasty. Spine 2014, 39, E1291–E1296. [Google Scholar] [CrossRef]
- Halonen, K.S.; Dzialo, C.M.; Mannisi, M.; Venäläinen, M.S.; de Zee, M.; Andersen, M.S. Workflow assessing the effect of gait alterations on stresses in the medial tibial cartilage—Combined musculoskeletal modelling and finite element analysis. Sci. Rep. 2017, 7, 17396. [Google Scholar] [CrossRef]
- Arjmand, H.; Nazemi, M.; Kontulainen, S.A.; McLennan, C.E.; Hunter, D.J.; Wilson, D.R.; Johnston, J.D. Mechanical Metrics of the Proximal Tibia are Precise and Differentiate Osteoarthritic and Normal Knees: A Finite Element Study. Sci. Rep. 2018, 8, 11478. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Zhang, K.; Zhu, L.; Wang, X.; Jiang, Q. Three-dimensional finite-element analysis of aggravating medial meniscus tears on knee osteoarthritis. J. Orthop. Transl. 2019, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chantarapanich, N.; Nanakorn, P.; Chernchujit, B.; Sitthiseripratip, K. A finite element study of stress distributions in normal and osteoarthritic knee joints. J. Med. Assoc. Thai. 2009, 92 (Suppl. S6), S97–S103. [Google Scholar]
- Chang, A.; Hayes, K.; Dunlop, D.; Hurwitz, D.; Song, J.; Cahue, S.; Genge, R.; Sharma, L. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis. Rheum. 2004, 50, 3897–3903. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Chang, A.H.; Jackson, R.D.; Nevitt, M.; Moisio, K.C.; Hochberg, M.; Eaton, C.; Kwoh, C.K.; Almagor, O.; Cauley, J.; et al. Varus thrust and incident and progressive knee osteoarthritis. Arthritis. Rheumatol. 2017, 69, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
