Course of Metal Ions after a Revision of Malfunctioning Metal-on-Metal Total Hip Prostheses
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analysis of Metals
2.3. Statistics
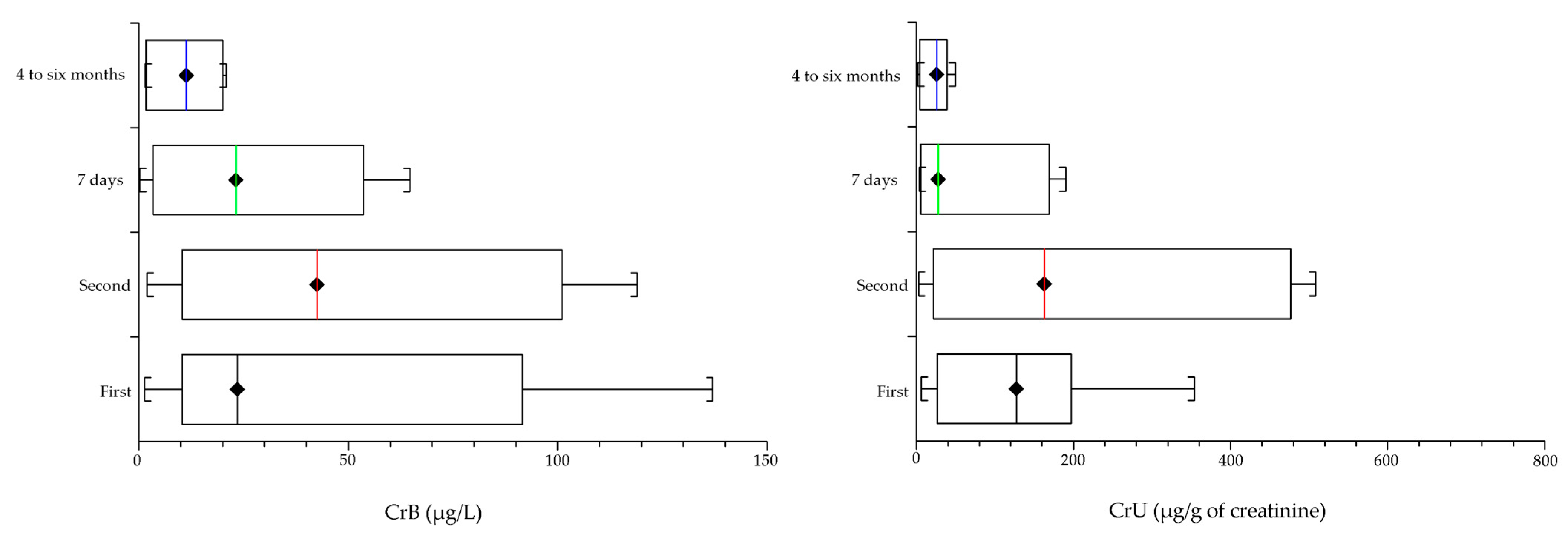
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willert, H.G.; Buchhorn, G.H.; Fayyazi, A.; Flury, R.; Windler, M.; Köster, G.; Lohmann, C.H. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J. Bone Jt. Surg. Am. 2005, 87, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, A.; Bisinella, G.; Padovani, G.; Vitella, A.; Chiara, F.; Trevisan, A. Predictivity and fate of metal ion release from metal-on-metal total hip prostheses. J. Arthroplast. 2014, 29, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, A.; Trevisan, A.; Bortoletti, I.; Pozzuoli, A.; Ruggieri, P.; Martinelli, A.; Gambalunga, A.; Carrieri, M. Biological monitoring of metal ions released from hip prostheses. Int. J. Environ. Res. Public Health 2020, 17, 3223. [Google Scholar] [CrossRef] [PubMed]
- Pozzuoli, A.; Berizzi, A.; Crimì, A.; Belluzzi, E.; Frigo, A.C.; Conti, G.; Nicolli, A.; Trevisan, A.; Biz, C.; Ruggieri, P. Metal ion release, clinical and radiological outcomes in large diameter metal-on-metal total hip arthroplasty at long-term follow-up. Diagnostics 2020, 10, 941. [Google Scholar] [CrossRef]
- Dover, C.; Kuiper, J.H.; Craig, P.; Shaylor, P. Ten years on: Increased metal ion levels in a cohort of patients who underwent uncemented metal-on-polyethylene total hip arthroplasty. Bone Jt. J. 2020, 102, 832–837. [Google Scholar] [CrossRef]
- van Raay, J.J. Metal-on-metal total hip arthroplasty: Known and unknown side effects. Orthopedics 2012, 35, 447–449. [Google Scholar] [CrossRef]
- Medical Device Alert. All metal-on-Metal (MoM) Hip Replacement. MHRA MDA/2012/008, Issued 28 February 2012. Available online: https://assets.publishing.service.gov.uk (accessed on 22 December 2020).
- Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure-An overview. Sci. Total Environ. 1994, 150, 1–6. [Google Scholar] [CrossRef]
- Barceloux, D.G. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–206. [Google Scholar] [CrossRef]
- Catalani, S.; Rizzetti, M.C.; Padovani, A.; Apostoli, P. Neurotoxicity of cobalt. Hum. Exp. Toxicol. 2012, 31, 421–437. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Galbraith, D.A.; Finley, B.L. Interpreting cobalt blood concentrations in hip implant patients. Clin. Toxicol. 2014, 52, 98–112. [Google Scholar] [CrossRef]
- Catalani, S.; Leone, R.; Rizzetti, M.C.; Padovani, A.; Apostoli, P. The role of albumin in human toxicology of cobalt: Contribution from a clinical case. ISRN Hematol. 2011, 690620. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Sklensky, M.; Janicek, P.; Lach, K. Severe cobalt intoxication following hip replacement revision: Clinical features and outcome. Clin. Toxicol. 2012, 50, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Wegner, R.; Baur, X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J. Arthroplasty 2009, 24, 825.e15–825.e20. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.R. Systemic toxicity related to metal hip prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar] [CrossRef]
- Vendittoli, P.-A.; Amzica, T.; Roy, A.G.; Lusignan, D.; Girard, J.; Lavigne, M. Metal ion release with large-diameter metal-on-metal hip arthroplasty. J. Arthroplast. 2011, 26, 282–288. [Google Scholar] [CrossRef]
- Maurer-Ertl, W.; Friesenbichler, J.; Sadoghi, P.; Pechmann, M.; Trennheuser, M.; Leithner, A. Metal ion levels in large-diameter total hip and resurfacing hip arthroplasty-Preliminary results of a prospective five year study after two years of follow-up. BMC Musculoskelet. Disord. 2012, 13, 56. [Google Scholar] [CrossRef]
- Catelas, I.; Wimmer, M.A. New insights into wear and biological effects of metal-on-metal bearings. J. Bone Jt. Surg. Am. 2011, 93, 76–83. [Google Scholar] [CrossRef]
- Granchi, D.; Savarino, L.M.; Ciapetti, G.; Baldini, N. Biological effects of metal degradation in hip arthroplasties. Crit. Rev. Toxicol. 2017, 48, 170–193. [Google Scholar] [CrossRef]
- Høl, P.J.; Hallan, G.; Indrekvam, K. Metal ion levels in the blood of patients with metal-on-metal hip prostheses. Tidsskr. Nor. Laegeforen. 2021, 141. [Google Scholar] [CrossRef]
- Waldstein, W.; Ulrich Koller, U.; Springer, B.; Kolbitsch, P.; Brodner, W.; Windhager, R.; Richard Lass, R. Serum cobalt concentrations remain at low levels at a minimum of 20 years following metal-on-metal total hip arthroplasty. Bone Jt. Res. 2020, 9, 146–151. [Google Scholar] [CrossRef]
- Witzleb, W.-C.; Ziegler, J.; Krummenauer, F.; Neumeister, V.; Guenther, K.-P. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006, 77, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Savarino, L.; Cadossi, M.; Chiarello, E.; Baldini, N.; Giannini, S. Do ion levels in metal-on-metal hip resurfacing differ from those in metal-on-metal THA at long-term followup? Clin. Orthop. Relat. Res. 2013, 471, 2964–2971. [Google Scholar] [CrossRef]
- Renner, L.; Faschingbauer, M.; Schmidt-Braekling, T.; Boettner, F. Cobalt serum levels differ in well functioning Birmingham resurfacing and Birmingham modular THA. Arch. Orthop. Trauma Surg. 2016, 136, 715–721. [Google Scholar]
