Efficacy and Safety of Sugammadex for the Reversal of Rocuronium-Induced Neuromuscular Blockade in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database and Literature Sources
2.2. Study Selection
2.3. Data Extraction
2.4. Assessment of Methodological Quality
2.5. Statistical Analysis
3. Results
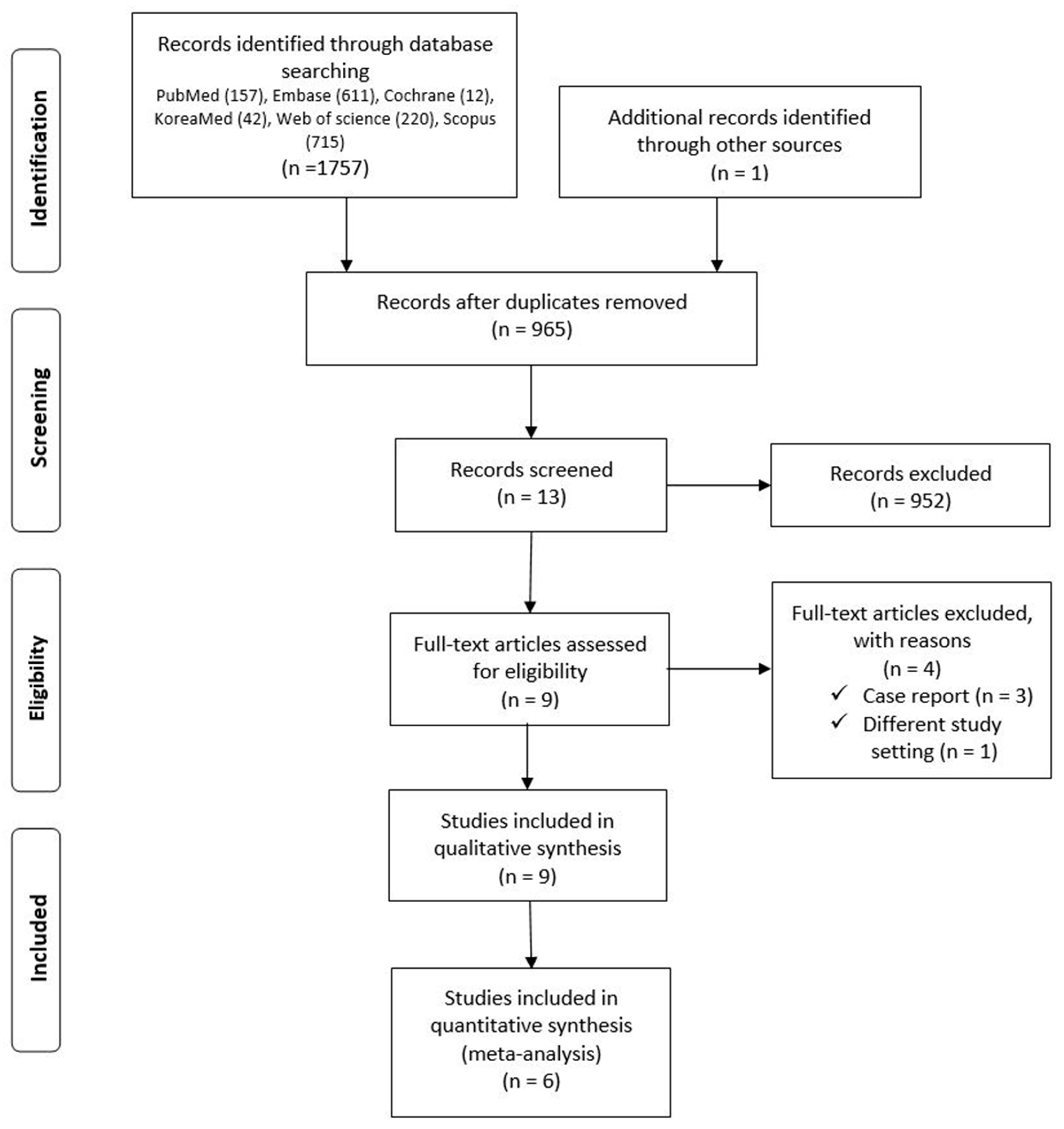
3.1. Identification of Studies
3.2. Study Characteristics and Patient Demographics
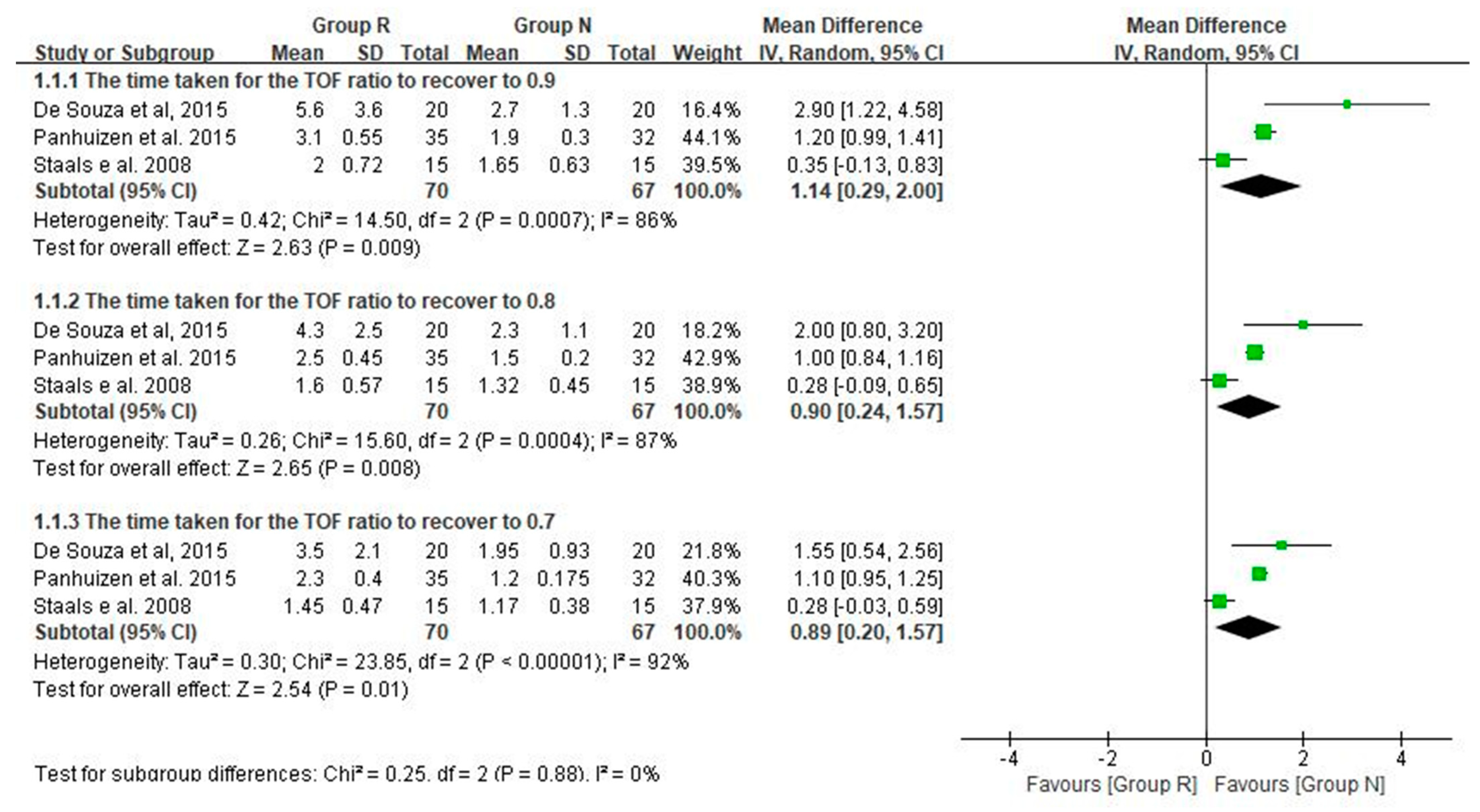
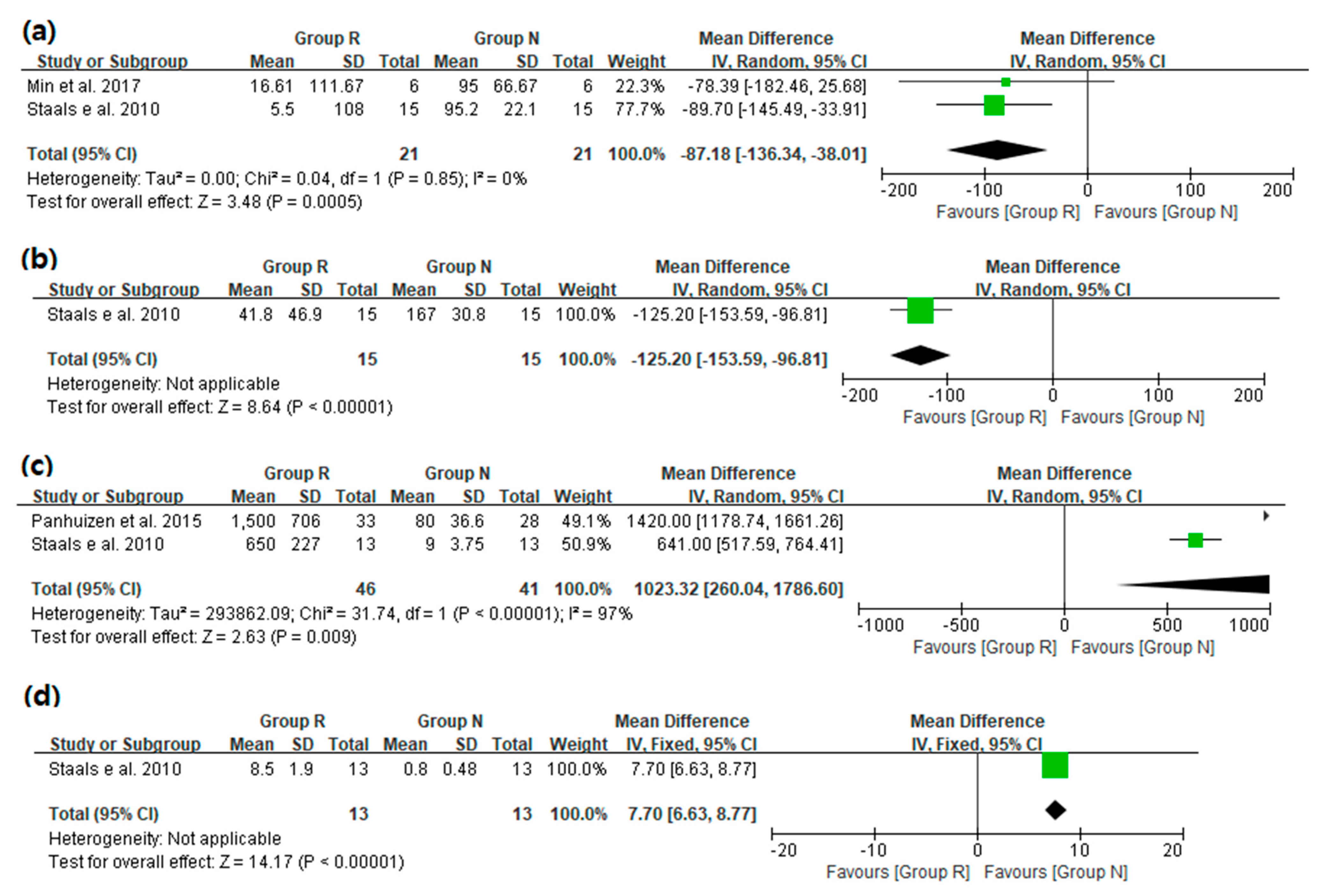
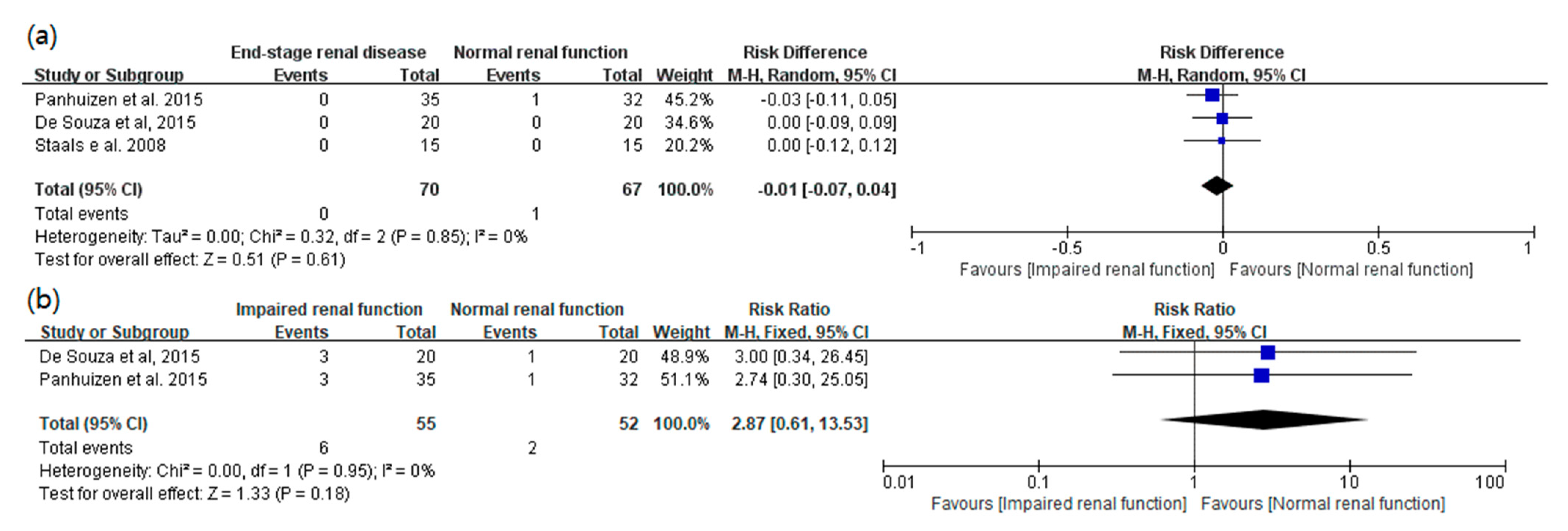
3.3. Main Results: The Primary and Secondary Outcomes of the Included Prospective Studies
3.4. The Results of the Included Retrospective Studies
3.5. Risk of Bias in the Prospective Case-Control Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; Van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. Engl. 2002, 41, 266–270. [Google Scholar] [CrossRef]
- Staals, L.M.; Snoeck, M.M.; Driessen, J.J.; van Hamersvelt, H.W.; Flockton, E.A.; van den Heuvel, M.W.; Hunter, J.M. Reduced clearance of rocuronium and sugammadex in patients with severe to end-stage renal failure: A pharmacokinetic study. Br. J. Anaesth. 2010, 104, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Epemolu, O.; Bom, A.; Hope, F.; Mason, R. Reversal of neuromuscular blockade and simultaneous increase in plasma rocuronium concentration after the intravenous infusion of the novel reversal agent Org 25969. Anesthesiology 2003, 99, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.; Passier, P.; Smeets, J.; Zwiers, A.; de Zwart, M.; van de Wetering-Krebbers, S.; van Iersel, M.; van Marle, S.; van den Dobbelsteen, D. Sugammadex is cleared rapidly and primarily unchanged via renal excretion. Biopharm. Drug Dispos. 2011, 32, 159–167. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration; Merck Sharp & Dohme Corp. BRIDION® (Sugammadex) Injection, for Intravenous Use; Initial U.S. Approval: Silver Spring, MD, USA, 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022225lbl.pdf (accessed on 5 December 2019).