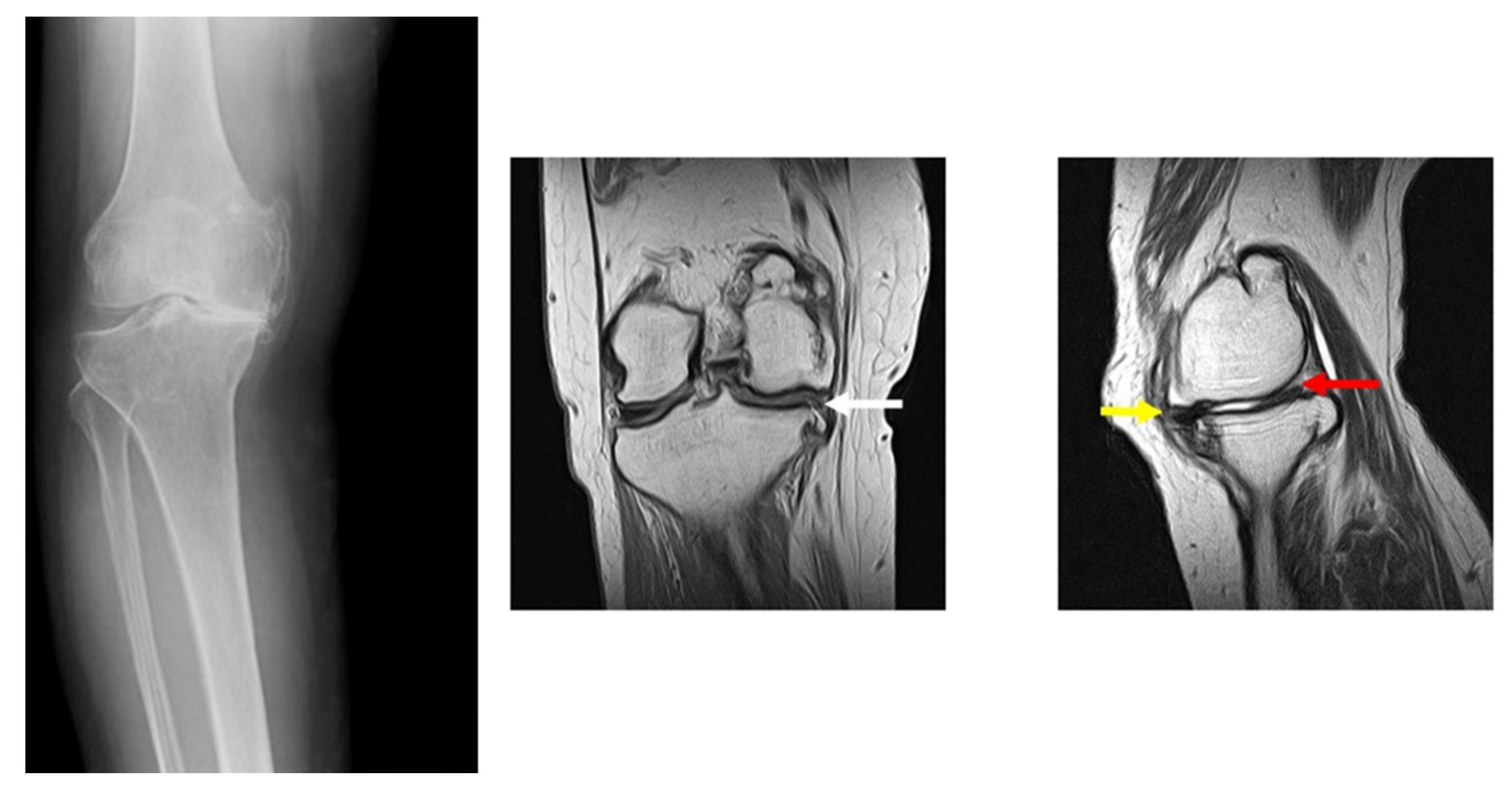
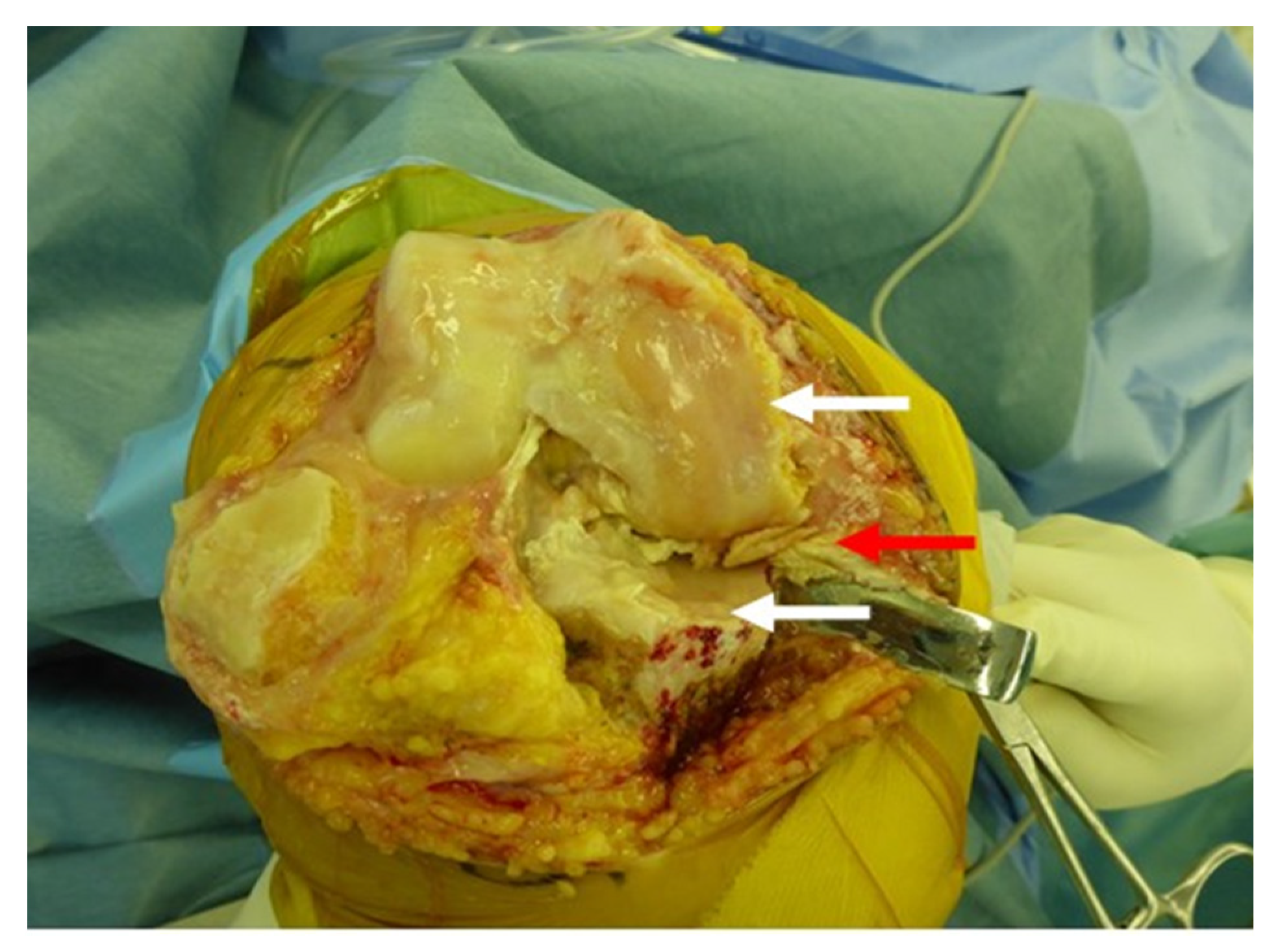
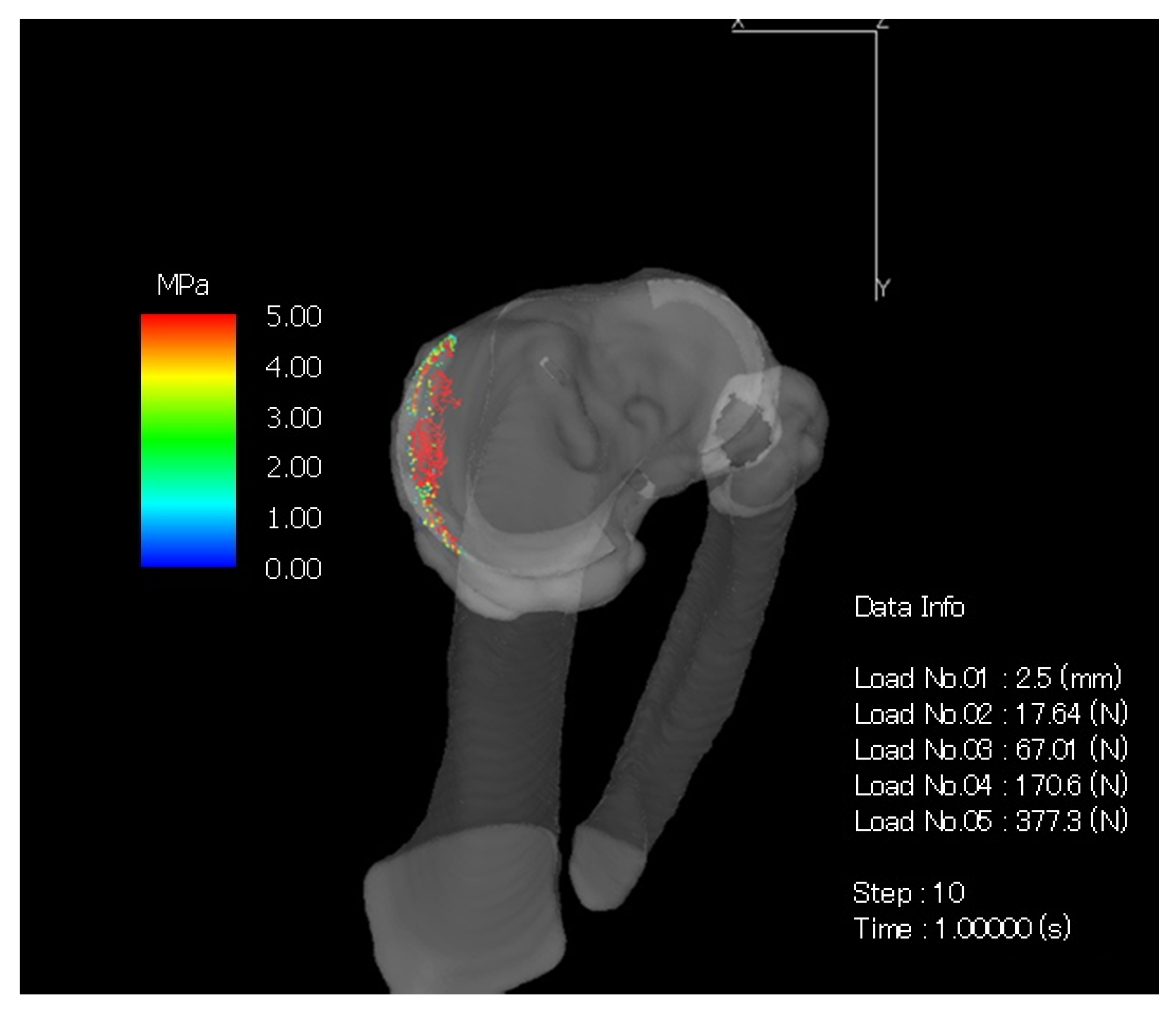
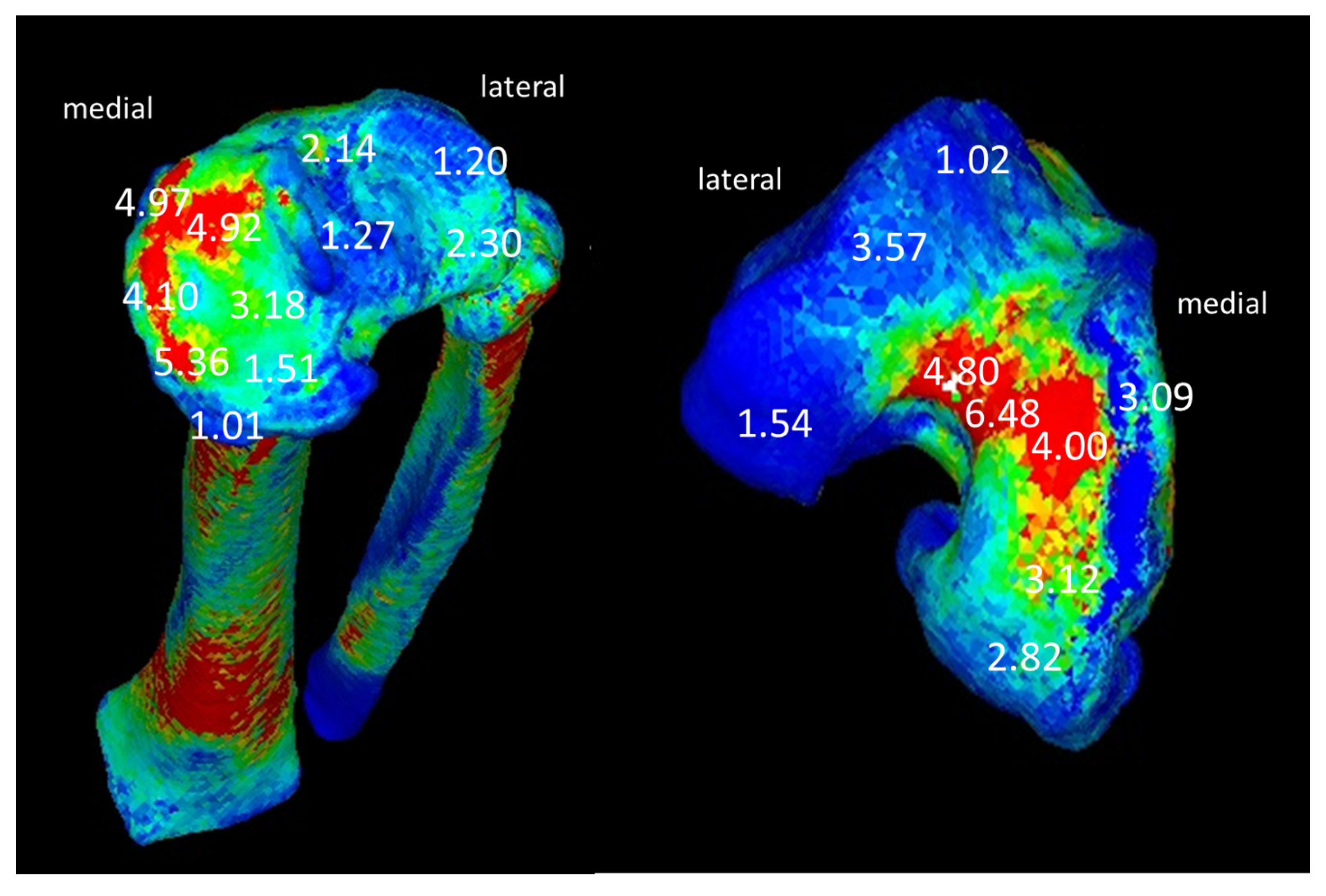
| Knee Joint Angles (Degrees) | Vertical Component of Ground Reaction Force (N) | |||
|---|---|---|---|---|
| Flexion | Adduction | External Rotation | ||
| KOA Subject | 0.3 | 16.2 | 11.9 | 371.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukaya, T.; Mutsuzaki, H.; Aoyama, T.; Watanabe, K.; Mori, K. A Simulation Case Study of Knee Joint Compressive Stress during the Stance Phase in Severe Knee Osteoarthritis Using Finite Element Method. Medicina 2021, 57, 550. https://doi.org/10.3390/medicina57060550
Fukaya T, Mutsuzaki H, Aoyama T, Watanabe K, Mori K. A Simulation Case Study of Knee Joint Compressive Stress during the Stance Phase in Severe Knee Osteoarthritis Using Finite Element Method. Medicina. 2021; 57(6):550. https://doi.org/10.3390/medicina57060550
Chicago/Turabian StyleFukaya, Takashi, Hirotaka Mutsuzaki, Toshiyuki Aoyama, Kunihiro Watanabe, and Koichi Mori. 2021. "A Simulation Case Study of Knee Joint Compressive Stress during the Stance Phase in Severe Knee Osteoarthritis Using Finite Element Method" Medicina 57, no. 6: 550. https://doi.org/10.3390/medicina57060550
APA StyleFukaya, T., Mutsuzaki, H., Aoyama, T., Watanabe, K., & Mori, K. (2021). A Simulation Case Study of Knee Joint Compressive Stress during the Stance Phase in Severe Knee Osteoarthritis Using Finite Element Method. Medicina, 57(6), 550. https://doi.org/10.3390/medicina57060550





