Association of Tinnitus with Depression in a Normal Hearing Population
Abstract
1. Introduction
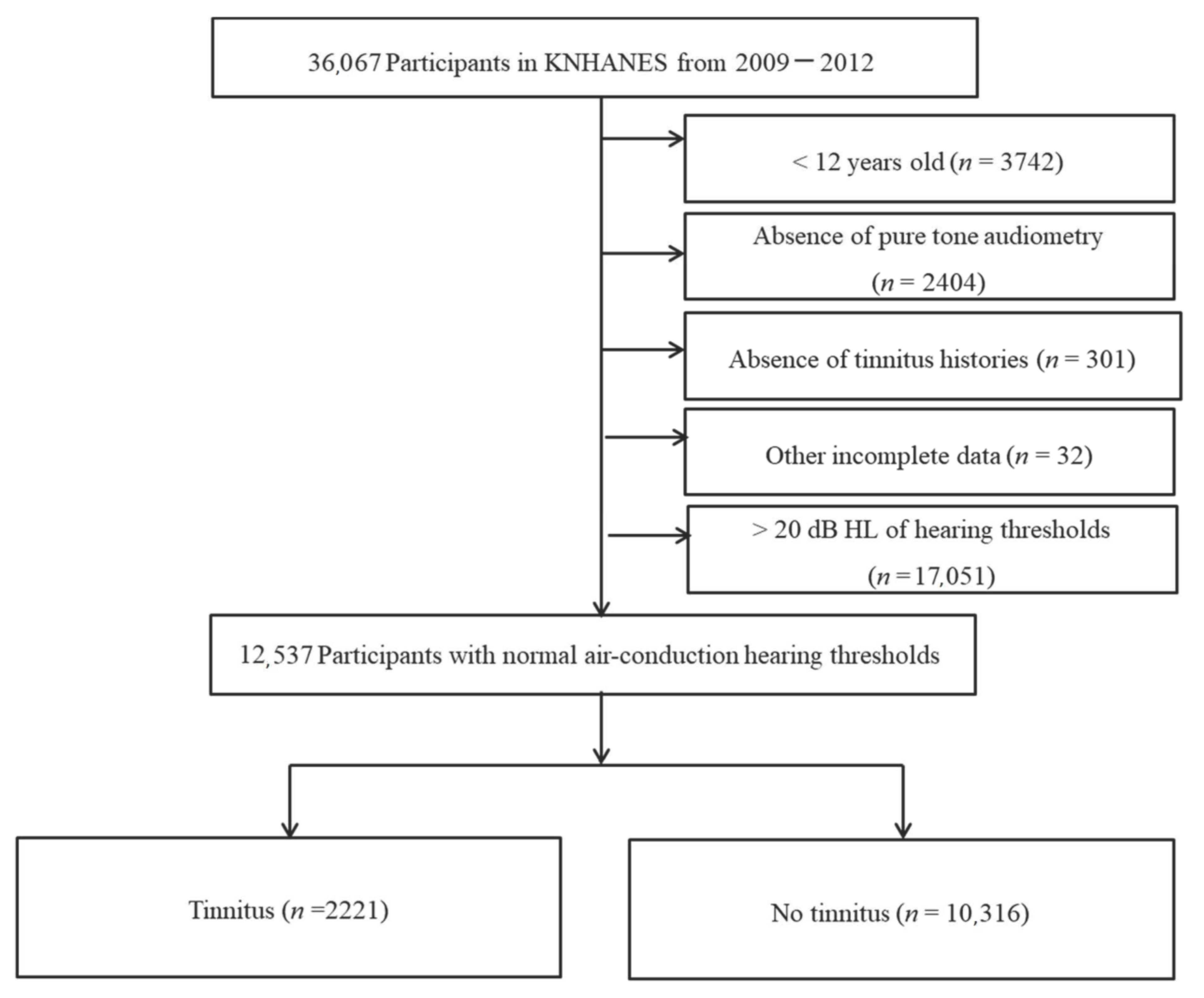
2. Materials and Methods
2.1. Ethical Considerations
2.2. Variables
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogo, R.; Farah, A.; Karlsson, K.K.; Pedersen, N.L.; Svartengren, M.; Skjönsberg, Å. Prevalence, Incidence Proportion, and Heritability for Tinnitus: A Longitudinal Twin Study. Ear Hear. 2017, 38, 292–300. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.-J.; An, S.-Y.; Sim, S.; Park, B.; Kim, S.W.; Lee, J.S.; Hong, S.K.; Choi, H.G. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS ONE 2015, 10, e0127578. [Google Scholar] [CrossRef]
- Wu, C.; Martel, D.T.; Shore, S.E. Increased Synchrony and Bursting of Dorsal Cochlear Nucleus Fusiform Cells Correlate with Tinnitus. J. Neurosci. 2016, 36, 2068–2073. [Google Scholar] [CrossRef]
- Hong, O.; Chin, D.L.; Phelps, S.; Joo, Y. Double Jeopardy: Hearing Loss and Tinnitus Among Noise-Exposed Workers. Work. Health Saf. 2016, 64, 235–242. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res. Rev. 2019, 56, 100963. [Google Scholar] [CrossRef]
- Wojtczak, M.; Beim, J.A.; Oxenham, A.J. Weak Middle-Ear-Muscle Reflex in Humans with Noise-Induced Tinnitus and Normal Hearing May Reflect Cochlear Synaptopathy. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Campbell, J.; Bean, C.; LaBrec, A. Normal hearing young adults with mild tinnitus: Reduced inhibition as measured through sensory gating. Audiol. Res. 2018, 8, 27–33. [Google Scholar] [CrossRef]
- Huang, Y.S.; Koo, M.; Chen, J.C.; Hwang, J.H. The association between tinnitus and the risk of ischemic cerebrovascular disease in young and middle-aged patients: A secondary case-control analysis of a nationwide, population-based health claims database. PLoS ONE 2017, 12, e0187474. [Google Scholar] [CrossRef] [PubMed]
- Rosing, S.N.; Schmidt, J.H.; Wedderkopp, N.; Baguley, D.M. Prevalence of tinnitus and hyperacusis in children and adolescents: A systematic review. BMJ Open 2016, 6, e010596. [Google Scholar] [CrossRef] [PubMed]
