Morphological Study of Fossa Ovalis in Formalin-Fixed Human Hearts and Its Clinical Importance
Abstract
1. Introduction
2. Materials and Methods
- -
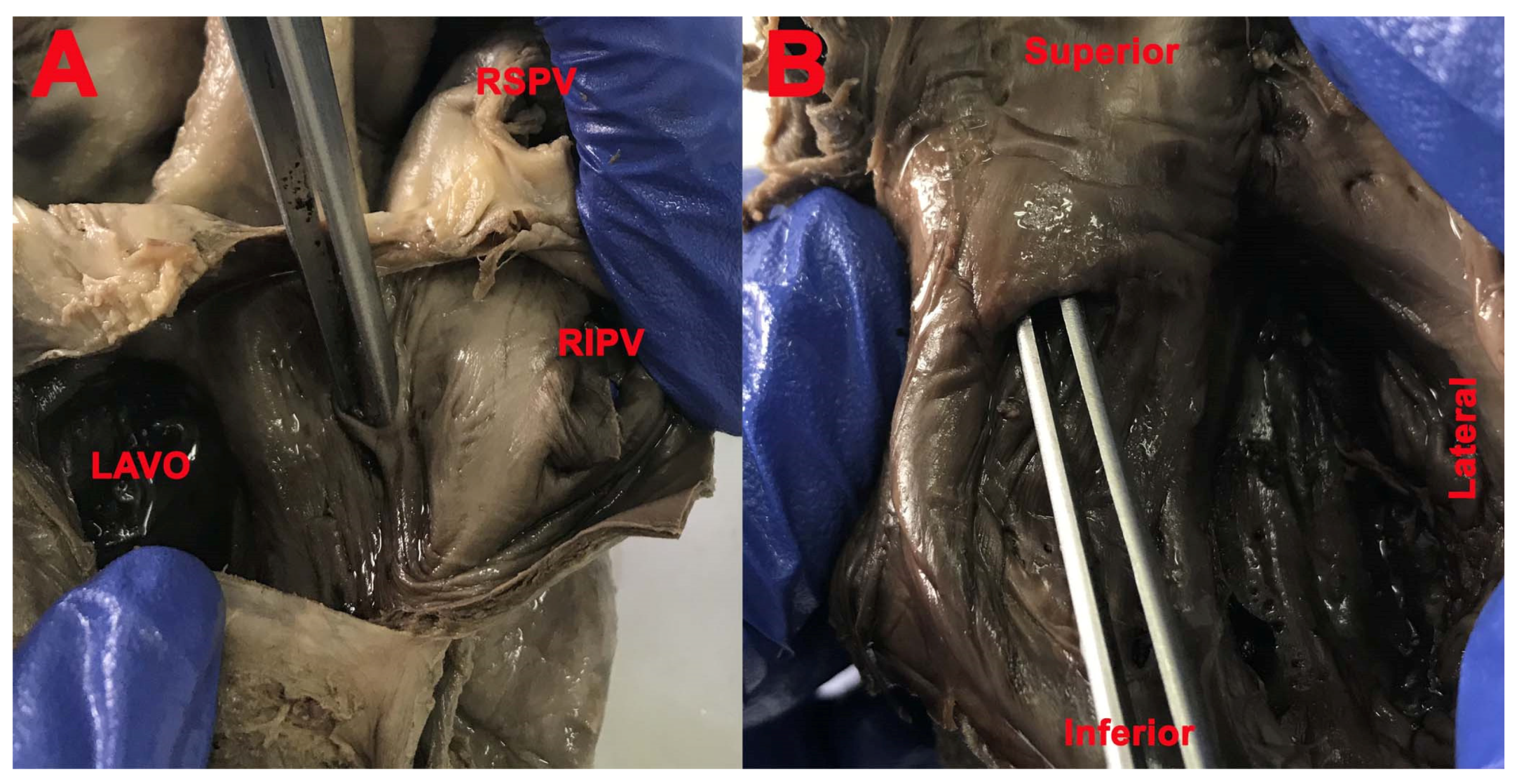
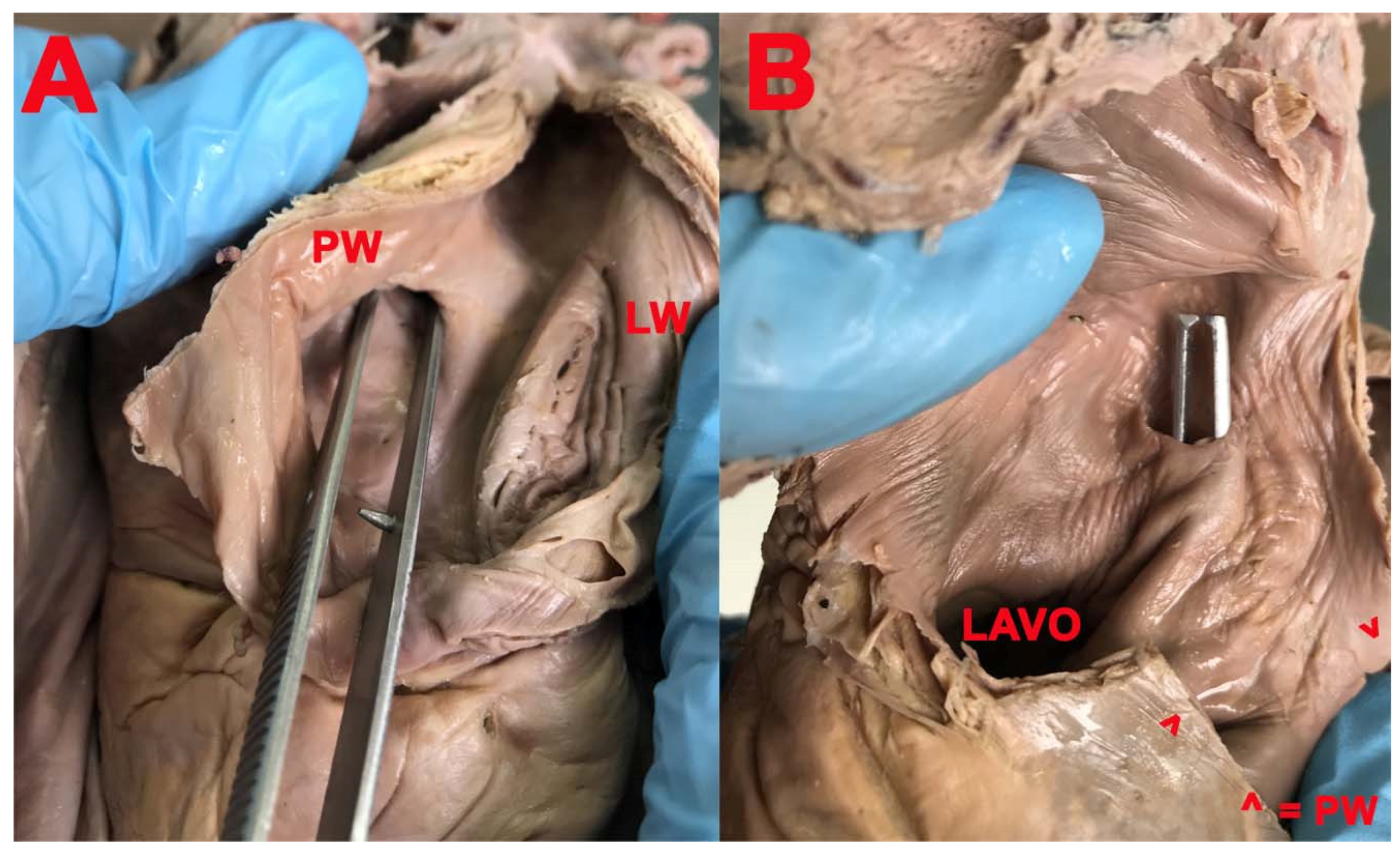
- Position of the FO in the interatrial septum;
- -
