Abstract
Beta-blockers are a class of drugs with important benefits in cardiovascular pathology. In this paper, we aim to highlight their adverse and therapeutic effects in the neuropsychiatric field. With respect to permeability, we would like to mention that most beta-blockers are lipophilic and can cross the blood–brain barrier. Observational studies show the presence of neuropsychiatric side effects when taking beta-blockers, and is the reason for which caution is recommended in their use in patients with depressive syndrome. From a therapeutic point of view, most current evidence is for the use of beta-blockers in migraine attacks, essential tremor, and akathisia. Beta-blockers appear to be effective in the treatment of aggressive behavior, beneficial in the prevention of posttraumatic stress syndrome and may play a role in the adjuvant treatment of obsessive–compulsive disorder, which is refractory to standard therapy. In conclusion, the relationship between beta-blockers and the central nervous system appears as a two-sided coin. Summarizing the neuropsychiatric side effects of beta-blockers, we suggest that clinicians pay special attention to the pharmacological properties of different beta-blockers.
1. Introduction
Beta-blockers are widely used for the treatment of cardiovascular and non-cardiovascular conditions [1,2,3]. Studies have shown that about 30% of the elderly are on beta-blocker treatment, hence the need to deepen the pharmacokinetic features [4]. The neuropsychiatric and cognitive effects of beta-blockers were proven many years ago. However, the evidence is limited by the use in trials of pharmaceutical preparations or different doses, but also by the application of different questionnaires of cognitive assessment [5,6,7]. The side effects on the central nervous system are heterogeneous, difficult to measure in clinical trials, and often require a prolonged follow-up. Both neuropsychiatric side effects and primary neurodegenerative diseases are common in patients with cardiovascular disease, making it difficult to attribute the symptoms to a concrete cause [8,9,10,11]. Therefore, this paper will summarize the pharmacokinetic and pharmacodynamic peculiarities of beta-blockers in relation to neuropsychiatric effects.
The lipophilic property of some beta-blockers is the ability of the drug to diffuse through the blood–brain barrier [12]. First, the agent must cross it and attach directly to the beta-adrenergic receptors, the suppression of which it mediates. Second, the drug must also interact with non-adrenergic receptors, blocking their signals or destabilizing the cell membrane [13]. Highly lipophilic beta-blockers, such as propranolol, diffuse rapidly through the brain tissue compared to hydrophilic beta-blockers, such as atenolol, which lacks this property [3].
2. Research Strategy
For this review, we employed the search strategy from well-known internationally accepted databases, namely PubMed, ScienceDirect, and Web of Science, using specific keywords, including beta-blockers side effects and psychiatric disorders associated with this medication. For investigation, a combination of keywords was used [beta-blockers; propranolol; metoprolol; atenolol; timolol; pindolol] + [fatigue; depression; sleep disorders; nightmares; hallucinations; delirium; Parkinson’s disease; risk of falling; migraine; tremor; akathisia; anxiety; posttraumatic stress syndrome; aggression; obsessive–compulsive disorder] + liposolubility. We included relevant reviews, meta-analyses, case reports, case studies, and comparative studies in the search strategy. When we started the research strategy, we discovered the lack of articles published in the last couple of years, this fact forcing us to include both articles dating back to 1975 up until 2020. Our research included prospective and retrospective cohorts and randomized clinical trials that included patients in treatment with lipophilic beta-blockers that had at least one neuropsychiatric symptoms (fatigue, depression, sleep disorders and nightmares, hallucinations, delirium, or other central nervous system therapeutic effects, such as migraine, tremor, akathisia, anxiety, aggression, or obsessive–compulsive disorder). Studies with patients under 18 years old, patients in treatment with hydrophilic beta-blockers, studies that did not mention or have psychiatric side effects, or small studies that included less than 50 patients were excluded. Beta-blockers are extremely frequently used in patients with cardiovascular disease, given it is recommended by all the guidelines, but we considered it useful to draw attention to their side effects, especially the ones in the psychiatric sphere. For example, a search with the keywords “beta-blockers side effects depression” yields 5 titles published in 2020, 14 titles in 2019, 6 in 2018, 9 in 2017, and 5 in 2016.
3. Pharmacological Characteristics
The physicochemical properties of molecules are of significant importance for therapeutic and side effects [14]. Although beta-blockers have similar pharmacotherapeutic effects, they may have different pharmacokinetic properties: gastrointestinal absorption rate, first-pass effect, lipid solubility, hepatic biotransformation rate, the pharmacological activity of metabolites, and clearance rate [15]. According to these pharmacokinetic properties, beta-blockers are divided into two major groups: agents eliminated from the body by hepatic metabolism and those excreted unchanged by the kidneys. First-class beta-blockers, such as propranolol or metoprolol, are almost completely absorbed in the small intestine, metabolized by the liver, and have a high solubility in lipids. In contrast, beta-blockers in the other category, such as atenolol or sotalol, are less or not at all soluble in lipids, incompletely absorbed and excreted unchanged by the kidney [16]. Beta-blockers were classified according to three fundamental characteristics for the understanding of the effects on the central nervous system: liposolubility, intrinsic sympathomimetic activity, and cardioselectivity (Table 1).

Table 1.
Beta-blockers classification regarding neuropsychiatric effects.