- Ono, Y.; Fujita, Y.; Kajiura, T.; Okawa, H.; Nakashima, J.; Isobe, H.; Fujiwara, Y. Efficacy and safety of sugammadex in patients undergoing renal transplantation. JA Clin. Rep. 2018, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.R.; Tollinche, L.E.; Yeoh, C.B.; Artman, J.; Mehta, M.; Phillips, D.; Fischer, G.W.; Quinlan, J.J.; Sakai, T. Short-term safety and effectiveness of sugammadex for surgical patients with end-stage renal disease: A two-centre retrospective study. Anaesthesia 2020, 75, 348–352. [Google Scholar] [CrossRef]
- Pfaff, K.; Tumin, D.; Tobias, J.D. Sugammadex for Reversal of Neuromuscular Blockade in a Patient With Renal Failure. J. Pediatr. Pharmacol. Ther. 2019, 24, 238–241. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J. Cochrane Handbook for Systematic Reviews of Interventions, Version 6; London, UK, 2019. Available online: https://training.cochrane.org/handbook/archive/v6 (accessed on 3 September 2021).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- De Souza, C.M.; Tardelli, M.A.; Tedesco, H.; Garcia, N.N.; Caparros, M.P.; Alvarez-Gomez, J.A.; de Oliveira, I.S., Jr. Efficacy and safety of sugammadex in the reversal of deep neuromuscular blockade induced by rocuronium in patients with end-stage renal disease: A comparative prospective clinical trial. Eur. J. Anaesthesiol. 2015, 32, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Panhuizen, I.F.; Gold, S.J.; Buerkle, C.; Snoeck, M.M.; Harper, N.J.; Kaspers, M.J.; van den Heuvel, M.W.; Hollmann, M.W. Efficacy, safety and pharmacokinetics of sugammadex 4 mg kg-1 for reversal of deep neuromuscular blockade in patients with severe renal impairment. Br. J. Anaesth. 