The Successful Use of Nitroglycerin for Uterine Hyperstimulation with Fetal Heart Rate Abnormality Caused by a Controlled-Release Dinoprostone Vaginal Delivery System (PROPESS): A Case Report
Abstract
:1. Introduction
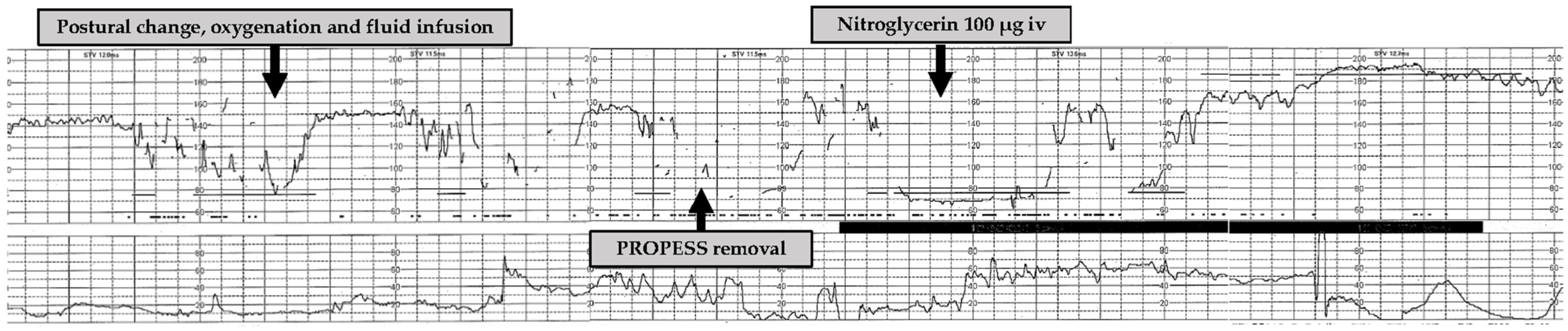
2. Case
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alfirevic, Z.; Kelly, A.J.; Dowswell, T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009, 2009, CD003246. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: Induction of labor. Obstet. Gynecol. 2009, 114, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Nooh, A.; Baghdadi, S.; Raouf, S. Induction of labour: How close to the evidence-based guidelines are we? J. Obstet. Gynaecol. 2005, 25, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Ishii, K.; Shigeta, N.; Itakura, A.; Hamada, H.; Nagamatsu, T.; Ishida, T.; Bungyoku, Y.; Falahati, A.; Tomisaka, M.; et al. Efficacy and safety of controlled-release dinoprostone vaginal delivery system (PROPESS) in Japanese pregnant women requiring cervical ripening: Results from a multicenter, randomized, double-blind, placebo-controlled phase III study. J. Obstet. Gynaecol. Res. 2021, 47, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xue, J.; Peprah, M.K.; Wen, S.W.; Walker, M.; Gao, Y.; Tang, Y. A systematic review and network meta-analysis comparing the use of Foley catheters, misoprostol, and dinoprostone for cervical ripening in the induction of labour. BJOG 2016, 123, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hong, S.; Liu, Y.; Duan, Y.; Yin, H. Controlled-release dinoprostone insert versus Foley catheter for labor induction: A meta-analysis. J. Matern. Fetal Neonatal. Med. 2016, 29, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, J.; Gillen, G.; Sanchez-Ramos, L.; Kaunitz, A.M. Do mechanical methods of cervical ripening increase infectious morbidity? A systematic review. Am. J. Obstet. Gynecol. 2008, 199, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Daykan, Y.; Biron-Shental, T.; Navve, D.; Miller, N.; Bustan, M.; Sukenik-Halevy, R. Prediction of the efficacy of dinoprostone slow release vaginal insert (PROPESS) for cervical ripening: A prospective cohort study. J. Obstet. Gynaecol. Res. 2018, 44, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Goeschen, K.; Fuchs, A.R.; Fuchs, F.; Rasmussen, A.B.; Rehnström, J.V.; Saling, E. Effect of beta-mimetics tocolysis on cervical ripening and plasma prostaglandin F2 alpha metabolite after endocervical application of prostaglandin E2. Obstet. Gynecol. 1985, 65, 166–171. [Google Scholar] [PubMed]
- Ohqvist, G. The use of nitrates in cardiac anaesthesia. Acta Anaesthesiol. Scand. Suppl. 1992, 97, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Axemo, P.; Fu, X.; Lindberg, B.; Ulmsten, U.; Wessen, A. Intravenous nitroglycerin for rapid uterine relaxation. Acta Obstet. Gynecol. Scand. 1998, 77, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, N.; Temming, L.A.; Doering, M.M.; Stoll, C.R.; Palanisamy, A.; Stout, M.J.; Colditz, G.A.; Cahill, A.G.; Tuuli, M.G. Maternal oxygen supplementation compared with room air for intrauterine resuscitation. JAMA Pediatr. 2021, 175, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Leathersich, S.J.; Vogel, J.P.; Tran, T.S.; Hofmeyr, G.J. Acute tocolysis for uterine tachysystole or suspected fetal distress. Cochrane Database Syst. Rev. 2018, 7, CD009770. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Dunn, L.; Tarnow-Mordi, W.; Flatley, C.; Flenady, V.; Kumar, S. Safety and efficacy of sildenafil citrate to reduce operative birth for intrapartum fetal compromise at term: A phase 2 randomized controlled trial. Am. J. Obstet. Gynecol. 2020, 222, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Dunn, L.; Kumar, S. Changes in fetoplacental doppler indices following intrapartum maternal sildenafil citrate treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 302–307. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takakura, S.; Tanaka, H.; Enomoto, N.; Maki, S.; Ikeda, T. The Successful Use of Nitroglycerin for Uterine Hyperstimulation with Fetal Heart Rate Abnormality Caused by a Controlled-Release Dinoprostone Vaginal Delivery System (PROPESS): A Case Report. Medicina 2021, 57, 478. https://doi.org/10.3390/medicina57050478
Takakura S, Tanaka H, Enomoto N, Maki S, Ikeda T. The Successful Use of Nitroglycerin for Uterine Hyperstimulation with Fetal Heart Rate Abnormality Caused by a Controlled-Release Dinoprostone Vaginal Delivery System (PROPESS): A Case Report. Medicina. 2021; 57(5):478. https://doi.org/10.3390/medicina57050478
Chicago/Turabian StyleTakakura, Sho, Hiroaki Tanaka, Naosuke Enomoto, Shintaro Maki, and Tomoaki Ikeda. 2021. "The Successful Use of Nitroglycerin for Uterine Hyperstimulation with Fetal Heart Rate Abnormality Caused by a Controlled-Release Dinoprostone Vaginal Delivery System (PROPESS): A Case Report" Medicina 57, no. 5: 478. https://doi.org/10.3390/medicina57050478
APA StyleTakakura, S., Tanaka, H., Enomoto, N., Maki, S., & Ikeda, T. (2021). The Successful Use of Nitroglycerin for Uterine Hyperstimulation with Fetal Heart Rate Abnormality Caused by a Controlled-Release Dinoprostone Vaginal Delivery System (PROPESS): A Case Report. Medicina, 57(5), 478. https://doi.org/10.3390/medicina57050478