Liposolubility is defined as the physicochemical property of a substance that determines the fundamental characteristics of a medicinal preparation, with an impact on the metabolic, pharmacokinetic, pharmacodynamic, and toxicological profiles. Currently, numerous clinical trials have reported a significant relationship between permeability and lipophilia. The more liposoluble and smaller the substance, the more permeable it is through biological membranes [17]. Lipid solubility is not the only factor responsible for central nervous system effects. Intrinsic sympathomimetic activity, also known as partial agonist activity, allows the agent a minimum degree of beta-stimulation at rest [18]. Cardioselectivity refers to the ability of a pharmacological agent to block beta-1 adrenergic receptors preferentially. This property is relative; selective beta-blockers used in high doses can also cause inhibition of beta-2 adrenergic receptors [19].
Liposolubility is the ability of a biochemical compound to dissolve in fats, oils, lipids, and nonpolar solvents, such as hexane or toluene. In vivo, it is the key element for the diffusion through the cell membrane and binding to protein sites. Most often, lipophilia overlaps with the term hydrophobicity used to describe nature, both of which are used to describe the force of dispersion. There are particular situations in which the two terms do not have the same meaning; compounds that are hydrophobic but not lipophilic: silicone, fluorocarbons [20]. Lipophilia can be measured by determining the partition coefficient (log P), a molecular parameter that describes the balance of a non-ionized solute between water and an immiscible organic solvent [21]. Data from the literature reported by Lipinski et al. show that a substance with log P < 5 has the characteristics of a lipophilic molecule [22]. At present, current evidence shows that a value of >1 and <3 of the partition coefficients provide a substance with appropriate physicochemical properties for therapeutic success and limited side effects [23]. The difference between lipophilic and hydrophilic beta-blockers can be observed in Figure 1.
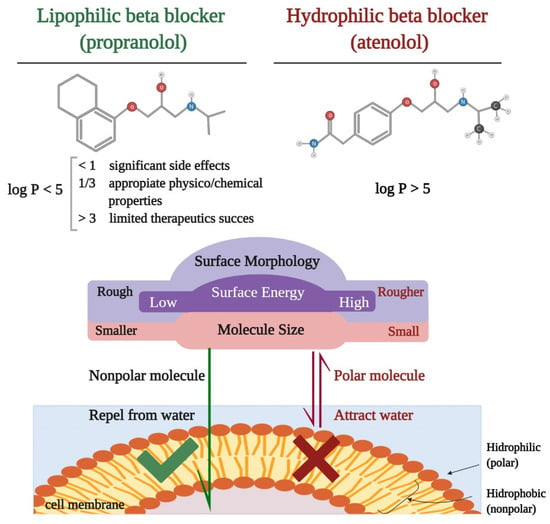
Figure 1.
Schematic representation of the difference between lipophilic and hydrophilic beta-blockers: chemical formula, partition coefficient (log P), physical properties, and permeability through a biological membrane.
The distribution of a drug in the central nervous system can also be explained by the structure of the interface between the blood system and the cerebrospinal system [24]. The blood–brain barrier is composed of endothelial vascular cells, astrocytes, and pericytes based in the choroid plexus, cerebral vascularity, and ependyma [24,25]. Capillary endothelial cells are interconnected by tight junctions containing efflux pumps (P-glycoprotein) with a role in limiting membrane permeability [26]. In general, small and highly lipophilic molecules can passively cross cell membranes that form the blood–brain barrier [27]. On the other hand, active transport mechanisms can influence the movement of the biological barrier to one side or the other; substances with high lipophilia have an increased affinity for certain efflux pumps [17]. Hence, getting across the blood–brain barrier is associated with various central nervous system consequences, as can be seen in Figure 2.
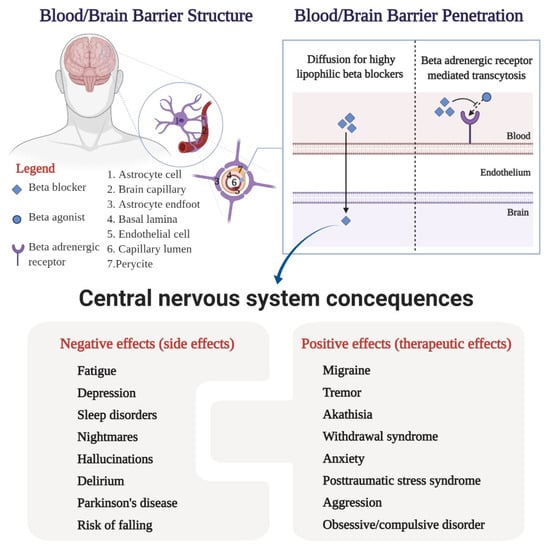
Figure 2.
Central nervous system consequences: angioarchitecture of the blood/brain barrier, delivery of the drugs to the brain, and interconnected negative and positive effects of the beta-blockers.
4. Central Nervous System Side Effects
Although beta-blockers have a high benefit/risk ratio, in some situations, they can negatively affect the quality of life through side effects. In general, side effects are reduced over time, are more common at high doses, and tend to be more frequent in elderly patients. By blocking beta-adrenergic receptors, the most common side effects are fatigue and cold extremities. Over time, beta-blocker treatment has been associated with a number of consequences for the central nervous system: fatigue, depression, sleep disorders and nightmares, visual hallucinations, delirium or psychosis, Parkinson’s disease, and the risk of falling [28].