2015, 114, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Staals, L.M.; Snoeck, M.M.; Driessen, J.J.; Flockton, E.A.; Heeringa, M.; Hunter, J.M. Multicentre, parallel-group, comparative trial evaluating the efficacy and safety of sugammadex in patients with end-stage renal failure or normal renal function. Br. J. Anaesth. 2008, 101, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Maeyama, A.; Nagasaka, H.; Matsumoto, N. Prolonged recovery time from muscle relaxation due to rocuronium in patients with severe chronic renal disease using sugammadex: 9AP4-6. Eur. J. Anaesthesiol. EJA 2014, 31, 152. [Google Scholar] [CrossRef] [Green Version]
- Min, K.C.; Lasseter, K.C.; Marbury, T.C.; Wrishko, R.E.; Hanley, W.D.; Wolford, D.G.; Udo de Haes, J.; Reitmann, C.; Gutstein, D.E. Pharmacokinetics of sugammadex in subjects with moderate and severe renal impairment. Int. J. Clin. Pharmacol. Ther. 2017, 55, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.; Porter, S.B.; Porter, I.E., 2nd; Renew, J.R. Sugammadex use in patients with end-stage renal disease: A historical cohort study. Can. J. Anaesth. 2020, 67, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Magoon, R.; Kashav, R.; Kohli, J.K. Sugammadex in end-stage renal disease: Too early for a “free-pass”. Can. J. Anaesth. 2021, 68, 264–265. [Google Scholar] [CrossRef]
- Carlos, R.V.; Torres, M.L.; de Boer, H.D. The use of rocuronium and sugammadex in paediatric renal transplantation: Two case reports. Eur. J. Anaesthesiol. 2016, 33, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Kurita, S.; Moriwaki, K.; Shiroyama, K.; Sanuki, M.; Toyota, Y.; Takebayashi, M. Rocuronium-sugammadex use for electroconvulsive therapy in a hemodialysis patient: A case report. JA Clin. Rep. 2016, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Renew, J.R.; Porter, S.B.; Porter, I.; Paredes, S. In reply: Sugammadex in end-stage renal disease: Too early for a “free-pass”. Can. J. Anaesth. 2021, 68, 266–267. [Google Scholar] [CrossRef]