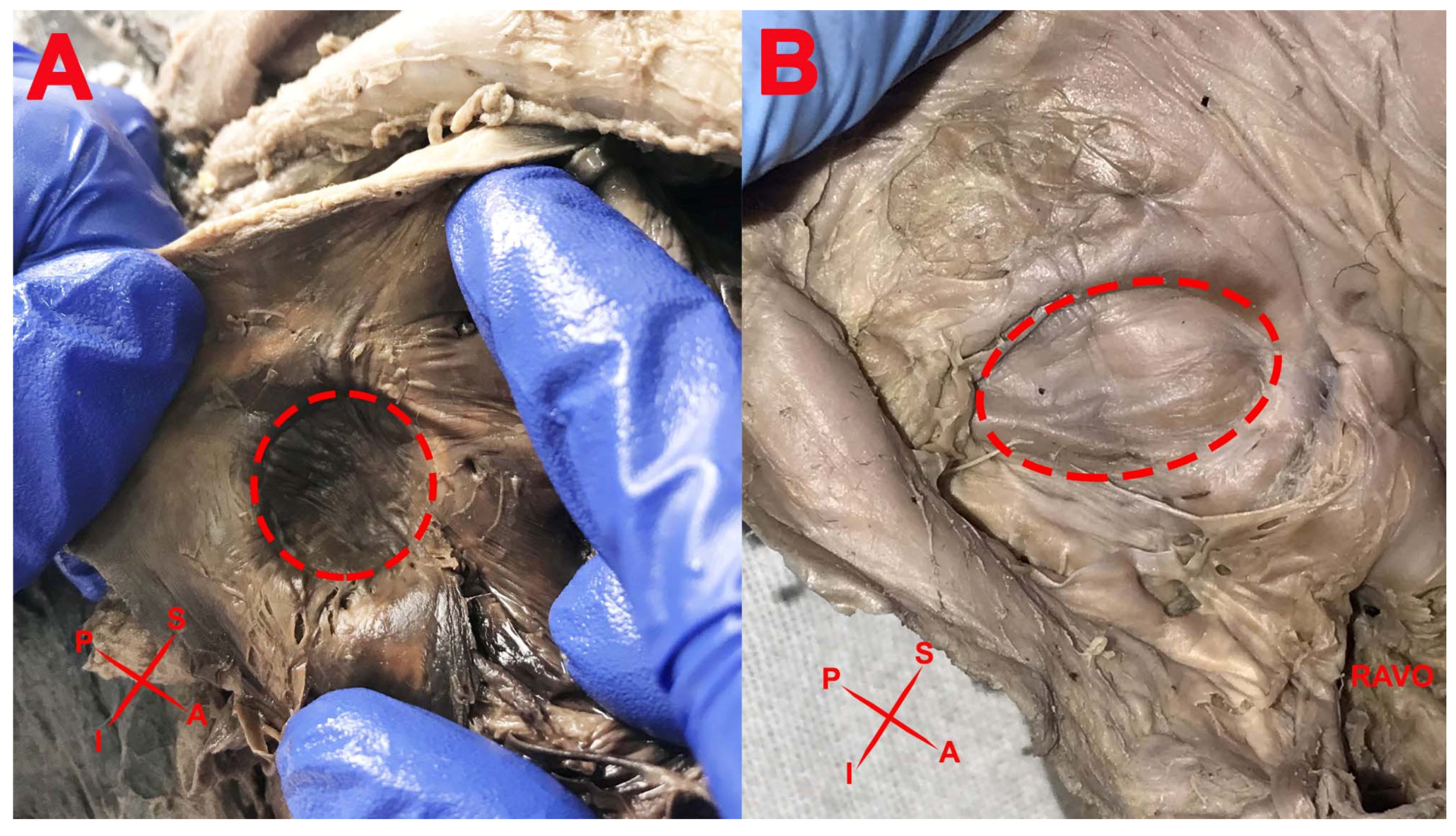
- Shape of the FO;
- -
- Aspect of the FO;
- -
- Presence and location of the limbus of the FO;
- -
