Emphysema-Predominant COPD Had a Greater 5-Year Mortality and a Worse Annual Decline in Lung Function Than Airway Obstruction-Predominant COPD or Asthma at Initial Same Degree of Airflow Obstruction
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Clinical Assessment
2.3. Scoring of High-Resolution Computed Tomography (HRCT)
2.4. Mortality
2.5. Statistical Analysis
3. Results
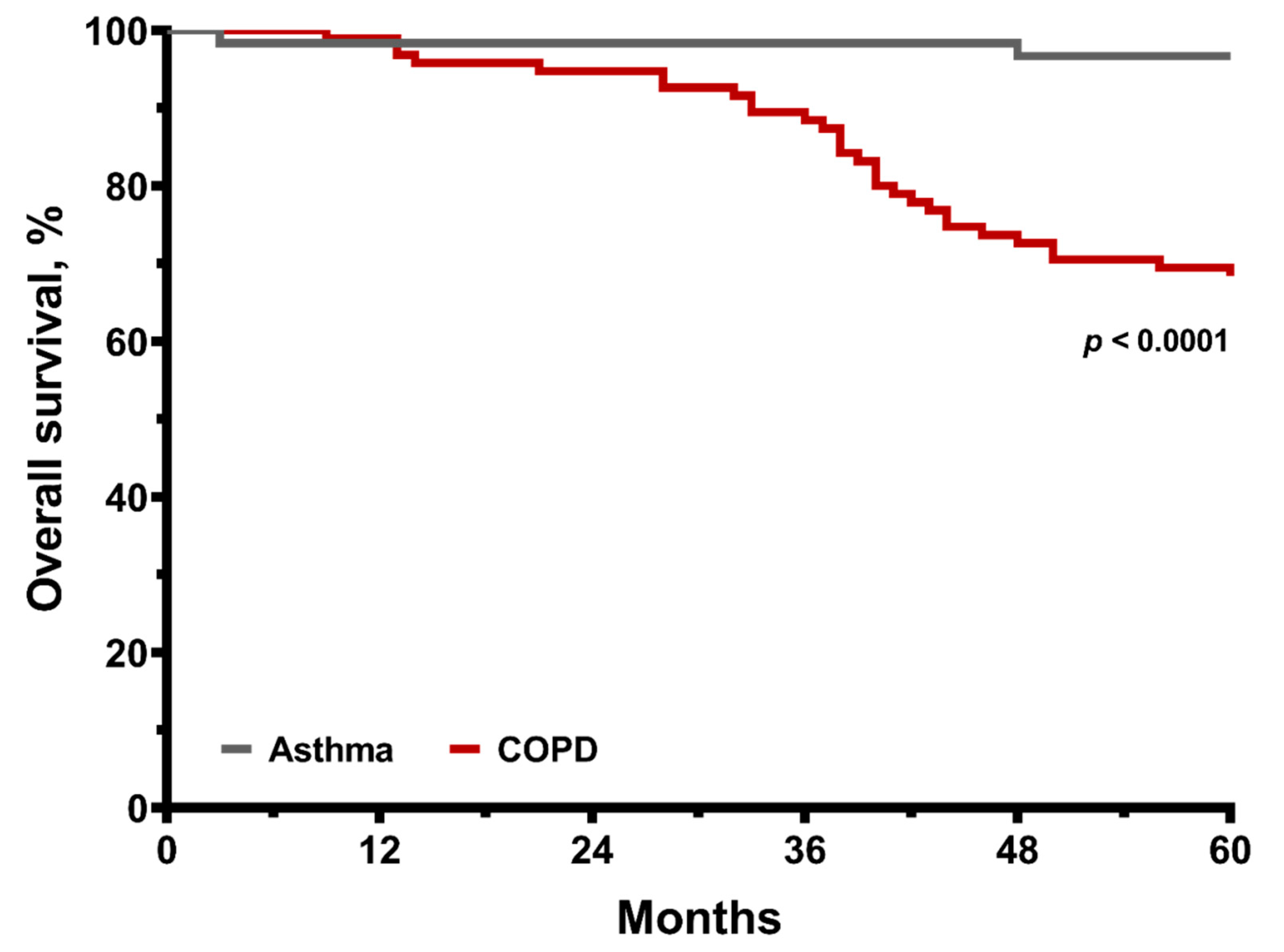
3.1. Conditions of Asthma and Overall COPD
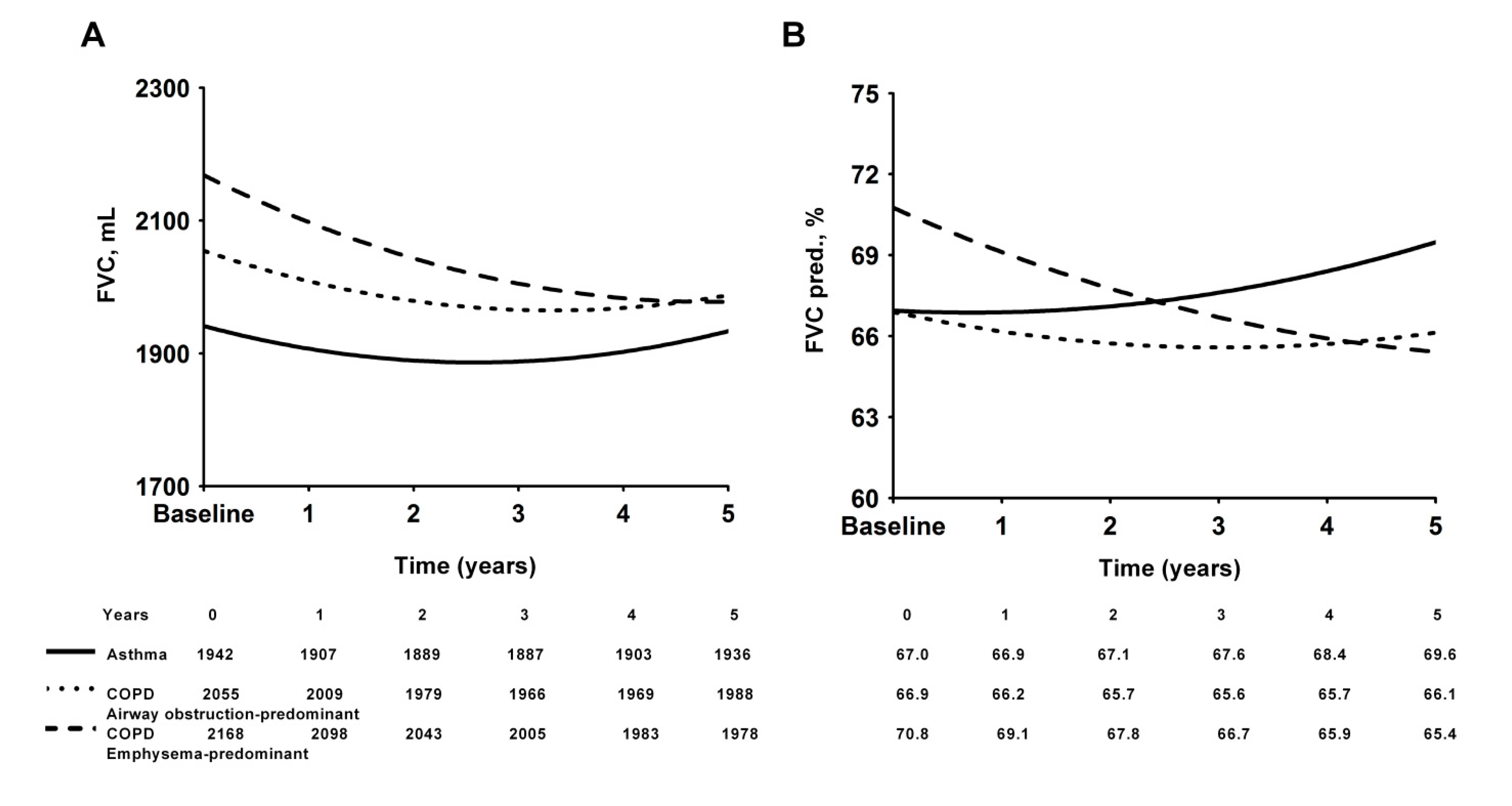
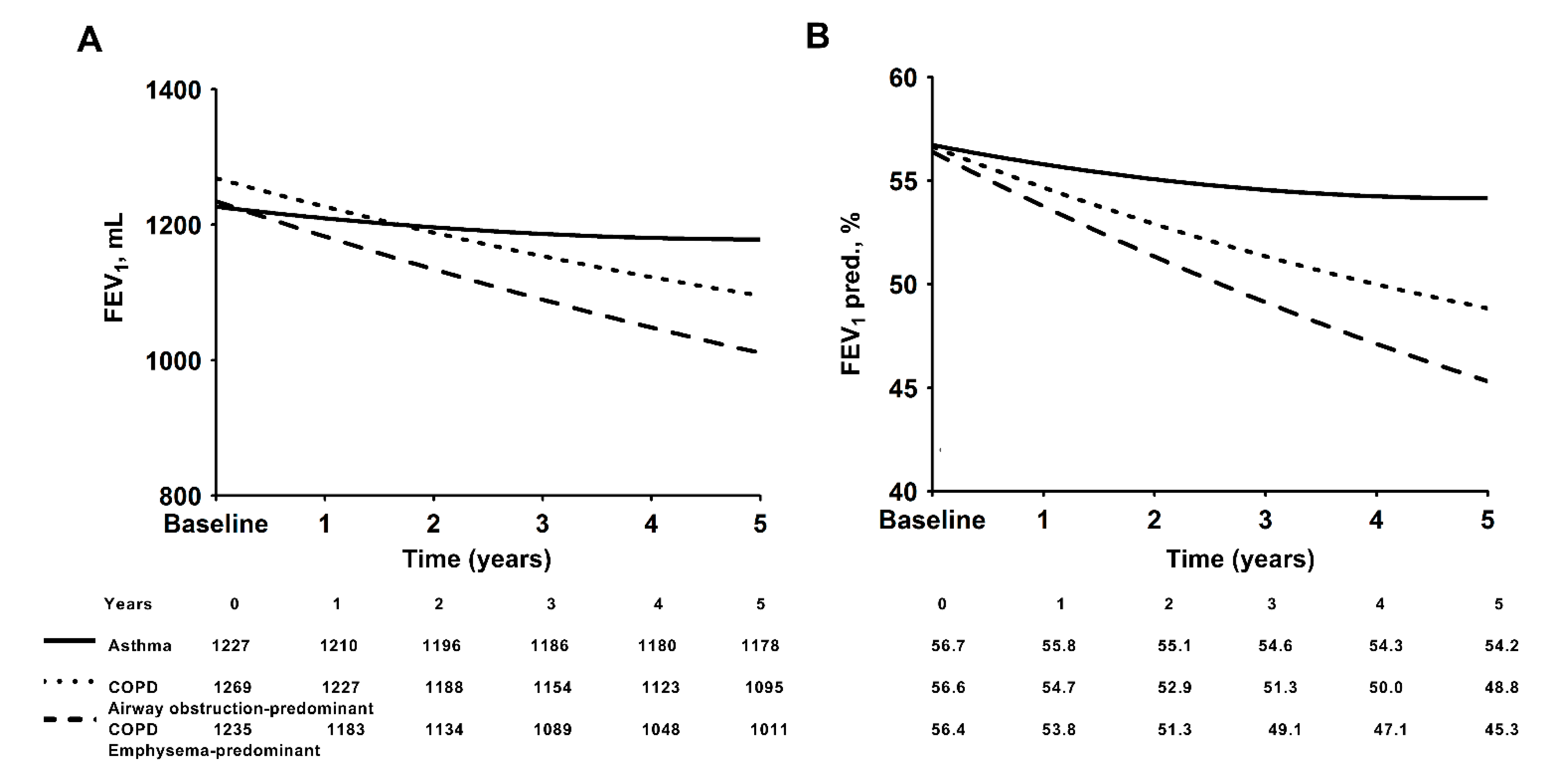
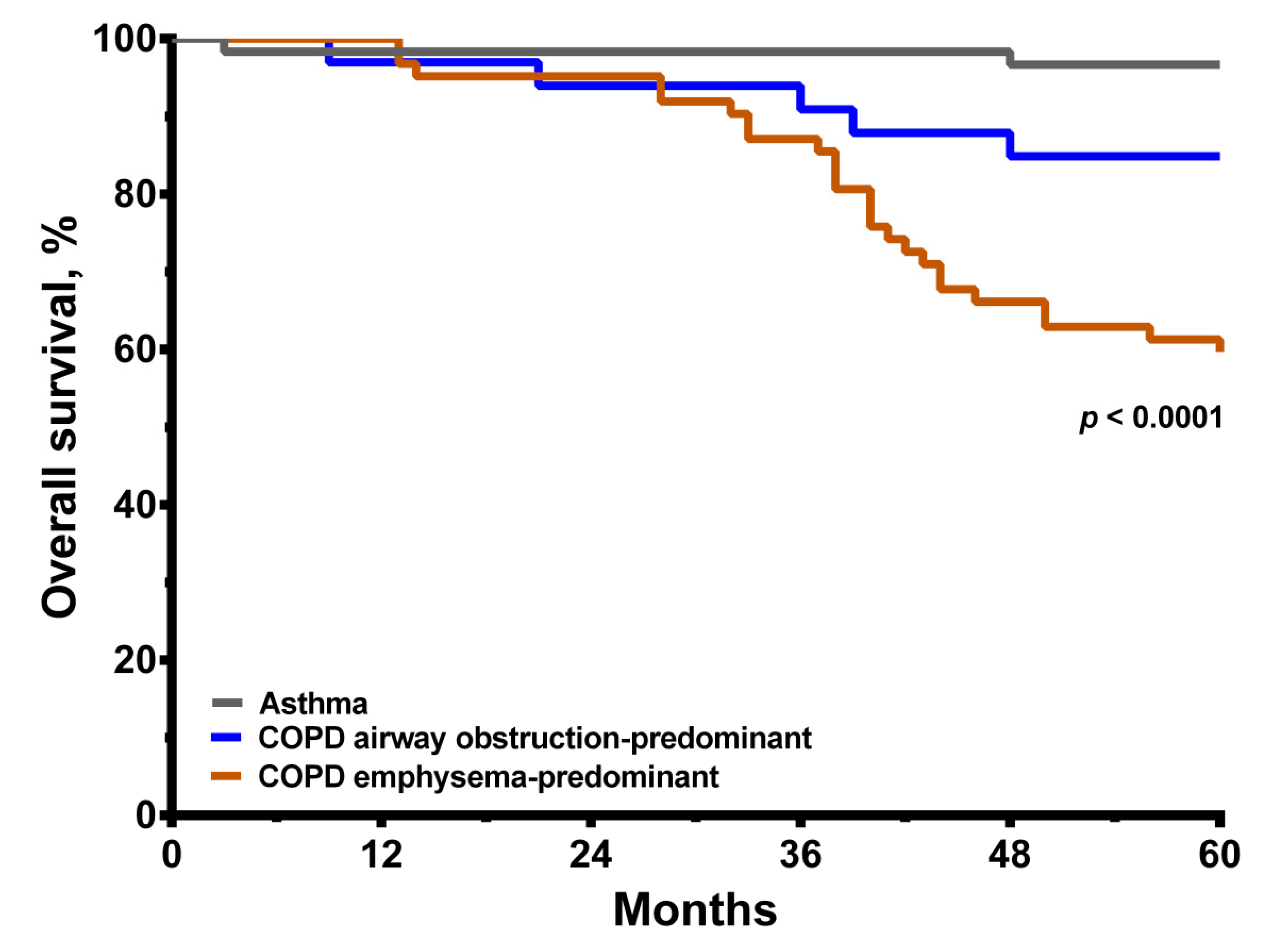
3.2. Conditions of Asthma and COPD with Predominant Emphysema or Predominant Airway Obstruction
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Sköld, C.M. Remodeling in asthma and COPD–differences and similarities. Clin. Respir. J. 2010, 4, 20–27. [Google Scholar] [CrossRef]
- Piqueras, M.C.; Cosio, M. Disease of the airways in chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 18, 41s–49s. [Google Scholar] [CrossRef]
- Saetta, M.; Turato, G. Airway pathology in asthma. Eur. Respir. J. 2001, 18, 18s–23s. [Google Scholar] [CrossRef]
- Saetta, M.; Turato, G.; Maestrelli, P.; Mapp, C.E.; Fabbri, L.M. Cellular and structural bases of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1304–1309. [Google Scholar] [CrossRef]
- Scichilone, N.; Bruno, A.; Marchese, R.; Vignola, A.M.; Togias, A.; Bellia, V. Association between reduced bronchodilatory effect of deep inspiration and loss of alveolar attachments. Respir. Res. 2005, 6, 55. [Google Scholar] [CrossRef][Green Version]
- Kesten, S.; Rebuck, A.S. Is the short-term response to inhaled β-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest 1994, 105, 1042–1045. [Google Scholar] [CrossRef]
- Ulrik, C.; Backer, V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur. Respir. J. 1999, 14, 892–896. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Ali, Z.; Dirks, C.G.; Ulrik, C.S. Long-term mortality among adults with asthma: A 25-year follow-up of 1075 outpatients with asthma. Chest 2013, 143, 1649–1655. [Google Scholar] [CrossRef]
- Backman, H.; Hedman, L.; Stridsman, C.; Jansson, S.A.; Lindberg, A.; Lundback, B.; Ronmark, E. A population-based cohort of adults with asthma: Mortality and participation in a long-term follow-up. Eur. Clin. Respir. J. 2017, 4, 1334508. [Google Scholar] [CrossRef]
