Associations between Physical Function, Bone Density, Muscle Mass and Muscle Morphology in Older Men with Sarcopenia: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Body Composition
2.3. Definition of Sarcopenia
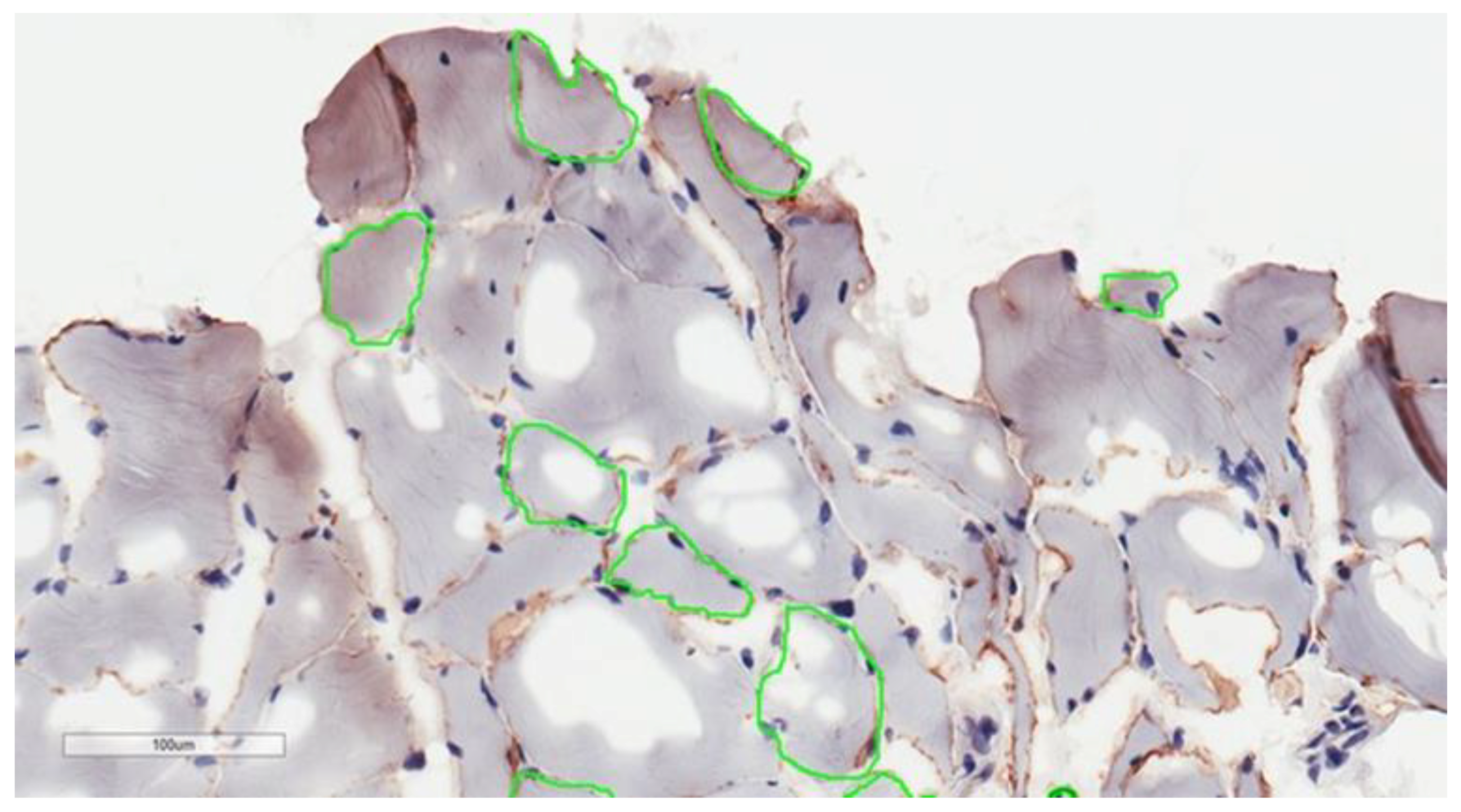
2.4. Muscle Biopsy
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Relationship between Bone Density and Body Composition
3.3. Association between Physical Performance, Bone Density and Lean Mass
3.4. Relationship between Strength, Bone Density and Lean Mass
3.5. Association between Muscle Morphology, Bone Density, Body Composition and Physical Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, I.H. Summary comments. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Morley, J.; Anker, S.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2018, 48, 48–56. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Soysal, P.; Smith, L.; Torbahn, G.; Schoene, D.; Schwingshackl, L.; Sieber, C.; Bauer, J.; Cesari, M.; et al. Sarcopenia and health-related outcomes: An umbrella review of observational studies. Eur. Geriatr. Med. 2019, 10, 853–862. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.-Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Henriksson-Larsén, K.; Winblad, B.; Sjöström, M. Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve 1983, 6, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R. Effects of Aging on Muscle Fibre Type and Size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Purves-Smith, F.M.; Sgarioto, N.; Hepple, R.T. Fiber typing in aging muscle. Exerc. Sport Sci. Rev. 2014, 42, 45–52. [Google Scholar] [CrossRef]
- Toomey, C.M.; Cremona, A.; Hughes, K.; Norton, C.; Jakeman, P. A Review of Body Composition Measurement in the Assessment of Health. Top. Clin. Nutr. 2015, 30, 16–32. Available online: https://journals.lww.com/topicsinclinicalnutrition/Fulltext/2015/01000/A_Review_of_Body_Composition_Measurement_in_the.3.aspx (accessed on 30 November 2020). [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Nguyen, U.D.T.; Nguyen, T.V. Association between Lean Mass, Fat Mass, and Bone Mineral Density: A Meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 30–38. [Google Scholar] [CrossRef]
- Westbury, L.D.; Syddall, H.E.; Fuggle, N.R.; Dennison, E.M.; Cauley, J.A.; Shiroma, E.J.; Fielding, R.A.; Newman, A.B.; Cooper, C. Long-term rates of change in musculoskeletal aging and body composition: Findings from the Health, Aging and Body Composition Study. Calcif. Tissue Int. 2020, 106, 616–624. [Google Scholar] [CrossRef]
- Kim, K.M.; Lim, S.; Oh, T.J.; Moon, J.H.; Choi, S.H.; Lim, J.Y.; Kim, K.W.; Park, K.S.; Jang, H.C. Longitudinal Changes in Muscle Mass and Strength, and Bone Mass in Older Adults: Gender-Specific Associations Between Muscle and Bone Losses. J. Gerontol. Ser. A 2017, 73, 1062–1069. [Google Scholar] [CrossRef]
- Frost, H.M. Perspectives: A proposed general model of the “mechanostat” (suggestions from a new skeletal-biologic paradigm). Anat. Rec. 1996, 244, 139–147. [Google Scholar] [CrossRef]