- Rosing, S.N.; Kapandais, A.; Schmidt, J.H.; Baguley, D.M. Demographic data, referral patterns and interventions used for children and adolescents with tinnitus and hyperacusis in Denmark. Int. J. Pediatr. Otorhinolaryngol. 2016, 89, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, O.A.; Hassaan, M.R. Psychoacoustic Characteristics of Tinnitus versus Temporal Resolution in Subjects with Normal Hearing Sensitivity. Int. Arch. Otorhinolaryngol. 2016, 21, 144–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, F.G.; Richardson, M.; Turner, K. Tinnitus Does Not Interfere with Auditory and Speech Perception. J. Neurosci. 2020, 40, 6007–6017. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, J.S.; Park, M.K. The Effects of Air Pollutants on the Prevalence of Common Ear, Nose, and Throat Diseases in South Korea: A National Population-Based Study. Clin. Exp. Otorhinolaryngol. 2019, 12, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, Y.S.; Chae, H.S.; Han, K. Nationwide analysis of the relationships between mental health, body mass index and tinnitus in premenopausal female adults in Korea: 2010–2012 KNHANES. Sci. Rep. 2018, 8, 7028. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, S.H.; Koo, J.-W.; Park, H.Y.; Lee, K.Y.; Choi, Y.S.; Oh, K.W.; Lee, A.; Yang, J.-E.; Woo, S.-Y.; et al. Prevalence and associated factors of tinnitus: Data from the Korean National Health and Nutrition Examination Survey 2009–2011. J. Epidemiol. 2014, 24, 417–426. [Google Scholar] [CrossRef]
- American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngol. Head Neck Surg. 1995, 113, 186–187. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef]
- Temugan, E.; Yildirım, R.B.; Onat, H.; Susuz, M.; Elden, C.; Unsal, S.; Gumus, N.M.; Kaya, M. Does Tinnitus Lead to Depression? Clin. Investig. Med. 2016, 39, 27505. [Google Scholar] [CrossRef]
- Kehrle, H.M.; Sampaio, A.L.; Granjeiro, R.C.; de Oliveira, T.S.; Oliveira, C.A. Tinnitus Annoyance in Normal-Hearing Individuals: Correlation With Depression and Anxiety. Ann. Otol. Rhinol. Laryngol. 2015, 125, 185–194. [Google Scholar] [CrossRef]
- Raj-Koziak, D.; Bieńkowska, K.; Gos, E.; Włodarczyk, E.; Skarzynski, H.; Skarżyński, P. Audiological and psychological profiles of children with tinnitus. Hear. Balance Commun. 2020, 18, 90–97. [Google Scholar] [CrossRef]
- Gordts, F.; Decreton, S. Tinnitus in children and adolescents. B-ENT 2007, 3 (Suppl. S7), 61–63. [Google Scholar] [PubMed]