- Huang, S.; Vasquez, M.M.; Halonen, M.; Martinez, F.D.; Guerra, S. Asthma, airflow limitation and mortality risk in the general population. Eur. Respir. J. 2015, 45, 338–346. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef]
- Lange, P.; Marott, J.L.; Vestbo, J.; Olsen, K.R.; Ingebrigtsen, T.S.; Dahl, M.; Nordestgaard, B.G. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: A study of the general population. Am. J. Respir. Crit. Care Med. 2012, 186, 975–981. [Google Scholar] [CrossRef]
- Cote, C.G.; Celli, B.R. Predictors of mortality in chronic obstructive pulmonary disease. Clin. Chest Med. 2007, 28, 515–524. [Google Scholar] [CrossRef]
- Kinney, G.L.; Santorico, S.A.; Young, K.A.; Cho, M.H.; Castaldi, P.J.; San Jose Estepar, R.; Ross, J.C.; Dy, J.G.; Make, B.J.; Regan, E.A.; et al. Identification of Chronic Obstructive Pulmonary Disease Axes That Predict All-Cause Mortality: The COPDGene Study. Am. J. Epidemiol. 2018, 187, 2109–2116. [Google Scholar] [CrossRef]
- Society, A.T. Committee on proficiency standards for clinical pulmonary function laboratories. ATS Statement Guidel. Six-Minute Walk Test Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar]
- Takigawa, N.; Tada, A.; Soda, R.; Date, H.; Yamashita, M.; Endo, S.; Takahashi, S.; Kawata, N.; Shibayama, T.; Hamada, N.; et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir. Med. 2007, 101, 561–567. [Google Scholar] [CrossRef]
- Waatevik, M.; Johannessen, A.; Real, F.G.; Aanerud, M.; Hardie, J.A.; Bakke, P.S.; Eagan, T.M.L. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur. Respir. J. 2016, 48, 82–91. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chou, P.C.; Wang, T.Y.; Lo, Y.L.; Joa, W.C.; Chen, L.F.; Sheng, T.F.; Chung, K.F.; Wang, C.H.; Kuo, H.P. Exercise-Induced Changes in Exhaled NO Differentiates Asthma with or without Fixed Airway Obstruction From COPD With Dynamic Hyperinflation. Medicine 2016, 95, e3400. [Google Scholar] [CrossRef]
- Baarnes, C.B.; Andersen, Z.J.; Tjonneland, A.; Ulrik, C.S. Incidence and long-term outcome of severe asthma-COPD overlap compared to asthma and COPD alone: A 35-year prospective study of 57,053 middle-aged adults. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 571–579. [Google Scholar] [CrossRef]