- Sparr, H.J.; Vermeyen, K.M.; Beaufort, A.M.; Rietbergen, H.; Proost, J.H.; Saldien, V.; Velik-Salchner, C.; Wierda, J.M. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: Efficacy, safety, and pharmacokinetics. Anesthesiology 2007, 106, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorgenfrei, I.F.; Norrild, K.; Larsen, P.B.; Stensballe, J.; Ostergaard, D.; Prins, M.E.; Viby-Mogensen, J. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: A dose-finding and safety study. Anesthesiology 2006, 104, 667–674. [Google Scholar] [CrossRef]
- Cammu, G.; Van Vlem, B.; van den Heuvel, M.; Stet, L.; el Galta, R.; Eloot, S.; Demeyer, I. Dialysability of sugammadex and its complex with rocuronium in intensive care patients with severe renal impairment. Br. J. Anaesth. 2012, 109, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tercan, M.; Yilmaz Inal, F.; Seneldir, H.; Kocoglu, H. Nephroprotective Efficacy of Sugammadex in Ischemia-Reperfusion Injury: An Experimental Study in a Rat Model. Cureus 2021, 13, e15726. [Google Scholar] [CrossRef] [PubMed]
| Study | Journal | Study Design | Center/Country | Group R (n) | Group N (n) | Age | Sugammadex Dose | NMB Monitoring |
|---|---|---|---|---|---|---|---|---|
| de Souza et al., 2015 [11] | European Journal of Anaesthe-siology | Prospective clinical trial | Two hospitals Brazil, and Spain | ClCr < 30 mL min−1 (20) | ClCr > 90 mL min−1 (20) | 18–65 | 4 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Panhuizen et al., 2015 [12] | British Journal of Anaesthesia | Case control comparative study | Eight centers in Europe | ClCr < 30 mL min−1 (35) | ClCr ≥ 80 mL min−1 (35) | ≥18 | 4 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Staals