- Isaacson, J.; Brotto, M. Physiology of Mechanotransduction: How Do Muscle and Bone “Talk” to One Another? Clin. Rev. Bone Miner. Metab. 2014, 12, 77–85. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Kerr, C.; Bottomley, C.; Shingler, S.; Giangregorio, L.; de Freitas, H.M.; Patel, C.; Randall, S.; Gold, D.T. The Importance of Physical Function to People with Osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2017, 28, 1597–1607. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where Bone, Muscle, and Fat Collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Kanis, J.A. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Synopsis of a WHO Report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, M.K.; Gallager, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Phu, S.; Kirk, B.; Bani Hassan, E.; Vogrin, S.; Zanker, J.; Bernardo, S.; Duque, G. The diagnostic value of the Short Physical Performance Battery for sarcopenia. BMC Geriatr. 2020, 20, 242. [Google Scholar] [CrossRef]
- Deyo, R.A.; Diehr, P.; Patrick, D.L. Reproducibility and responsiveness of health status measures statistics and strategies for evaluation. Control. Clin. Trials 1991, 12, S142–S158. [Google Scholar] [CrossRef]
- Verschueren, S.; Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Ward, K.A.; Wu, F.C.; Szulc, P.; Laurent, M.; Claessens, F.; et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos. Int. 2013, 24, 87–98. [Google Scholar] [CrossRef]
- Patel, H.P.; Dawson, A.; Westbury, L.D.; Hasnaoui, G.; Syddall, H.E.; Shaw, S.; Sayer, A.A.; Cooper, C.; Dennison, E.M. Muscle Mass, Muscle Morphology and Bone Health Among Community-Dwelling Older Men: Findings from the Hertfordshire Sarcopenia Study (HSS). Calcif. Tissue Int. 2018, 103, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Hars, M.; Biver, E.; Chevalley, T.; Herrmann, F.; Rizzoli, R.; Ferrari, S.; Trombetti, A. Low Lean Mass Predicts Incident Fractures Independently From FRAX: A Prospective Cohort Study of Recent Retirees. J. Bone Miner. Res. 2016, 31, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Intriago, M.; Maldonado, G.; Guerrero, R.; Messina, O.D.; Rios, C. Bone Mass Loss and Sarcopenia in Ecuadorian Patients. J. Aging Res. 2020, 2020, 1072675. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Fu, L.; Jia, L.; Han, P.; Kang, L.; Yu, H.; Chen, X.; Yu, X.; Hou, L.; Wang, L.; et al. Muscle strength rather than muscle mass is associated with osteoporosis in older Chinese adults. J. Formos. Med Assoc. 2018, 117, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Ilich, J.Z.; Brummel-Smith, K.; Ghosh, S. New insight into fat, muscle and bone relationship in women: Determining the threshold at which body fat assumes negative relationship with bone mineral density. Int. J. Prev. Med. 2014, 5, 1452–1463. [Google Scholar]
- Lee, I.; Cho, J.; Jin, Y.; Ha, C.; Kim, T.; Hyunsik, K. Body Fat and Physical Activity Modulate the Association between Sarcopenia and Osteoporosis in Elderly Korean Women. J. Sports Sci. Med. 2016, 15, 477–482. [Google Scholar]