4.1. Fatigue
Fatigue is a subjective clinical manifestation expressed by a deficiency of mental or physical energy. The mechanism of production is complex and poorly understood at present, involving both the central and the peripheral nervous systems [29,30,31]. Fatigue depends on intrinsic and extrinsic factors, such as the patient’s general mood or weather situation [32]. The Na+/K+-ATPase pump that controls the movement of ions between muscle cells and plasma may be one of the pathogenic pathways involved in the development of fatigue in patients treated with beta-blockers [33]. Both cardio selective and first-generation beta-blockers have a higher rate of association with fatigue compared to non-selective and new-generation beta-blockers [34]. The duration of treatment has a significant influence. Acute treatment with beta-blockers increases the risk of inducing fatigue and is often poorly tolerated by the patient, which can lead to therapeutic noncompliance. In contrast, in hypertensive patients undergoing beta-blocker treatment for long periods of time, the fatigue seems to disappear spontaneously [35].
4.2. Depression
Although the association between the use of beta-blocker treatment and the development of depression has been widely described over time, it is still a controversial topic nowadays. Several case reports and small general reports have suggested a relationship between propranolol and depression [28]. Specialists have proposed several mechanisms to explain a possible association between beta-blockers and depression in elderly patients, both through side effects regarding the sleep and circadian cycle regulation, as well as through sympathetic mediated feedback [36]. Two clinical trials have shown that antidepressants are prescribed at a significant rate in patients treated with propranolol [37,38].
On the contrary, a study on 312 patients receiving propranolol treatment did not show a substantial association with depressive symptoms after one year of follow-up [39]. A comprehensive overview of 5800 patients showed that the use of propranolol predisposes to depression in rare situations and that such symptoms occur after prolonged administration [40]. The most extensive analysis in relation to the association between beta-blockers and depression, a meta-analysis of 15 clinical trials (over 35,000 patients), showed that there was no significant increase in the incidence of depressive symptoms [34]. Jin et al. followed the 1-year incidence of major depressive disorder in patients without a history of antidepressant treatment or a diagnosis of depression in which beta-blocker was initiated for cardiovascular disease. Recently, the results of an observational study were published that showed the fact that the use of non-selective beta-blockers, female sex, a history of anxiety, and common chronic diseases (gastrointestinal and musculoskeletal) were predictive [41].
In patients with heart failure, data from the literature report an 11–45% prevalence of adverse events, such as anxiety and depression, in the cohort of patients undergoing beta-blocker treatment [42]. In the case of patients with chronic coronary syndrome, a study by Burkauskas et al. demonstrated an association between beta-blocker treatment and psychological symptoms [43]. Emotional stress caused by anxiety or depression can degenerate into a burnout syndrome clinically expressed through fatigue and exhaustion [1].
4.3. Sleep Disorders and Nightmares
The sympathetic vegetative nervous system is involved in inducing and maintaining sleep, while serotonin plays an important role in the physiology of normal sleep [44]. Beta-blockers, by their action of antagonizing beta-adrenergic and serotonergic receptors, suppress rapid eye movement (REM) sleep and can induce disorders up to insomnia [45]. Patients with insomnia have difficulty inducing sleep, maintaining sleep, or early morning awakening without the ability to fall back asleep [46]. Chronic insomnia occurs in 9–15% of patients with sleep disorders and can lead to significant daytime consequences, such as fatigue, sleepiness, inattention, concentration deficiency, impaired performance, and mood disturbance [47].
Melatonin plays a key role in regulating the circadian rhythm and sleep–wake cycle. The synthesis and secretion of melatonin in the systemic circulation are influenced by norepinephrine through beta 1-adrenergic receptors. Beta-blockers reduce the level of melatonin in the body and can thus induce sleep disorders and nightmares [48]. Nightmares are dysphoric dreams that generally occur during REM sleep in the last third of the night. Nightmares become pathological when they are frequent and disabling, affecting the individual from a social, occupational, and emotional point of view [44]. A systematic review showed that one-third of symptomatic patients with nightmares were on beta-blocker treatment [49]. Unlike pindolol, which decreases REM sleep time, acebutolol rather increases this time, so it has been concluded that sympathomimetic activity is not involved in the pathogenesis of sleep disorders [45].
4.4. Hallucinations and Delirium
Hallucinations are a mental distortion manifested in the form of imaginary sensory experiences that seem real to the patient experiencing them [50]. Current data regarding the relationship between beta-blockers and hallucinations are limited. Several studies have shown that lipophilic beta-blockers may have a causal role, with the remission of symptoms after the cessation of treatment. Goldner et al. showed that there is a relationship between metoprolol and visual hallucinations, a side effect generally attributed to dreams or nightmares, consequently underdiagnosed. At the same time, patients are reluctant to report such symptoms. Therefore, it is difficult to estimate a real incidence of hallucinations [51]. Sirois et al. reported that hallucinations occur in patients treated with metoprolol as an isolated symptom, but in elderly patients or those with preexisting cognitive deficits may degenerate into delirium [52]. Hallucinations caused by metoprolol usually disappear a few days after stopping the treatment [51].
Delirium is a clinical syndrome characterized by pathological disturbance of consciousness and perception manifested through hallucinations, delusions, or illusions [53]. Beta-blockers can cause delirium, especially in the elderly or those with preexisting cognitive dysfunction [54]. Katznelson R et al. showed that beta-blocker treatment is associated with doubling the risk of delirium after vascular surgery [55]. Harrison et al. demonstrated that patients who receive beta-blocker treatment had a significantly higher incidence of delirium compared to patients treated with calcium channel blockers [56]. Moreover, a directly proportional relationship between the beta-blocker dose and the risk of delirium-compatible symptoms has been reported. The incriminated mechanism is related to the antagonistic action of beta-blockers on the serotonin-sensitive adenylate cyclase system [57]. Psychosis, usually in the context of delirium, has been reported rarely in patients treated with propranolol, metoprolol, and atenolol [57,58,59].