- Golpe, R.; Suarez-Valor, M.; Martin-Robles, I.; Sanjuan-Lopez, P.; Cano-Jimenez, E.; Castro-Anon, O.; Perez de Llano, L.A. Mortality in COPD patients according to clinical phenotypes. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1433–1439. [Google Scholar] [CrossRef]
- Zulueta, J.J.; Wisnivesky, J.P.; Henschke, C.I.; Yip, R.; Farooqi, A.O.; McCauley, D.I.; Chen, M.; Libby, D.M.; Smith, J.P.; Pasmantier, M.W.; et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012, 141, 1216–1223. [Google Scholar] [CrossRef]
- Han, M.K.; Tayob, N.; Murray, S.; Woodruff, P.G.; Curtis, J.L.; Kim, V.; Criner, G.; Galban, C.J.; Ross, B.D.; Hoffman, E.A.; et al. Association between Emphysema and Chronic Obstructive Pulmonary Disease Outcomes in the COPDGene and SPIROMICS Cohorts: A Post Hoc Analysis of Two Clinical Trials. Am. J. Respir. Crit. Care Med. 2018, 198, 265–267. [Google Scholar] [CrossRef]
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am. Rev. Respir. Dis. 1987, 136, 225–244. [Google Scholar]
- Kohlhaufl, M.; Brand, P.; Rock, C.; Radons, T.; Scheuch, G.; Meyer, T.; Schulz, H.; Pfeifer, K.J.; Haussinger, K.; Heyder, J. Noninvasive diagnosis of emphysema. Aerosol morphometry and aerosol bolus dispersion in comparison to HRCT. Am. J. Respir. Crit. Care Med. 1999, 160, 913–918. [Google Scholar] [CrossRef]
- Miniati, M.; Filippi, E.; Falaschi, F.; Carrozzi, L.; Milne, E.N.; Sostman, H.D.; Pistolesi, M. Radiologic evaluation of emphysema in patients with chronic obstructive pulmonary disease. Chest radiography versus high resolution computed tomography. Am. J. Respir. Crit. Care Med. 1995, 151, 1359–1367. [Google Scholar] [CrossRef]
- Lu, T.H.; Shau, W.Y.; Shih, T.P.; Lee, M.C.; Chou, M.C.; Lin, C.K. Factors associated with errors in death certificate completion. A national study in Taiwan. J. Clin. Epidemiol. 2001, 54, 232–238. [Google Scholar] [CrossRef]
- Lu, T.H.; Lee, M.C.; Chou, M.C. Accuracy of cause-of-death coding in Taiwan: Types of miscoding and effects on mortality statistics. Int. J. Epidemiol. 2000, 29, 336–343. [Google Scholar] [CrossRef]