- Holm Hjorth, M.; Mechlenburg, I.; Soballe, K.; Roemer, L.; Stilling, M. The correlation between activity level, serum-ion concentrations and pseudotumours in patients with metal-on-metal hip articulations and metal-on-polyethylene total hip articulations. J. Orthop. Translat. 2018, 18, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Takahashi, K.; Kabata, T.; Sakagoshi, D.; Tomita, K.; Yamada, M. Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve 2010, 42, 140–143. [Google Scholar] [CrossRef]
- Ebreo, D.; Khan, A.; El-Meligy, M.; Armstrong, C.; Peter, V. Metal ion levels decrease after revision for metallosis arising from large-diameter metal-on-metal hip arthroplasty. Acta Orthop. Belg. 2011, 77, 777–781. [Google Scholar]
- Leikin, J.B.; Karydes, H.C.; Whiteley, P.M.; Wills, B.K.; Cumpston, K.L.; Jacobs, J.J. Outpatient toxicology clinic experience of patients with hip implants. Clin. Toxicol. 2013, 51, 230–236. [Google Scholar] [CrossRef]
- Merritt, K.; Brown, S.A. Distribution of cobalt chromium wear and corrosion products and biologic reactions. Clin. Orthop. Relat. Res. 1996, 329, S233–S243. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology-a brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Goullé, J.P.; Saussereau, E.; Grosjean, J.; Doche, C.; Mahieu, L.; Thouret, J.M.; Guebert, M.; Lacroix, C. Accidental potassium dichromate poisoning. Toxicokinetics of chromium by ICP-MS-CRC in biological fluids and in hair. Forensic Sci. Int. 2012, 217, e8–e12. [Google Scholar] [CrossRef]
- Urban, R.M.; Tomlinson, M.J.; Hall, D.J.; Jacobs, J.J. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J. Arthroplast. 2004, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Hannemann, F.; Lützner, J.; Seidler, A.; Drexler, H.; Günther, K.-P.; Schmitt, J. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing--systematic review of clinical and epidemiological studies. PLoS ONE 2013, 8, e70359. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Well-Functioning THP (N = 27) | Malfunctioning THP (N = 7 *) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| CoB (µg/L) | 2.8 ± 2.4 | 91.2 ± 84.4 |
| CoU (µg/g of creatinine) | 18.9 ± 21.6 | 515.5 ± 536.5 |
| CrB (µg/L) | 1.4 ± 1.0 | 48.1 ± 46.3 |
| CrU (µg/g of creatinine) | 4.3 ± 4.9 | 213.7 ± 216.0 |
| Age at surgery (years) | 53 ± 7 | 57 ± 10 |
| Age at analysis (years) | 57 ± 7 | 63 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolli, A.; Bortoletti, I.; Maso, S.; Trevisan, A. Course of Metal Ions after a Revision of Malfunctioning Metal-on-Metal Total Hip Prostheses. Medicina 2021, 57, 115. https://doi.org/10.3390/medicina57020115
Nicolli A, Bortoletti I, Maso S, Trevisan A. Course of Metal Ions after a Revision of Malfunctioning Metal-on-Metal Total Hip Prostheses. Medicina. 2021; 57(2):115. https://doi.org/10.3390/medicina57020115
Chicago/Turabian StyleNicolli, Annamaria, Isabella Bortoletti, Stefano Maso, and Andrea Trevisan. 2021. "Course of Metal Ions after a Revision of Malfunctioning Metal-on-Metal Total Hip Prostheses" Medicina 57, no. 2: 115. https://doi.org/10.3390/medicina57020115
APA StyleNicolli, A., Bortoletti, I., Maso, S., & Trevisan, A. (2021). Course of Metal Ions after a Revision of Malfunctioning Metal-on-Metal Total Hip Prostheses. Medicina, 57(2), 115. https://doi.org/10.3390/medicina57020115