4.5. Parkinson’s Disease
Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s disease, but the fastest growing in its category with a worldwide increase in the number of patients with the disease in recent decades [60]. Alpha-synucleine, a protein encoded by the SNCA gene, is the major constituent of Lewy bodies deposited in brain tissue [61]. Deposition of Lewy bodies in the brain tissue is the main pathogenic mechanism involved in the dementia of patients with Parkinson’s disease [62]. A human cell model that used neuroblastoma showed that beta-adrenergic agonists suppress SNCA gene expression, in contrast to beta-adrenergic antagonists that overexpress this gene and thus increase alpha-synucleine concentration [63]. Currently, the association between Parkinson’s disease and beta-blockers is considered a causal relationship between dose and duration of treatment [64]. The potential risk of beta-adrenergic antagonists is similar to the risk of pesticide exposure and overlaps with the most common genetic determination of Parkinson’s disease [65,66]. Patients treated with propranolol have been shown to have a reduced risk of developing Parkinson’s disease. For this reason, most clinicians do not change their therapeutic strategy [67].
4.6. The Risk of Falling
In the aging population, fall incidents form a growing healthcare problem [68]. Among patients over the age of 65, one out of three has at least one fall each year [69]. Falls significantly increase morbidity and mortality; they are associated with reduced quality of life and lead to increased health care costs [70]. One of the risk factors for falls is the use of certain drugs, including beta-adrenergic antagonists [71]. The mechanisms by which beta-blockers may increase the risk of falls refer to the induction of bradycardia, reducing the cardiac output, hypotension, and associated dizziness [72]. Pharmacological effects and adverse events may vary between beta-blocking agents, mainly related to the degree of lipid solubility, intrinsic sympathomimetic activity, and cardioselectivity [72,73]. A meta-analysis that included 2917 patients showed that treatment with non-selective beta-blockers significantly increased the risk of falling in elderly patients, in contrast to cardio selective beta-blockers that did not involve this risk. Ham A.C. et al. argued that the fall risk should be considered when prescribing a beta-blocker in the age group, and the pros and cons for beta-blockers classes should be taken into consideration [74].
5. Central Nervous System Therapeutic Effects
5.1. Migraine
The migraine, characterized by recurrent episodes of moderate–severe headache lasting 4–72 h, is a common disease that affects 15% of the general population [75,76]. Prophylactic therapy is aimed at patients with at least two episodes lasting more than 24 h/month and side effects despite the acute treatment of the headache [77]. Data from randomized clinical trials show that beta-blockers, in particular propranolol, metoprolol, and timolol, are effective in the prevention of chronic migraine. For hypertensive, non-smoking patients under the age of 60, beta-blockers are a reasonable option as first-line prophylactic medications [78]. A recently published meta-analysis evaluating the efficacy of various therapeutic classes in the management of vestibular migraine (antiepileptics, calcium channel blockers, beta-blockers, and serotonin reuptake inhibitors) showed that beta-blockers were associated with the best improvement regarding the symptoms [79].
5.2. Tremor
The tremor is defined as the involuntary, rhythmic, and oscillating movement of a body segment [80]. Essential tremor, the most common cause of acting tremor in adults, can be partially treated symptomatically. Propranolol monotherapy has been shown to be effective. Doses of 120–240 mg/day may reduce the severity of limbs tremor associated with essential tremor [81]. According to the results of randomized clinical trials, propranolol may reduce tremor amplitude by approximately 55%, measured with the accelerometer. A clinical trial showed that the association of primidone reduced the tremor amplitude by 60%, thus providing additional benefits [82]. The dual combination of propranolol and primidone has been shown to be more effective than monotherapy in the treatment of postural and kinetic tremor [83].
5.3. Akathisia and Alcohol or Benzodiazepine Withdrawal Syndrome
Akathisia is a clinical syndrome characterized by the feeling of inner discomfort and an imperative need for movement [84]. Acute akathisia most often occurs as a side effect of drugs, such as neuroleptics, selective serotonin reuptake inhibitors, or calcium channel blockers. Symptoms with a prolonged duration over 3 months characterize chronic forms, late akathisia, or abstinence [85]. Treatment of akathisia is difficult. Prevention and proper dose adjustment for drugs with this side effect are the most important strategies. Propranolol is the first line of treatment for acute forms of akathisia [86]. Atenolol, by reducing the symptoms of autonomous hyperactivity, is only an adjuvant measure in patients with alcohol or benzodiazepine withdrawal syndrome, with no proven benefit over delirium or seizures [87].
5.4. Anxiety and Posttraumatic Stress Syndrome
Propranolol was the first anxiolytic generally studied, but the interest has declined as selective serotonin reuptake inhibitors have become the first-line pharmacological treatment across the range of anxiety disorders [88]. Propranolol selectively blocks protein synthesis, thereby prohibiting the ‘reconsolidation’ of the fear memory while sparing declarative memory [89]. A meta-analysis suggests that propranolol has potential for the treatment of anxiety disorders that are rooted in the presence of disturbing memories, particularly posttraumatic stress disorder [90]. The administration of propranolol in the first 6 h after trauma significantly reduces the risk of developing posttraumatic stress disorder [91,92]. Propranolol is currently considered an amnesic agent used to reduce traumatic memory rather than a general anxiolytic [93].