- Chen, L.F.; Wang, C.H.; Chou, P.C.; Ho, S.C.; Joa, W.C.; Sheng, T.F.; Kuo, H.P. Association Between Emphysema Score, Six-Minute Walk and Cardiopulmonary Exercise Tests in COPD. Open Respir. Med. J. 2012, 6, 104–110. [Google Scholar] [CrossRef][Green Version]
- Gagnon, P.; Guenette, J.A.; Langer, D.; Laviolette, L.; Mainguy, V.; Maltais, F.; Ribeiro, F.; Saey, D. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 187–201. [Google Scholar]
- O’Donnell, D.E.; Lam, M.; Webb, K.A. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 542–549. [Google Scholar] [CrossRef]
- Hogg, J.C.; McDonough, J.E.; Suzuki, M. Small airway obstruction in COPD: New insights based on micro-CT imaging and MRI imaging. Chest 2013, 143, 1436–1443. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef]
- Tanabe, N.; Vasilescu, D.M.; McDonough, J.E.; Kinose, D.; Suzuki, M.; Cooper, J.D.; Pare, P.D.; Hogg, J.C. Micro-Computed Tomography Comparison of Preterminal Bronchioles in Centrilobular and Panlobular Emphysema. Am. J. Respir. Crit. Care Med. 2017, 195, 630–638. [Google Scholar] [CrossRef]
- Soriano, J.B.; Visick, G.T.; Muellerova, H.; Payvandi, N.; Hansell, A.L. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005, 128, 2099–2107. [Google Scholar] [CrossRef]
- Tantucci, C.; Modina, D. Lung function decline in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 95. [Google Scholar] [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef]
- Vestbo, J.; Edwards, L.D.; Scanlon, P.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; et al. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 2011, 365, 1184–1192. [Google Scholar] [CrossRef]
- Cerveri, I.; Corsico, A.G.; Grosso, A.; Albicini, F.; Ronzoni, V.; Tripon, B.; Imberti, F.; Galasso, T.; Klersy, C.; Luisetti, M.; et al. The rapid FEV(1) decline in chronic obstructive pulmonary disease is associated with predominant emphysema: A longitudinal study. COPD 2013, 10, 55–61. [Google Scholar] [CrossRef]
- Mohamed Hoesein, F.A.; de Hoop, B.; Zanen, P.; Gietema, H.; Kruitwagen, C.L.; van Ginneken, B.; Isgum, I.; Mol, C.; van Klaveren, R.J.; Dijkstra, A.E.; et al. CT-quantified emphysema in male heavy smokers: Association with lung function decline. Thorax 2011, 66, 782–787. [Google Scholar] [CrossRef]