et al., 2008 [13] | British Journal of Anaesthesia | Prospective clinical trial | Three centers in Europe | ClCr < 30 mL min−1 (15) | ClCr ≥ 80 mL min−1 (15) | ≥18 | 2 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Staals et al., 2010 [2] | British Journal of Anaesthesia | Prospective clinical trial | Three centers in Europe | ClCr < 30 mL min−1 (15) | ClCr ≥ 80 mL min−1 (15) | ≥18 | 2 mg kg−1 | Acceleromyography at the adductor pollicis muscle |
| Maeyama et al., 2014 [14] | European Journal of Anaesthesiology | Prospective clinical trial | University hospital, Japan | ClCr < 15mL min−1 (13) | ClCr > 90 mL min−1 (14) | ≥18 | 4 mg kg−1 | Not mentioned |
| Min et al., 2017 [15] | International Journal of Clinical Pharmacology and Therapeutics | Open label, two parts, phase 1 study | Clinical pharma-cology of Miami, USA | ClCr < 30 mL min−1 (6) | ClCr ≥ 80 mL min−1 (6) | ≥18 | 4 mg kg−1 | None |
| Study ID | Journal | Study Design | Center/Country | Enrolled Criteria (n) | Age | NMB Reversal Agent |
|---|---|---|---|---|---|---|
| Adams et al., 2020 [7] | Anaesthesia | Two centers retrospective study | Pittsburgh Medical Center, Memorial Sloan Kettering Cancer Center, USA | End-stage renal disease which is mandatory for renal replacement therapy (158) | ≥18 | sugammadex |
| Paredes et al., 2020 [16] | Canadian Journal of Anaesthesia | Historical cohort study, three-distinct geographic locations | Scottsdale, AZ, Jacksonville, FL, Rochester, MN, USA | eGFR value < 15 mL min−1 (219) | ≥18 | sugammadex |
| Ono et al., 2018 [6] | Journal of Anesthesia Clinical Reports | Retrospective study | Aichi Medical University, Nagakute, Japan | Diagnosed severe with renal failure and underwent renal transplantation (99) | not mentioned | sugammadex |
| Study | Confounding | Selection | Classification of Interventions | Deviation from Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | ROBINS-I Overall |