Treatment with atenolol in association with scopolamine (antimuscarinic agent) has been proposed as a fast-acting anxiolytic, useful in the management of acute anxiety, especially before medical procedures (dental, minimally invasive) [94]. Recent data from a retrospective study suggest that atenolol may achieve the control of the symptoms in patients with anxiety or posttraumatic stress disorder, with reported efficacy of up to 86% at doses of 100 mg/day. The main advantage is the better tolerability to propranolol, but the disadvantage is that, so far, there are no randomized clinical trials regarding this topic [95].
5.5. Aggression
Aggression is a motor and visceral social behavior that consists of directing aggressive stimuli, physical or mental, of attacking an individual or for defense. Overall, the evidence for any successful treatment of aggression with any agent, or class of agents, is limited. Beta-blockers appear to be a well-tolerated class of drugs in patients with aggression related to traumatic brain injury [96]. Furthermore, beta-blockers appear to be effective in reducing aggression among patients with a variety of neuropsychiatric conditions, such as schizophrenia, dementia, or behavioral disorders [97,98,99].
5.6. Obsessive–Compulsive Disorder
Obsessive–compulsive disorder is characterized by intrusive and inappropriate obsessions accompanied by repetitive behavioral or mental compulsions [100]. Specific treatment with selective serotonin reuptake inhibitors or tricyclic antidepressants is effective in 80% of cases, and 20% of them have a partial response [101]. Pindolol, although it has antagonistic action on presynaptic 5-hydroxytryptamine 1A receptors, may have serotonergic effects due to its sympathomimetic activity [102]. Pindolol amplifies the effect of selective serotonin reuptake inhibitors and thus has been used adjunctively to enhance the benefits of selective serotonin reuptake inhibitors in obsessive–compulsive disorder and panic disorders refractory to standard therapy [101,102,103].
6. Limitations and Strengths
This paper has several limitations. First, the use of non-selective and lipophilic beta-blockers might decrease over time, also reducing the associated side effects. Second, the outcomes reflecting neuropsychiatric disorders will be underestimated because of the reduced addressability of the patients to a specialist. Third, the therapeutic effect of beta-blockers in psychiatric conditions is hard to prove through randomized clinical trials because there are already first-class recommendations of guidelines for other drugs than beta-blockers in different pathologies. However, data from observational studies offer hope regarding this topic. The development of the current society has determined an increase in the number of patients with psychiatric disorders, and through this article, we draw attention to the use of cardiovascular medication in this special category of patients. The main strength of this narrative review is to highlight the pros and cons of using beta-blockers, where lipophilia plays a leading role. Thus, beta-blockers are recognized as having an integrative role both in neuropsychiatry and cardiovascular specialties. As far as we know, this is one of the few articles that includes such extensive research in multiple databases regarding the lipophilicity of beta-blockers and their neuropsychiatric side effects.
7. Future Perspectives and Conclusions
Beta-blockers are a class of drugs with important benefits in cardiovascular pathology, mainly by reducing mortality. The number of patients with psychiatric disorders is increasing, and the elderly are one of the most affected categories. Thus, for the recommendation of beta-blockers in various pathologies (migraine, tremor, akathisia, anxiety, aggression, or obsessive–compulsive disorder), there are both pros and cons. Starting from these neuropsychiatric consequences (fatigue, depression, sleep disorders and nightmares, hallucinations, delirium, Parkinson’s disease, or the risk of falling), we suggest to the clinicians to apply special attention to the pharmacological characteristics of the different agents in the class. Hydrophilic beta-blockers may be an option in selecting the appropriate beta-blocker for the elderly patient, but further studies are needed to accurately identify patients at high risk of side effects on the central nervous system. However, the therapeutic effect of some lipophilic beta-blockers in the management of neuropsychiatric disorders is not negligible. At the same time, it is important to use beta-blockers in well-established and correct indications, in appropriate doses for each patient, and to monitor side effects throughout the treatment. Further studies regarding the benefits of lipophilic beta-blockers are needed. Thus, competent European and worldwide committees could take them into account and maybe include all the above in a guideline as a higher-class recommendation. Moreover, this paper is a place to start for researchers in the field, highlighting the fact that not only the type of beta-blocker matters but also the dosage, all being individualized for each patient profile.