- Qi, H.; Sheng, Y.; Chen, S.; Wang, S.; Zhang, A.; Cai, J.; Lai, B.; Ding, G. Bone mineral density and trabecular bone score in Chinese subjects with sarcopenia. Aging Clin. Exp. Res. 2019, 31, 1549–1556. [Google Scholar] [CrossRef]
- Lindblad, A.; Dahlin-Ivanoff, S.; Bosaeus, I.; Rothenberg, E. Body Composition and Hand Grip Strength in Healthy Community-dwelling Older Adults in Sweden. J. Aging Res. Clin. Pract. 2015, 4, 54–58. [Google Scholar]
- Charlton, K.; Batterham, M.; Langford, K.; Lateo, J.; Brock, E.; Walton, K.; Lyons-Wall, P.; Eisenhauer, K.; Green, N.; McLean, C. Lean Body Mass Associated with Upper Body Strength in Healthy Older Adults While Higher Body Fat Limits Lower Extremity Performance and Endurance. Nutrients 2015, 7, 7126–7142. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, C.; Celi, M.; Lecce, D.; Baldi, J.; Rastelli, E.; Lena, E.; Massa, R.; Tarantino, U. Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporos. Int. 2013, 24, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Sarcopenia and osteoporosis are interrelated in geriatric inpatients. Z. Gerontol. Geriatr. 2019, 52, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Leung, J.; Woo, J. Incremental Predictive Value of Sarcopenia for Incident Fracture in an Elderly Chinese Cohort: Results From the Osteoporotic Fractures in Men (MrOs) Study. J. Am. Med Dir. Assoc. 2014, 15, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Avin, K.G.; Bloomfield, S.A.; Gross, T.S.; Warden, S.J. Biomechanical Aspects of the Muscle-Bone Interaction. Curr. Osteoporos. Rep. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.-Y.; Beaudart, C.; Buckinx, F.; Bruyère, O. Osteoporosis and Sarcopenia: Two Diseases or One? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef]
| Characteristics | All Subjects (n = 151) | No Sarcopenia (n = 106) | Sarcopenia (n = 45) | p-Value * |
|---|---|---|---|---|
| Age, years | 72.9 ± 8.02 | 70.33 ± 6.47 | 79.09 ± 8.05 | <0.001 |
| Height, cm | 172.73 ± 6.73 | 173.56 ± 6.56 | 170.74 ± 6.79 | 0.006 |
| Weight, kg | 81.5 ± 13.85 | 84.56 ± 13.44 | 74.15 ± 12.05 | <0.001 |
| BMI, kg/m2 | 27.24 ± 4.07 | 28.01 ± 4 | 25.4 ± 3.66 | 0.001 |
| Total fat mass, kg | 24.75 ± 8.83 | 25.86 ± 8.87 | 21.99 ± 7.91 | 0.01 |
| Android fat mass, kg (median (IQR)) | 2.57 (1.8–3.22) | 2.71 (2.03–3.44) | 2.3 (1.5–3.04) | 0.023 |
| Gynoid fat mass, kg (median (IQR)) | 3.35 (2.77–4.06) | 3.5 (2.96–4.17) | 3.06 (2.27–3.67) | 0.006 |
| Lean mass, kg | 54.01 ± 6.61 | 55.88 ± 6.06 | 49.37 ± 5.7 | <0.001 |
| Appendicular lean mass, kg | 24.32 ± 3.68 | 25.5 ± 3.34 | 21.51 ± 2.88 | <0.001 |
| Arm lean mass, kg | 6.78 ± 1.12 | 7.12 ± 1 | 5.85 ± 0.9 | <0.001 |
| Leg lean mass, kg | 17.61 ± 2.69 | 18.42 ± 2.47 | 15.66 ± 2.13 | <0.001 |
| SMMI, aSM/m2 | 8.13 ± 0.94 | 8.44 ± 0.82 | 7.37 ± 0.78 | <0.001 |
| Handgrip strength, kg | 31.54 ± 10.37 | 35.76 ± 8.66 | 21.39 ± 6.32 | <0.001 |
| Balance test, s (median (IQR)) | 10 (10–10) | 10 (10–10) | 10 (7.65–10) | <0.001 |