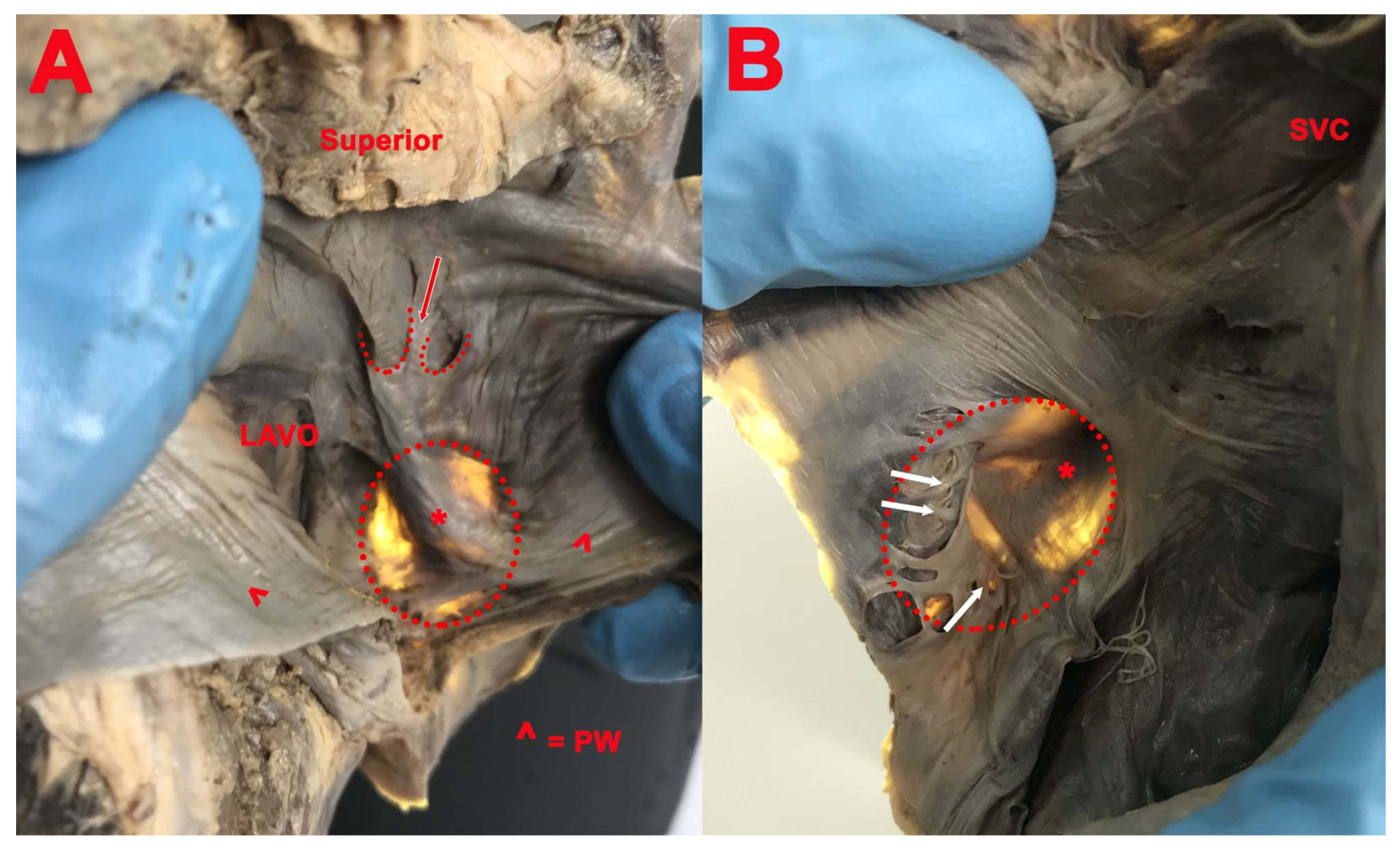
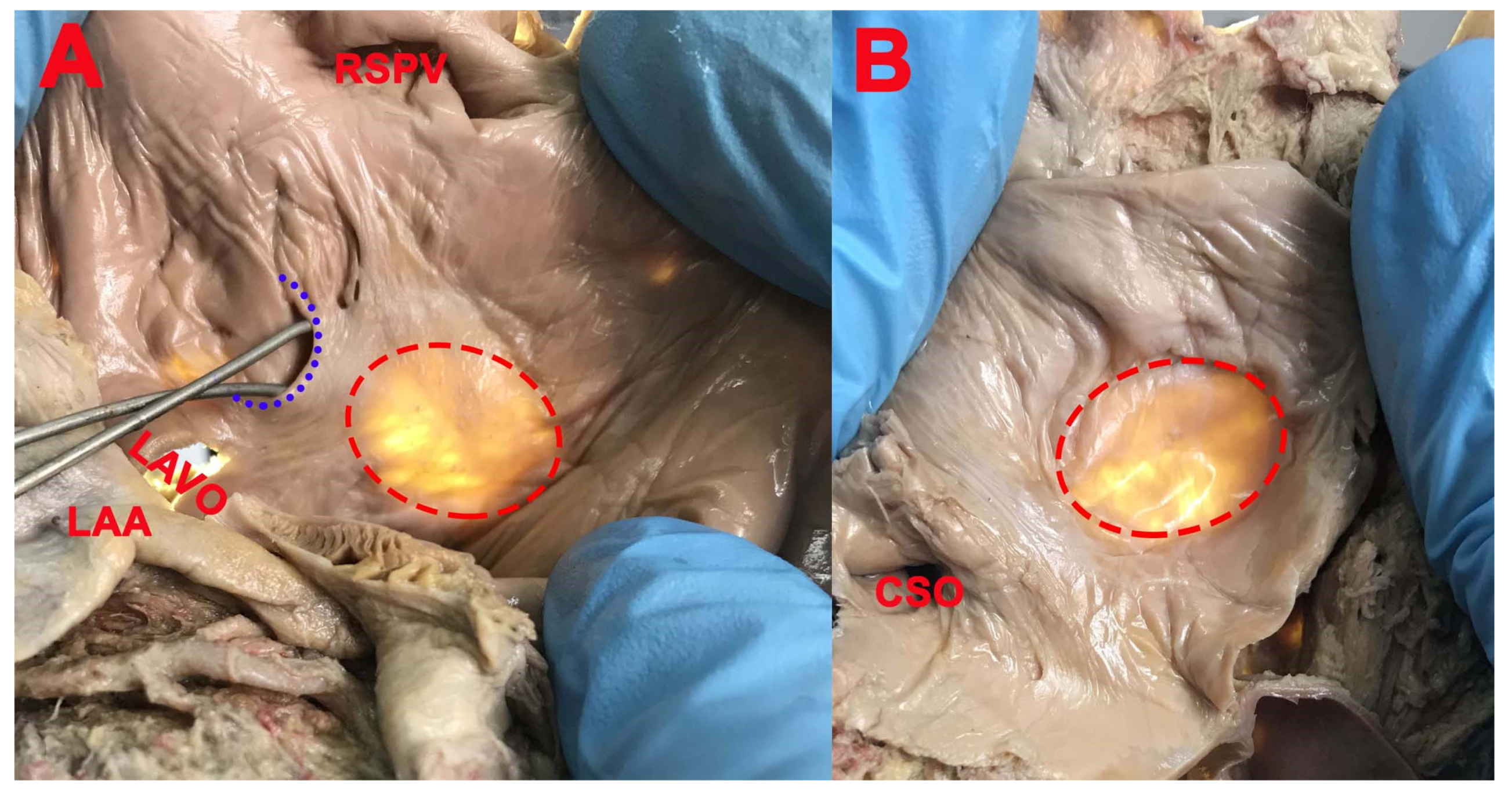
- Presence of the septal pouch (SP), right SP opened into the right atrium, left SP opened into the left atrium, and the position of the SP in interatrial septum;
- -
- Presence of patent foramen ovale (PFO).