Author Contributions
Conceptualization, M.M.L.-C. and F.M.; methodology, R.A.S. and C.S.; investigation, S.A.C.; resources, A.M.; writing—original draft preparation, S.A.C.; writing—review and editing, A.M.; visualization, M.M.L.-C.; supervision, F.M. All authors have equal contributions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McAinsh, J.; Cruickshank, J.M. Beta-blockers and central nervous system side effects. Pharmacol. Ther. 1990, 46, 163–197. [Google Scholar] [CrossRef]
- Weber, M.A. The role of the new beta-blockers in treating cardiovascular disease. Am. J. Hypertens. 2005, 18, 169S–176S. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Babar, A.; Patel, A.; Dortonne, R.; Jordan, J. Metoprolol-Associated Central Nervous System Complications. Cureus 2020, 12, e8236. [Google Scholar] [CrossRef] [PubMed]
- Márquez, P.H.P.; Torres, O.H.; San-José, A.; Vidal, X.; Agustí, A.; Formiga, F.; López-Soto, A.; Ramírez-Duque, N.; Fernández-Moyano, A.; Ruiz, D.; et al. Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ Project. Potentially Inappropriate Antihypertensive Prescriptions to Elderly Patients: Results of a Prospective, Observational Study. Drugs Aging. 2017, 34, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Stuhec, M.; Keuschler, J.; Serra-Mestres, J.; Isetta, M. Effects of different antihypertensive medication groups on cognitive function in older patients: A systematic review. Eur. Psychiatry 2017, 46, 1–15. [Google Scholar] [CrossRef]
- de Quervain, D.J.; Aerni, A.; Roozendaal, B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am. J. Psychiatry 2007, 164, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, J.G. Depression and cardiovascular disease: An update on how course of illness may influence risk. Curr. Psychiatry Rep. 2014, 16, 492. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Frishman, W.H. Neuropsychiatric effects of cardiovascular drug therapy. Cardiol. Rev. 2003, 11, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; Oh, J.M.; Harding, S.; Xu, G.; Hillmer, A.T.; Wooten, D.W.; Murali, D.; Barnhart, T.E.; et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, J.; Kovacs, G.G. Prevalence of mixed pathologies in the aging brain. Alzheimers Res. Ther. 2014, 6, 82. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Marchionni, N.; Fornasari, D. β-blockers: Their new life from hypertension to cancer and migraine. Pharmacol. Res. 2020, 151, 104587. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E.; Bruck, H.; Leineweber, K. Cardiac adrenoceptors: Physiological and pathophysiological relevance. J. Pharmacol. Sci. 2006, 100, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score–A comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2018, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W.H.; Lazar, E.J.; Gorodokin, G. Pharmacokinetic optimisation of therapy with beta-adrenergic blocking agents. Clin. Pharmacokinet. 1991, 20, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 1. Pharmacodynamic and pharmacokinetic properties. Am. Heart J. 1979, 97, 663–670. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Magder, S.; Sami, M.; Ripley, R.; Lisbona, R. Comparison of the effects of pindolol and propranolol on exercise performance in patients with angina pectoris. Am. J. Cardiol. 1987, 59, 1289–1294. [Google Scholar] [CrossRef]
- Lertora, J.J.; Mark, A.L.; Johannsen, J.; Wilson, W.R.; Abboud, F.M. Selective beta-1 receptor blockade with oral practolol in man. A dose-related phenomenon. J. Clin. Investig. 1975, 56, 719–724. [Google Scholar] [CrossRef][Green Version]
- Leeson, P.D.; Davis, A.M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 2004, 47, 6338–6348. [Google Scholar] [CrossRef]
- Testa, B.; Crivori, P.; Reist, M.; Carrupt, P.A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug Discov. Des. 2000, 19, 179–211. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Krämer, S.D.; Wunderli-Allenspach, H. Physicochemical properties in pharmacokinetic lead optimization. Farmaco 2001, 56, 145–148. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 1998, 251, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Huffman, J.C.; Stern, T.A. Neuropsychiatric consequences of cardiovascular medications. Dialogues Clin. Neurosci. 2007, 9, 29–45. [Google Scholar] [CrossRef]
- Kaminska, M.; Kimoff, R.J.; Schwartzman, K.; Trojan, D.A. Sleep disorders and fatigue in multiple sclerosis: Evidence for association and interaction. J. Neurol. Sci. 2011, 302, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Chervin, R.D. Fatigue in multiple sclerosis: Mechanisms, evaluation, and treatment. Sleep 2010, 33, 1061–1067. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Hall, P.E.; Kendall, M.J.; Smith, S.R. Beta blockers and fatigue. J. Clin. Hosp. Pharm. 1984, 9, 283–291. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, R.S.; Jones, N.L.; Heigenhauser, G.J. Factors contributing to increased muscle fatigue with beta-blockers. Can. J. Physiol. Pharmacol. 1991, 69, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Sedrakyan, A.; Curtis, J.P.; Krumholz, H.M. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002, 288, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Fellenius, E. Muscle fatigue and beta-blockers—A review. Int. J. Sports Med. 1983, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agustini, B.; Mohebbi, M.; Woods, R.L.; McNeil, J.J.; Nelson, M.R.; Shah, R.C.; Murray, A.M.; Ernst, M.E.; Reid, C.M.; Tonkin, A.; et al. on behalf of the ASPREE Investigator Group. The association of antihypertensive use and depressive symptoms in a large older population with hypertension living in Australia and the United States: A cross-sectional study. J. Hum. Hypertens. 2020, 34, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, B.Q.; Wallace, S.M.; Blackburn, J.L.; Wilson, T.W.; Bergman, U. Increased prescribing of antidepressants subsequent to beta-blocker therapy. Arch. Intern. Med. 1990, 150, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Hallas, J. Evidence of depression provoked by cardiovascular medication: A prescription sequence symmetry analysis. Epidemiology 1996, 7, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Stable, E.J.; Halliday, R.; Gardiner, P.S.; Baron, R.B.; Hauck, W.W.; Acree, M.; Coates, T.J. The effects of propranolol on cognitive function and quality of life: A randomized trial among patients with diastolic hypertension. Am. J. Med. 2000, 108, 359–365. [Google Scholar] [CrossRef]