| 5 chair stands, s | 15.18 ± 5.45 | 14.18 ± 4.1 | 17.17 ± 7.61 | 0.017 |
| Gait speed, m/s (median (IQR)) | 0.88 (0.72–1) | 1 (0.85–1.08) | 0.66 (0.57–0.77) | <0.001 |
| SPPB, score (median (IQR)) | 9 (8–9) | 9 (8–9) | 8 (7.5–8.5) | <0.001 |
| Whole body BMD, g/cm2 | 1.19 ± 0.14 | 1.22 ± 0.13 | 1.11 ± 0.14 | 0.001 |
| Lumbar spine BMD, g/cm2 | 1.22 ± 0.23 | 1.2 ± 0.23 | 1.17 ± 0.33 | 0.157 |
| Femoral neck BMD, g/cm2 | 0.92 ± 0.15 | 0.97 ± 0.14 | 0.83 ± 0.12 | <0.001 |
| Whole body T-score (median (IQR)) | −0.1 (−1–0.85) | 0.1 (−0.7–1.1) | −1.1 (−1.7–0.1) | <0.001 |
| Lumbar spine BMD T-score (median (IQR)) | −0.4 (−1.3–1.3) | −0.4 (−1.2–1.3) | −0.6 (−1.55–0.6) | 0.17 |
| Femoral neck BMD T-score (median (IQR)) | −1.2 (−1.9–−0.4) | −0.9 (−1.6–−0.1) | −1.9 (−2.57–−1.3) | <0.001 |
| T-score ≤ −1.0, number (%) | 97 (64.2) | 58 (54.7) | 39 (86.7) | <0.001 |
| Whole body BMC, kg | 3 ± 0.47 | 3.11 ± 0.44 | 2.73 ± 0.45 | <0.001 |
| Number of comorbidities (median (IQR)) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.503 |
| Number of medications (median (IQR)) | 1 (1–2) | 1 (1–2) | 1.5 (1–3) | 0.277 |
| Lean Mass Parameters | Bone Density Measures | |||
|---|---|---|---|---|
| Whole Body BMD, g/cm2 | Femoral Neck BMD, g/cm2 | Whole Body T-Score | Femoral Neck T-Score | |
| Lean mass, kg | 0.37 * | 0.418 * | 0.37 * | 0.446 * |
| Appendicular lean mass, kg | 0.44 * | 0.5 * | 0.442 * | 0.52 * |
| Arm lean mass, kg | 0.451 * | 0.576 * | 0.457 * | 0.564 * |
| Leg lean mass, kg | 0.382 * | 0.435 * | 0.382 * | 0.411 * |
| SMMI, aSM/m2 | 0.382 * | 0.347 * | 0.372 * | 0.362 * |
| Physical performance | ||||
| SPPB, score | 0.57 | 0.223 * | 0.082 | 0.219 * |
| Gait speed, m/s | 0.14 | 0.301 | 0.05 | 0.46 * |
| Balance test, s | 0.024 | 0.442 * | 0.368 * | 0.45 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastavičiūtė, A.; Kilaitė, J.; Petroška, D.; Laurinavičius, A.; Tamulaitienė, M.; Alekna, V. Associations between Physical Function, Bone Density, Muscle Mass and Muscle Morphology in Older Men with Sarcopenia: A Pilot Study. Medicina 2021, 57, 156. https://doi.org/10.3390/medicina57020156
Mastavičiūtė A, Kilaitė J, Petroška D, Laurinavičius A, Tamulaitienė M, Alekna V. Associations between Physical Function, Bone Density, Muscle Mass and Muscle Morphology in Older Men with Sarcopenia: A Pilot Study. Medicina. 2021; 57(2):156. https://doi.org/10.3390/medicina57020156
Chicago/Turabian StyleMastavičiūtė, Asta, Justina Kilaitė, Donatas Petroška, Arvydas Laurinavičius, Marija Tamulaitienė, and Vidmantas Alekna. 2021. "Associations between Physical Function, Bone Density, Muscle Mass and Muscle Morphology in Older Men with Sarcopenia: A Pilot Study" Medicina 57, no. 2: 156. https://doi.org/10.3390/medicina57020156
APA StyleMastavičiūtė, A., Kilaitė, J., Petroška, D., Laurinavičius, A., Tamulaitienė, M., & Alekna, V. (2021). Associations between Physical Function, Bone Density, Muscle Mass and Muscle Morphology in Older Men with Sarcopenia: A Pilot Study. Medicina, 57(2), 156. https://doi.org/10.3390/medicina57020156