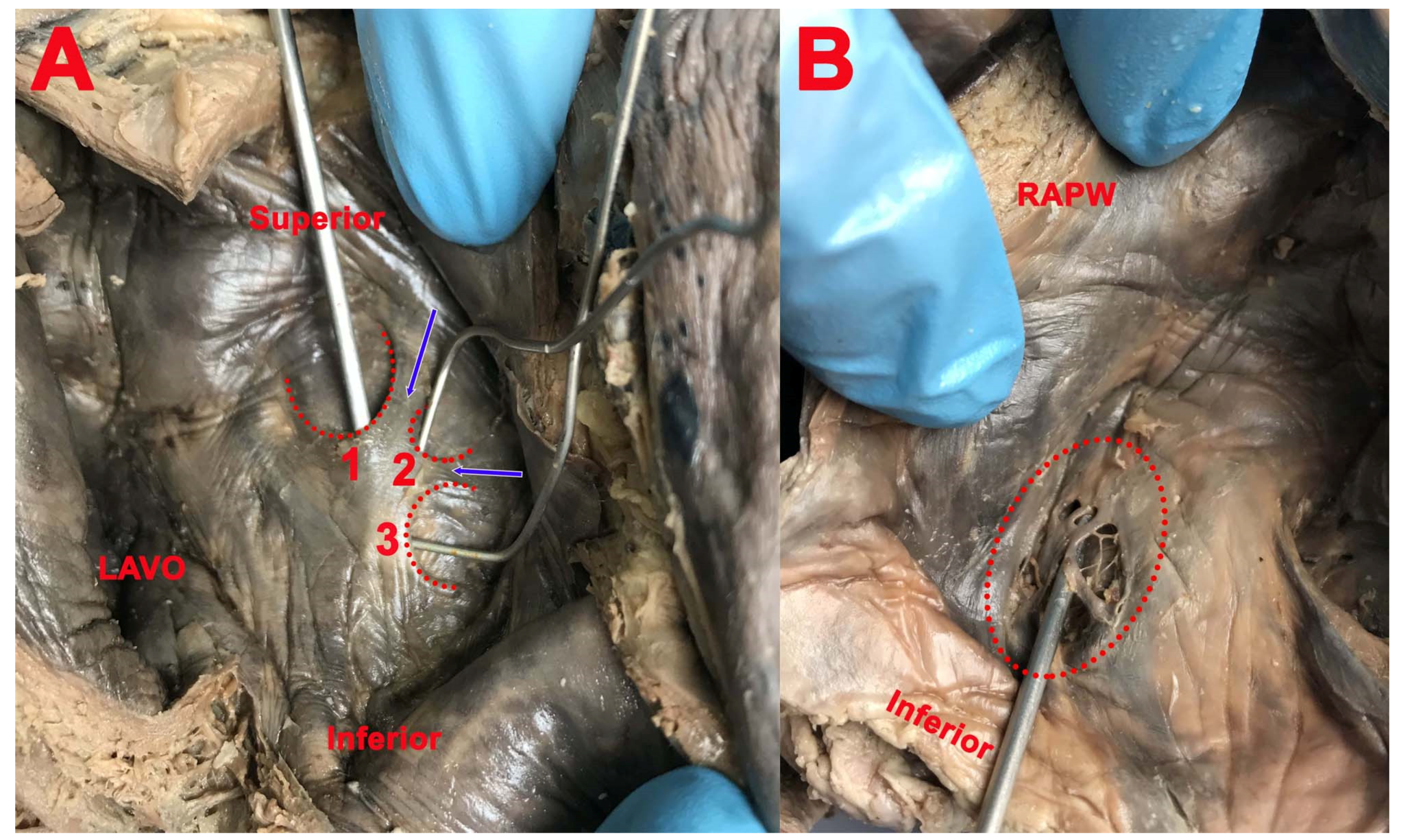
3. Results
- (1)
- Six specimens (37.5%), on the entire surface of the FO;
- (2)
- Six specimens (37.5%), on the posteroinferior part of the FO;
- (3)
- Four specimens (25%), only on the inferior part of the FO.
- (1)
- All around the circumference in 2 cases (6.89%);
- (2)
- Superior in one case (3.44%);
- (3)
- Superior and anterior in 3 cases (10.35%);
- (4)
- Superior, posterior, and inferior in 13 cases (44.82%);
- (5)
- Anterior, superior, and posterior in 7 cases (24.14%);
- (6)
- Superior and posterior in 3 cases (10.35%).