- Kaltenbach, J.A. The dorsal cochlear nucleus as a participant in the auditory, attentional and emotional components of tinnitus. Hear. Res. 2006, 216–217, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Deniz, M.; Bayazit, Y.A.; Celenk, F.; Karabulut, H.; Yilmaz, A.; Gunduz, B.; Saridogan, C.; Dagli, M.; Erdal, E.; Menevse, A. Significance of serotonin transporter gene polymorphism in tinnitus. Otol. Neurotol. 2010, 31, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.W.; Tzounopoulos, T. Imaging the neural correlates of tinnitus: A comparison between animal models and human studies. Front. Syst. Neurosci. 2012, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.R.; de Azevedo, A.A.; Penido Nde, O. Tinnitus and arterial hypertension: A systematic review. Eur. Arch. Oto-Rhino-Laryngology 2014, 272, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Al-Swiahb, J.N.; Hwang, E.S.; Kong, J.S.; Kim, W.J.; Yeo, S.W.; Park, S.N. Clinical and audiologic characteristics of patients with sensorineural tinnitus and its association with psychological aspects: An analytic retrospective study. Eur. Arch. Oto-Rhino-Laryngology 2016, 273, 4161–4165. [Google Scholar] [CrossRef] [PubMed]
- Swierniak, W.; Gos, E.; Skarzynski, P.H.; Czajka, N.; Skarzynski, H. Personal Music Players Use and Other Noise Hazards among Children 11 to 12 Years Old. Int. J. Environ. Res. Public Health 2020, 17, 6934. [Google Scholar] [CrossRef]
- Weisz, N.; Hartmann, T.; Dohrmann, K.; Schlee, W.; Norena, A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear. Res. 2006, 222, 108–114. [Google Scholar] [CrossRef]
- Ralli, M.; Greco, A.; Turchetta, R.; Altissimi, G.; De Vincentiis, M.; Cianfrone, G. Somatosensory tinnitus: Current evidence and future perspectives. J. Int. Med. Res. 2017, 45, 933–947. [Google Scholar] [CrossRef]
- Ralli, M.; Altissimi, G.; Turchetta, R.; Mazzei, F.; Salviati, M.; Cianfrone, F.; Orlando, M.P.; Testugini, V.; Cianfrone, G. Somatosensory Tinnitus: Correlation between Cranio-Cervico-Mandibular Disorder History and Somatic Modulation. Audiol. Neurotol. 2016, 21, 372–382. [Google Scholar] [CrossRef]
- Song, K.; Shin, S.A.; Chang, D.S.; Lee, H.Y. Audiometric Profiles in Patients With Normal Hearing and Bilateral or Unilateral Tinnitus. Otol. Neurotol. 2018, 39, e416–e421. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Participants | |
|---|---|---|
| Tinnitus (n, %) | No Tinnitus (n, %) | |
| Number | 2221 | 10,316 |
| Age (years old) | 38.58 (16.53) | 39.09 (15.80) |
| Sex | ||
| Male | 811 (36.5) | 5842 (56.6) |
| Female | 1410 (63.5) | 4474 (43.4) |
| Diabetes mellitus | ||
| Yes | 87 (4.0) | 399 (3.9) |
| No | 2113 (96.0) | 9831 (96.1) |
| Hypertension | ||
| Yes | 240 (10.9) | 1158 (11.3) |
| No | 1960 (89.1) | 9072 (88.7) |