- Stoudemire, A.; Brown, J.T.; Harris, R.T.; Blessing-Feussner, C.; Roberts, J.H.; Nichols, J.C.; Houpt, J.L. Propranolol and depression: A reevaluation based on a pilot clinical trial. Psychiatr. Med. 1984, 2, 211–218. [Google Scholar]
- Jin, S.; Kostka, K.; Posada, J.D.; Kim, Y.; Seo, S.I.; Lee, D.Y.; Shah, N.H.; Roh, S.; Lim, Y.H.; Chae, S.G.; et al. Prediction of Major Depressive Disorder Following Beta-Blocker Therapy in Patients with Cardiovascular Diseases. J. Pers. Med. 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Willgoss, T.G.; Baldwin, R.C.; Connolly, M.J. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: Prevalence, relevance, clinical implications and management principles. Int. J. Geriatr. Psychiatry 2010, 25, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Burkauskas, J.; Noreikaite, A.; Bunevicius, A.; Brozaitiene, J.; Neverauskas, J.; Mickuviene, N.; Bunevicius, R. Beta-1-Selective Beta-Blockers and Cognitive Functions in Patients with Coronary Artery Disease: A Cross-Sectional Study. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Kostis, J.B.; Rosen, R.C. Central nervous system effects of beta-adrenergic-blocking drugs: The role of ancillary properties. Circulation 1987, 75, 204–212. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, 13–15 June 2005. Sleep 2005, 28, 1049–1057. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Thompson, D.F.; Pierce, D.R. Drug-induced nightmares. Ann. Pharmacother. 1999, 33, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.; Larøi, F.; McGuire, P.K.; Aleman, A. The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 2008, 32, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Goldner, J.A. Metoprolol-induced visual hallucinations: A case series. J. Med. Case Rep. 2012, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Sirois, F.J. Visual hallucinations and metoprolol. Psychosomatics 2006, 47, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R. Delirium in Hospitalized Older Adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Wiens, C.A. An approach to drug induced delirium in the elderly. Postgrad. Med. J. 2004, 80, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, R.; Djaiani, G.; Mitsakakis, N.; Lindsay, T.F.; Tait, G.; Friedman, Z.; Wasowicz, M.; Beattie, W.S. Delirium following vascular surgery: Increased incidence with preoperative beta-blocker administration. Can. J. Anaesth. 2009, 56, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Luciano, S.; Colbourne, L. Rates of delirium associated with calcium channel blockers compared to diuretics, renin-angiotensin system agents and beta-blockers: An electronic health records network study. J. Psychopharmacol. 2020, 34, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.A.; Davis, M.; Jeffery, I. Acute delirium induced by metoprolol. Cardiovasc. Drugs Ther. 2002, 16, 161–165. [Google Scholar] [CrossRef]
- Love, J.N.; Handler, J.A. Toxic psychosis: An unusual presentation of propranolol intoxication. Am. J. Emerg. Med. 1995, 13, 536–537. [Google Scholar] [CrossRef]
- Viadero, J.J.; Wong, S.H.; White, W.B. Acute psychotic behavior associated with atenolol. Am. J. Psychiatry 1983, 140, 1382. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Braak, H.; Bohl, J.R.; Müller, C.M.; Rüb, U.; de Vos, R.A.; Del Tredici, K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov. Disord. 2006, 21, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Bjørnevik, K.; Im, D.S.; Flierl, A.; Dong, X.; Locascio, J.J.; Abo, K.M.; Long, E.; Jin, M.; Xu, B.; et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science 2017, 357, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Norton, G.; Radinsky, K.; Shalev, V. Chronic Use of β-Blockers and the Risk of Parkinson’s Disease. Clin. Drug Investig. 2019, 39, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J.P. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism. Relat. Disord. 2016, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Nalls, M.A.; Hallgrímsdóttir, I.B.; Hunkapiller, J.; van der Brug, M.; Cai, F.; Kerchner, G.A.; Ayalon, G.; Bingol, B.; Sheng, M.; et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Höglinger, G.U.; Kuhlenbäumer, G.; Pottegård, A.; Wod, M.; Christensen, K.; Tanner, C.M.; Deuschl, G. β-adrenoreceptors and the risk of Parkinson’s disease. Lancet Neurol. 2020, 19, 247–254. [Google Scholar] [CrossRef]
- Cumming, R.G. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Doucette, J.; Claus, E.; Marottoli, R. Risk factors for serious injury during falls by older persons in the community. J. Am. Geriatr. Soc. 1995, 43, 1214–1221. [Google Scholar] [CrossRef]
- Hartholt, K.A.; van Beeck, E.F.; Polinder, S.; van der Velde, N.; van Lieshout, E.M.; Panneman, M.J.; van der Cammen, T.J.; Patka, P. Societal consequences of falls in the older population: Injuries, healthcare costs, and long-term reduced quality of life. J. Trauma. 2011, 71, 748–753. [Google Scholar] [CrossRef]