- (1)
- Right and double SP had the opening in the right atrium in 19 cases (61.29%) and the concavity was located in the inferior part (12 cases, 63.15%), anteroinferior part (4 cases, 21.05%), and posteroinferior part (3 cases, 15.8%) of the circumference of the FO;
- (2)
- Left and double SP had the opening in the left atrium in 25 cases (80.64%). The concavity was located in the superior part (15 cases, 60%), posteroinferior part (2 cases, 8%), posterosuperior part (2 cases, 8%), anterior part (3 cases, 12%), posterior part (1 case, 4%), and anterosuperior part (2 cases, 8%) of the circumference of the FO.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asirvatham, S.J.; Stevenson, W.G. Editor’s perspective: The interatrial septum. Circ. Arrhythm. Electrophysiol. 2013, 6, e75–e76. [Google Scholar] [CrossRef][Green Version]
- Anderson, R.H.; Brown, N.A.; Webb, S. Development and structure of the atrial septum. Heart 2002, 88, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Piotrowska, W.; Hołda, M.K.; Koziej, M.; Piątek, K.; Hołda, J. Anatomy of the true interatrial septum for transseptal access to the left atrium. Ann. Anat 2016, 205, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Dudek, R.W. High.-Yield Embryology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1996; pp. 26–31. [Google Scholar]
- Sadler, T.W. Cardiovascular system. In Langman’s Medical Embryology, 13th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2015; pp. 184–188. [Google Scholar]
- Jensen, B.; Wang, T.; Moorman, A.F.M. Evolution and Development of the Atrial Septum. Anat. Rec. 2019, 302, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Spicer, D.E.; Sheppard, M.N.; Anderson, R.H. Development of the atrial septum in relation to postnatal anatomy and interatrial communications. Heart 2017, 103, 456–462. [Google Scholar] [CrossRef]
- Naqvi, N.; McCarthy, K.P.; Ho, S.Y. Anatomy of the atrial septum and interatrial communications. J. Thorac. Dis. 2018, 10, S2837–S2847. [Google Scholar] [CrossRef]
- Papilian, V.; Papilian, V.V. Manual Practic de Disecție și Descoperiri Anatomice; Dacia: Mioveni, Romania, 1994. [Google Scholar]
- Kerut, E.K.; Norfleet, W.T.; Plotnick, G.D.; Giles, T.D. Patent foramen ovale: A review of associated conditions and the impact of physiological size. J. Am. Coll. Cardiol. 2001, 38, 613–623. [Google Scholar] [CrossRef]
- Loukas, M.; Sullivan, A.; Tubbs, R.S.; Weinhaus, A.J.; Derderian, T.; Hanna, M. Chiari’s network: Review of the literature. Surg. Radiol. Anat. 2010, 32, 895–901. [Google Scholar] [CrossRef]
- Joshi, S.D.; Chawre, H.K.; Joshi, S.S. Morphological study of fossa ovalis and its clinical relevance. Indian Heart J. 2016, 68, 147–152. [Google Scholar] [CrossRef][Green Version]
- Chan, K.C.; Godman, M.J. Morphological variations of fossa ovalis atrial septal defects (secundum): Feasibility for transcutaneous closure with the clam-shell device. Br. Heart J. 1993, 69, 52–55. [Google Scholar] [CrossRef]
- Hołda, M.K.; Pietsch-Fulbiszewska, A.; Trybus, M.; Koziej, M. Morphological variations of the interatrial septum in ovine heart. PLoS ONE 2018, 13, e0209604. [Google Scholar] [CrossRef]
- Shizukuda, Y.; Muth, J.; Chaney, C.; Attari, M. Anomalous ridge on the left atrial side of the atrial septum. Ann. Card Anaesth. 2012, 15, 161–162. [Google Scholar] [CrossRef]
- Zisa, D.; Faletra, F.F.; Wessler, B.S.; Halin, N.J.; Reddy, P.; Patel, A.R.; Pandian, N.G. Ridges and Pouches: A Case Series of Anomalous Atrial Septal Fusion. Case 2019, 4, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.; Koutsouflianiotis, K.; Iliou, K. The first descriptions of various anatomical structures and embryological remnants of the heart: A systematic overview. Int. J. Cardiol. 2017, 227, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Filipoiu, F.M. Atlas of Heart Anatomy; Springer: London, UK; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands, 2014; pp. 129–130. [Google Scholar]