| Dyslipidemia | ||
| Yes | 177 (8.0) | 700 (6.8) |
| No | 2023 (92.0) | 9530 (93.2) |
| Ischemic heart diseases | ||
| Yes | 40 (1.9) | 94 (1.0) |
| No | 2050 (98.1) | 9567 (99.0) |
| Stroke | ||
| Yes | 14 (0.6) | 60 (0.6) |
| No | 2186 (99.4) | 10,170 (99.4) |
| Chronic renal diseases | ||
| Yes | 6 (0.3%) | 21 (0.2%) |
| No | 2194 (99.7) | 10,209 (99.8) |
| Depression | ||
| Yes | 107 (4.9) | 290 (2.8) |
| No | 2093 (95.1) | 9940 (97.2) |
| Characteristics | Tinnitus | |||
|---|---|---|---|---|
| Crude | p-Value | Adjusted † | p-Value | |
| Age | 1.00 (0.99–1.00) | 0.028 | 0.99 (0.99–1.00) | 0.004 |
| Sex | 1.49 (1.32–1.67) | <0.001 * | 1.75 (1.53–1.99) | <0.001 * |
| Diabetes | 1.01(0.75–1.37) | 0.929 | 1.07 (0.77–1.48) | 0.694 |
| Hypertension | 0.91 (0.75–1.10) | 0.313 | 0.92 (0.74–1.15) | 0.246 |
| Dyslipidemia | 1.28 (1.03–1.58) | 0.025 * | 1.42 (1.11–1.82) | 0.008 * |
| Ischemic heart diseases | 1.60 (1.03–2.49) | 0.035 | 1.70 (1.05–2.76) | 0.030 |
| Stroke | 1.27 (0.63–2.58) | 0.507 | 1.31 (0.62–2.80) | 0.479 |
| Chronic renal diseases | 0.86 (0.29–2.55) | 0.785 | 0.70 (0.21–2.32) | 0.560 |
| Depression | 1.97 (1.45–2.67) | <0.001 * | 1.89 (1.37–2.60) | <0.001 * |
| Characteristics | Tinnitus | |||
|---|---|---|---|---|
| Crude | p-Value | Adjusted † | p-Value | |
| Male | 2.93(1.59–5.40) | 0.001 * | 2.88 (1.57–5.29) | 0.001 * |
| Female | 1.52 (1.09–2.11) | 0.014 * | 1.66 (1.18–2.33) | 0.004 * |
| Young (12–29 years) | 1.67 (0.85–3.25) | 0.134 | 1.77 (0.88–3.57) | 0.111 |
| Middle (30–59 years) | 1.97 (1.45–2.67) | <0.001 * | 1.89 (1.37–2.60) | <0.001 * |
| Old (≥60 years) | 1.26 (0.60–2.62) | 0.541 | 1.28 (0.58–2.80) | 0.545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Lee, C.H.; Kim, S.Y. Association of Tinnitus with Depression in a Normal Hearing Population. Medicina 2021, 57, 114. https://doi.org/10.3390/medicina57020114
Choi J, Lee CH, Kim SY. Association of Tinnitus with Depression in a Normal Hearing Population. Medicina. 2021; 57(2):114. https://doi.org/10.3390/medicina57020114
Chicago/Turabian StyleChoi, Jay, Chang Ho Lee, and So Young Kim. 2021. "Association of Tinnitus with Depression in a Normal Hearing Population" Medicina 57, no. 2: 114. https://doi.org/10.3390/medicina57020114
APA StyleChoi, J., Lee, C. H., & Kim, S. Y. (2021). Association of Tinnitus with Depression in a Normal Hearing Population. Medicina, 57(2), 114. https://doi.org/10.3390/medicina57020114