- Suissa, S.; Ernst, P.; Benayoun, S.; Baltzan, M.; Cai, B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N. Engl. J. Med. 2000, 343, 332–336. [Google Scholar] [CrossRef]
- Busse, W.W.; Bateman, E.D.; Caplan, A.L.; Kelly, H.W.; O’Byrne, P.M.; Rabe, K.F.; Chinchilli, V.M. Combined Analysis of Asthma Safety Trials of Long-Acting beta2-Agonists. N. Engl. J. Med. 2018, 378, 2497–2505. [Google Scholar] [CrossRef]
- Martinez, F.J.; Foster, G.; Curtis, J.L.; Criner, G.; Weinmann, G.; Fishman, A.; DeCamp, M.M.; Benditt, J.; Sciurba, F.; Make, B.; et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am. J. Respir. Crit. Care Med. 2006, 173, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Andrianopoulos, V.; Celli, B.R.; Franssen, F.M.; Pinto-Plata, V.M.; Calverley, P.M.; Vanfleteren, L.E.; Vogiatzis, I.; Vestbo, J.; Agusti, A.; Bakke, P.S.; et al. Determinants of exercise-induced oxygen desaturation including pulmonary emphysema in COPD: Results from the ECLIPSE study. Respir. Med. 2016, 119, 87–95. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; D’Arsigny, C.; Fitzpatrick, M.; Webb, K.A. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am. J. Respir. Crit. Care Med. 2002, 166, 663–668. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 199–208. [Google Scholar] [PubMed]
- Thompson, A.A.; Dickinson, R.S.; Murphy, F.; Thomson, J.P.; Marriott, H.M.; Tavares, A.; Willson, J.; Williams, L.; Lewis, A.; Mirchandani, A.; et al. Hypoxia determines survival outcomes of bacterial infection through HIF-1alpha dependent re-programming of leukocyte metabolism. Sci. Immunol. 2017, 2, eaal2861. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.F.; Tambuwala, M.M.; Bruning, U.; Schaible, B.; Scholz, C.C.; Byrne, A.; O’Connor, A.; Gallagher, W.M.; Lenihan, C.R.; Garvey, J.F.; et al. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. J. Immunol. 2011, 186, 1091–1096. [Google Scholar] [CrossRef]
- Diaz, A.A.; Pinto-Plata, V.; Hernandez, C.; Pena, J.; Ramos, C.; Diaz, J.C.; Klaassen, J.; Patino, C.M.; Saldias, F.; Diaz, O. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir. Med. 2015, 109, 882–889. [Google Scholar] [CrossRef]