- Krishnan, S.C.; Salazar, M. Septal pouch in the left atrium: A new anatomical entity with potential for embolic complications. JACC Cardiovasc. Interv. 2010, 3, 98–104. [Google Scholar] [CrossRef]
- Mazur, M.; Jasinska, K.A.; Walocha, J.A. The morphology, clinical significance and imaging methods of the atrialseptal pouch: A critical review. Transl. Res. Anat. 2018, 13, 7–11. [Google Scholar]
- Hołda, M.K.; Koziej, M.; Hołda, J.; Piątek, K.; Tyrak, K.; Chołopiak, W.; Bolechala, F.; Walocha, J.A.; Klimek-Piotrowska, W. Atrial septal pouch—Morphological features and clinical considerations. Int. J. Cardiol. 2016, 220, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S. The Atrial Septal Pouch—A New Source of Thrombus? Medscape, 29 January 2010. [Google Scholar]
- Berthet, K.; Lavergne, T.; Cohen, A.; Guize, L.; Bousser, M.G.; Le Heuzey, J.Y.; Amarenco, P. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke 2000, 31, 398–403. [Google Scholar] [CrossRef]
- Tugcu, A.; Okajima, K.; Jin, Z.; Rundek, T.; Homma, S.; Sacco, R.L.; Elkind, M.S.V.; Di Tullio, M.R. Septal pouch in the left atrium and risk of ischemic stroke. JACC Cardiovasc. Imaging 2010, 3, 1276–1283. [Google Scholar] [CrossRef]
- Hołda, M.K.; Koziej, M. Left-Sided Atrial Septal Pouch as a Risk Factor of Cryptogenic Stroke: A Systematic Review and Meta-Analysis. Cereb. Dis. 2018, 46, 223–229. [Google Scholar] [CrossRef]
- Kuwaki, H.; Takeuchi, M.; Kaku, K.; Haruki, N.; Yoshitani, H.; Tamura, M.; Okazaki, M.; Abe, H.; Otsuji, Y. Thrombus attached to the left atrial septal pouch assessed on 3-dimensional transesophageal echocardiography. Circ. J. 2011, 75, 2280–2281. [Google Scholar] [CrossRef] [PubMed]
- Loza, G.; Américo, C.; Gómez, A.; Janssen, B.; Pazos, A.; Parma, G.; Florio, L. Prevalence of septal pouch in a cohort derived for transesophageal echocardiography. Rev. Urug. Cardiol. 2019, 34, 156–162. [Google Scholar]
- Wong, J.M.; Fisher, M. The potential role of the left atrial septal pouch in cryptogenic stroke. Expert Rev. Cardiovasc. 2016, 14, 1–3. [Google Scholar] [CrossRef]
- Kabirdas, D.; Nekkanti, R. Webbed left atrial septal pouch-A new anatomical variant. Echocardiography 2018, 35, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Strachinaru, M.; Castro-Rodriguez, J.; Verbeet, T.; Gazagnes, M.D. The left atrial septal pouch as a risk factor for stroke: A systematic review. Arch. Cardiovasc. Dis. 2017, 110, 250–258. [Google Scholar] [CrossRef]
- Terpenning, S.; Ketai, L.H.; Rissing, S.M.; Teague, S.D. Correlation of Left Atrial Septal Pouch with the Prevalence of Patent Foramen Ovale: A Retrospective Review. Cardiol. Angiol. Int. J. 2015, 3, 122–129. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaida, M.A.; Streian, C.G.; Gug, C.; Damen, N.S.; Jianu, A.M.; Grigoriță, A.; Grigoriță, L. Morphological Study of Fossa Ovalis in Formalin-Fixed Human Hearts and Its Clinical Importance. Medicina 2021, 57, 1254. https://doi.org/10.3390/medicina57111254
Vaida MA, Streian CG, Gug C, Damen NS, Jianu AM, Grigoriță A, Grigoriță L. Morphological Study of Fossa Ovalis in Formalin-Fixed Human Hearts and Its Clinical Importance. Medicina. 2021; 57(11):1254. https://doi.org/10.3390/medicina57111254
Chicago/Turabian StyleVaida, Monica Adriana, Caius Glad Streian, Cristina Gug, Nawwaf Sebastian Damen, Adelina Maria Jianu, Andreea Grigoriță, and Laura Grigoriță. 2021. "Morphological Study of Fossa Ovalis in Formalin-Fixed Human Hearts and Its Clinical Importance" Medicina 57, no. 11: 1254. https://doi.org/10.3390/medicina57111254
APA StyleVaida, M. A., Streian, C. G., Gug, C., Damen, N. S., Jianu, A. M., Grigoriță, A., & Grigoriță, L. (2021). Morphological Study of Fossa Ovalis in Formalin-Fixed Human Hearts and Its Clinical Importance. Medicina, 57(11), 1254. https://doi.org/10.3390/medicina57111254