|---|---|---|---|---|---|---|---|---|
| de Souza et al., 2015 [11] | Moderate | Moderate | Moderate | Moderate | Moderate | Serious | Low | Serious |
| Panhuizen et al., 2015 [12] | Moderate | Low | Moderate | Low | Moderate | Serious | Low | Serious |
| Staals et al., 2008 [13] | Moderate | Moderate | Low | Low | Low | Serious | Low | Serious |
| Staals et al., 2010 [2] | Moderate | Moderate | Low | Low | Moderate | Serious | Low | Serious |
| Maeyama et al., 2014 [14] | Moderate | Serious | Low | Low | Low | Low | Low | Serious |
| Min et al., 2017 [15] | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Lim, B.-G.; Won, Y.-J.; Oh, S.-K.; Oh, J.-S.; Cho, S.-A. Efficacy and Safety of Sugammadex for the Reversal of Rocuronium-Induced Neuromuscular Blockade in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 1259. https://doi.org/10.3390/medicina57111259
Kim Y-S, Lim B-G, Won Y-J, Oh S-K, Oh J-S, Cho S-A. Efficacy and Safety of Sugammadex for the Reversal of Rocuronium-Induced Neuromuscular Blockade in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Medicina. 2021; 57(11):1259. https://doi.org/10.3390/medicina57111259
Chicago/Turabian StyleKim, Young-Sung, Byung-Gun Lim, Young-Ju Won, Seok-Kyeong Oh, Jung-Suk Oh, and Soo-Ah Cho. 2021. "Efficacy and Safety of Sugammadex for the Reversal of Rocuronium-Induced Neuromuscular Blockade in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis" Medicina 57, no. 11: 1259. https://doi.org/10.3390/medicina57111259
APA StyleKim, Y.-S., Lim, B.-G., Won, Y.-J., Oh, S.-K., Oh, J.-S., & Cho, S.-A. (2021). Efficacy and Safety of Sugammadex for the Reversal of Rocuronium-Induced Neuromuscular Blockade in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Medicina, 57(11), 1259. https://doi.org/10.3390/medicina57111259