- Boutou, A.K.; Shrikrishna, D.; Tanner, R.J.; Smith, C.; Kelly, J.L.; Ward, S.P.; Polkey, M.I.; Hopkinson, N.S. Lung function indices for predicting mortality in COPD. Eur. Respir. J. 2013, 42, 616–625. [Google Scholar] [CrossRef] [PubMed]

| COPD Total (N = 95) | COPD Emphysema- Predominant (N = 62) | COPD Airway Obstruction- Predominant (N = 33) | Asthma (N = 60) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 67.1 ± 9.1 | 67.1 ± 9.0 | 67.3 ± 9.3 | 64.8 ± 12 | 0.387 |
| Gender, female | 8 | 2 | 5 | 20 | <0.001 |
| Body mass index, kg/m2 | 23.0 ± 3.5 | 22.6 ± 3.4 | 23.7 ± 3.8 | 25.3 ± 3.1 | <0.001 |
| Smoking habit | 84 | 56 | 28 | 6 | <0.001 |
| HRCT score | 22.3 ± 19.6 | 30.8 ± 19.5 | 6.4 ± 1.7 | 1.8 ± 3.2 | <0.001 |
| Appearance of comorbidities Ischemic heart disease | 0.992 | ||||
| Ischemic heart disease | 14 | 8 | 6 | 9 | 0.788 |
| Cerebrovascular disease | 4 | 2 | 2 | 2 | 0.803 |
| Diabetes | 13 | 9 | 4 | 11 | 0.704 |
| Liver disease | 2 | 1 | 1 | 2 | 0.822 |
| Chronic kidney disease | 7 | 5 | 2 | 5 | 0.948 |
| Pulmonary function (pre-bronchodilator) | |||||
| FVC, L | 2.13± 0.54 | 2.15± 0.55 | 2.10 ± 0.51 | 2.00± 0.50 | 0.315 |
| FVC, % pred. | 69.61 ± 17.45 | 69.75 ± 18.58 | 69.34± 15.36 | 72.70± 13.82 | 0.510 |
| FEV1, L | 1.25 ± 0.44 | 1.23 ± 0.41 | 1.28 ± 0.48 | 1.20 ± 0.38 | 0.672 |
| FEV1, % pred. | 56.70 ± 21.89 | 55.90 ± 22.16 | 58.22 ± 21.63 | 59.04 ± 19.66 | 0.702 |
| FEV1/FVC, % | 58.10 ± 11.99 | 57.19 ± 11.29 | 59.8 2± 13.21 | 60.61± 14.06 | 0.318 |
| FEV1: <35% | 15 | 9 | 6 | 7 | 0.769 |
| FEV1: 35~50% | 20 | 16 | 4 | 15 | |
| FEV1: 50~75% | 44 | 28 | 16 | 28 | |
| FEV1: >75% | 16 | 9 | 7 | 10 | |
| MMEF, % pred. | 25.23 ± 14.43 | 25.10± 14.84 | 25.48 ± 13.84 | 25.29 ± 14.98 | 0.994 |
| Pre-6MWT IC, L | 1.50 ± 0.45 | 1.51 ± 0.47 | 1.48± 0.43 | 1.57 ± 0.36 | 0.588 |
| Post-6MWT IC, L | 1.39 ± 0.43 * | 1.34± 0.40 | 1.49± 0.48 | 1.57 ± 0.42 | 0.029 |
| ΔIC (post-pre) | −0.11 ± 0.27 * | -0.18 ± 0.19 | 0.01 ± 0.34 | 0.00 ± 0.25 | 0.001 |
| 6MWD, M | 387.7 ± 101.0 * | 380.4 ± 107.2 | 404.4 ± 87.3 | 432.0 ± 111.9 | 0.028 |
| SpO2 saturation | |||||
| Pre-exercise, % | 95.2 ± 2.1 | 95.0 ± 2.3 | 95.3 ± 1.6 | 95.5 ± 2.3 | 0.535 |
| Post-exercise, % | 87.1 ± 7.0 ** | 86.4 ± 6.7 | 88.3 ± 7.4 | 89.7 ± 3.9 | 0.006 |
| Post, SpO2 | |||||
| FEV1: <35% | 84.0 (66, 91) ** | 83.0 (66, 91) | 86.5 (80, 90) | 89.0 (85, 91) | 0.016 |