- Boyle, N.; Naganathan, V.; Cumming, R.G. Medication and falls: Risk and optimization. Clin. Geriatr. Med. 2010, 26, 583–605. [Google Scholar] [CrossRef]
- Reiter, M.J. Cardiovascular drug class specificity: Beta-blockers. Prog. Cardiovasc. Dis. 2004, 47, 11–33. [Google Scholar] [CrossRef]
- Frishman, W.H.; Alwarshetty, M. Beta-adrenergic blockers in systemic hypertension: Pharmacokinetic considerations related to the current guidelines. Clin. Pharmacokinet. 2002, 41, 505–516. [Google Scholar] [CrossRef]
- Ham, A.C.; van Dijk, S.C.; Swart, K.M.; Enneman, A.W.; van der Zwaluw, N.L.; Brouwer-Brolsma, E.M.; van Schoor, N.M.; Zillikens, M.C.; Lips, P.; de Groot, L.C.; et al. Beta-blocker use and fall risk in older individuals: Original results from two studies with meta-analysis. Br. J. Clin. Pharmacol. 2017, 83, 2292–2302. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000, 55, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.J.; Levy, D.A.; Nguyen, S.A.; Brennan, E.; Rizk, H.G. Treatment of Vestibular Migraine: A Systematic Review and Meta-analysis. Laryngoscope 2021, 131, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G. Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Haubenberger, D.; Hallett, M. Essential Tremor. N. Engl. J. Med. 2018, 378, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Raethjen, J.; Hellriegel, H.; Elble, R. Treatment of patients with essential tremor. Lancet Neurol. 2011, 10, 148–161. [Google Scholar] [CrossRef]
- Heilman, K.M. Orthostatic tremor. Arch. Neurol. 1984, 41, 880–881. [Google Scholar] [CrossRef] [PubMed]
- Musco, S.; McAllister, V.; Caudle, I. Dopamine-receptor blocking agent-associated akathisia: A summary of current understanding and proposal for a rational approach to treatment. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320937575. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S. Neuroleptic-induced movement disorders: An overview. Psychiatr. Clin. 2005, 28, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Sethuram, K.; Gedzior, J. Akathisia: Case Presentation and Review of Newer Treatment Agents. Psychiatr. Ann. 2014, 44, 391–396. [Google Scholar] [CrossRef]
- Horwitz, R.I.; Gottlieb, L.D.; Kraus, M.L. The efficacy of atenolol in the outpatient management of the alcohol withdrawal syndrome. Results of a randomized clinical trial. Arch. Intern. Med. 1989, 149, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.S.; Anderson, I.M.; Nutt, D.J.; Allgulander, C.; Bandelow, B.; den Boer, J.A.; Christmas, D.M.; Davies, S.; Fineberg, N.; Lidbetter, N.; et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. 2014, 28, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Kindt, M.; Soeter, M.; Vervliet, B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat. Neurosci. 2009, 12, 256–258. [Google Scholar] [CrossRef]
- Lonergan, M.H.; Olivera-Figueroa, L.A.; Pitman, R.K.; Brunet, A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: A meta-analysis. J. Psychiatry Neurosci. 2013, 38, 222–231. [Google Scholar] [CrossRef]
- Vaiva, G.; Ducrocq, F.; Jezequel, K.; Averland, B.; Lestavel, P.; Brunet, A.; Marmar, C.R. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol. Psychiatry 2003, 54, 947–949. [Google Scholar] [CrossRef]
- Pitman, R.K.; Sanders, K.M.; Zusman, R.M.; Healy, A.R.; Cheema, F.; Lasko, N.B.; Cahill, L.; Orr, S.P. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol. Psychiatry. 2002, 51, 189–192. [Google Scholar] [CrossRef]
- Steenen, S.A.; van Wijk, A.J.; van der Heijden, G.J.; van Westrhenen, R.; de Lange, J.; de Jongh, A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J. Psychopharmacol. 2016, 30, 128–139. [Google Scholar] [CrossRef]
- Thomas, T.; Dooley, T. Treatment of anxiety prior to a medical procedure using an atenolol-scopolamine combination drug. J. Depress. Anxiety 2018, 7, 303. [Google Scholar] [CrossRef]
- Armstrong, C.; Kapolowicz, M.R. A Preliminary Investigation on the Effects of Atenolol for Treating Symptoms of Anxiety. Mil. Med. 2020, 185, e1954–e1960. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S.; Greenwood, R.J.; Oliver, D.L. Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database Syst. Rev. 2006, 4, CD003299. [Google Scholar] [CrossRef] [PubMed]
- Haspel, T. Beta-blockers and the treatment of aggression. Harv. Rev. Psychiatry 1995, 2, 274–281. [Google Scholar] [CrossRef]
- Fava, M. Psychopharmacologic treatment of pathologic aggression. Psychiatr. Clin. 1997, 20, 427–451. [Google Scholar] [CrossRef]
- Peskind, E.R.; Tsuang, D.W.; Bonner, L.T.; Pascualy, M.; Riekse, R.G.; Snowden, M.B.; Thomas, R.; Raskind, M.A. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease: A placebo-controlled study. Alzheimer Dis. Assoc. Disord. 2005, 19, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Bloch, M.H.; Mathews, C.A. Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. JAMA 2017, 317, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Dannon, P.N.; Sasson, Y.; Hirschmann, S.; Iancu, I.; Grunhaus, L.J.; Zohar, J. Pindolol augmentation in treatment-resistant obsessive compulsive disorder: A double-blind placebo controlled trial. Eur. Neuropsychopharmacol. 2000, 10, 165–169. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Zhu, D.; Chen, J.; Qin, B.; Zhang, Y.; Wang, X.; Yang, D.; Meng, H.; Luo, Q.; et al. Is pindolol augmentation effective in depressed patients resistant to selective serotonin reuptake inhibitors? A systematic review and meta-analysis. Hum. Psychopharmacol. 2015, 30, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, S.; Dannon, P.N.; Iancu, I.; Dolberg, O.T.; Zohar, J.; Grunhaus, L. Pindolol augmentation in patients with treatment-resistant panic disorder: A double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2000, 20, 556–559. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).