| FEV1: 35~50% | 88.0 (74, 95) | 87.0 (74, 93) | 91.0 (79, 95) | 90.0 (77, 95) | 0.156 |
| FEV1: 50~75% | 90.5 (71, 97) | 90.0 (71, 95) | 91.0 (72, 97) | 90.5 (81, 98) | 0.801 |
| FEV1: >75% | 91.5 (59, 93) | 89.0 (72, 93) | 93.0 (59, 93) | 91.5 (84, 94) | 0.459 |
| Overall mortality event | 0.0001 | ||||
| All-cause death, n (%) | 30 (31.6) | 25 (40.3) | 5 (15.2) | 2 (3.3) | <0.0001 |
| Cardiovascular death, n (%) | 4 (4.2) | 4 (6.5) | 0 (0.0) | 0 (0.0) | 0.046 |
| Lung cancer death, n (%) | 3 (3.2) | 3 (4.8) | 0 (0.0) | 0 (0.0) | 0.101 |
| Pulmonary cause, n (%) | 17 (17.9) ** | 14 (22.6) | 3 (9.1) | 0 (0.0) | 0.0003 |
| Other death, n (%) | 6 (6.3) | 4 (6.4) | 2 (6.1) | 2 (3.3) | 0.714 |
| Disease-specific death (pulmonary, cardiovascular and lung cancer), n (%) | 24 (25.3) ** | 21 (33.9) | 3 (9.1) | 0 (0.0) | <0.0001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Groups | ||||||
| Asthma | 1 (ref) | 1 (ref) | ||||
| COPD | 11.036 | 2.636–46.203 | 0.001 | 6.979 | 0.852–57.173 | 0.070 |
| Age, years | 1.055 | 1.013–1.099 | 0.011 | 1.055 | 1.000–1.113 | 0.048 |
| Gender | ||||||
| Female | 1 (ref) | |||||
| Male | 7.199 | 0.983–52.741 | 0.052 | |||
| Body mass index | 0.885 | 0.796–0.983 | 0.023 | 1.016 | 0.906–1.139 | 0.791 |
| FEV1 (%) per 10% decrease | 1.000 | 0.843–1.185 | 0.999 | |||
| MMEF % pred. value | 0.998 | 0.972–1.025 | 0.880 | |||
| HRCT score | 1.031 | 1.016–1.046 | <0.001 | 1.021 | 1.000–1.043 | 0.048 |
| 6MWD per 10 m increase | 0.954 | 0.926–0.983 | 0.002 | 0.994 | 0.957–1.033 | 0.767 |
| Nadir saturation during 6MWT | ||||||
| SpO2: ≥90% | 1 (ref) | 1 (ref) | ||||
| SpO2: 80–89% | 2.738 | 1.616–6.461 | 0.021 | 2.120 | 0.761–5.902 | 0.150 |
| SpO2: <80% | 7.019 | 2.669–18.252 | <0.001 | 4.918 | 1.556–15.550 | 0.007 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Groups | ||||||
| Asthma | 1 (ref) | 1 (ref) | ||||
| COPD HRCT score ≤ 10 | 4.929 | 0.956–25.407 | 0.057 | 4.947 | 0.531–46.134 | 0.160 |
| COPD HRCT score > 10 | 14.726 | 3.485–26.229 | <0.001 | 9.817 | 1.110–86.785 | 0.040 |
| Age, years | 1.055 | 1.013–1.099 | 0.011 | 1.056 | 1.001–1.115 | 0.046 |
| Gender | ||||||
| Female | 1 (ref) | |||||
| Male | 7.199 | 0.983–52.741 | 0.052 | |||
| Body mass index | 0.885 | 0.796–0.983 | 0.023 | 1.009 | 0.895–1.136 | 0.887 |
| FEV1 (%) per 10% decrease | 1.000 | 0.843–1.185 | 0.999 | |||
| MMEF % pred. value | 0.998 | 0.972–1.025 | 0.880 | |||
| HRCT score | 1.031 | 1.016–1.046 | <0.001 | 1.014 | 0.989–1.040 | 0.283 |
| 6MWD per 10 m increase | 0.954 | 0.926–0.983 | 0.002 | 0.999 | 0.961–1.039 | 0.966 |
| Nadir saturation during 6MWT | ||||||
| SpO2: ≥90% | 1 (ref) | 1 (ref) | ||||
| SpO2: 80–89% | 2.738 | 1.161–6.461 | 0.021 | 2.403 | 0.736–5.675 | 0.170 |
| SpO2: <80% | 7.019 | 2.699–18.252 | <0.001 | 4.859 | 1.537–15.353 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Huang, H.-Y.; Chung, F.-T.; Lo, C.-Y.; Huang, Y.-C.; Wang, T.-W.; Yang, L.-Y.; Pan, Y.-B.; Chung, K.F.; Wang, C.-H. Emphysema-Predominant COPD Had a Greater 5-Year Mortality and a Worse Annual Decline in Lung Function Than Airway Obstruction-Predominant COPD or Asthma at Initial Same Degree of Airflow Obstruction. Medicina 2021, 57, 1261. https://doi.org/10.3390/medicina57111261
Lin C-W, Huang H-Y, Chung F-T, Lo C-Y, Huang Y-C, Wang T-W, Yang L-Y, Pan Y-B, Chung KF, Wang C-H. Emphysema-Predominant COPD Had a Greater 5-Year Mortality and a Worse Annual Decline in Lung Function Than Airway Obstruction-Predominant COPD or Asthma at Initial Same Degree of Airflow Obstruction. Medicina. 2021; 57(11):1261. https://doi.org/10.3390/medicina57111261
Chicago/Turabian StyleLin, Chang-Wei, Hung-Yu Huang, Fu-Tsai Chung, Chun-Yu Lo, Yu-Chen Huang, Ting-Wen Wang, Lan-Yan Yang, Yu-Bin Pan, Kian Fan Chung, and Chun-Hua Wang. 2021. "Emphysema-Predominant COPD Had a Greater 5-Year Mortality and a Worse Annual Decline in Lung Function Than Airway Obstruction-Predominant COPD or Asthma at Initial Same Degree of Airflow Obstruction" Medicina 57, no. 11: 1261. https://doi.org/10.3390/medicina57111261
APA StyleLin, C.-W., Huang, H.-Y., Chung, F.-T., Lo, C.-Y., Huang, Y.-C., Wang, T.-W., Yang, L.-Y., Pan, Y.-B., Chung, K. F., & Wang, C.-H. (2021). Emphysema-Predominant COPD Had a Greater 5-Year Mortality and a Worse Annual Decline in Lung Function Than Airway Obstruction-Predominant COPD or Asthma at Initial Same Degree of Airflow Obstruction. Medicina, 57(11), 1261. https://doi.org/10.3390/medicina57111261






