Prenatal Diagnosis and Outcome of Tracheal Agenesis as Part of Congenital High Airway Obstruction Syndrome. Case Presentation and Literature Review
Abstract
:1. Introduction
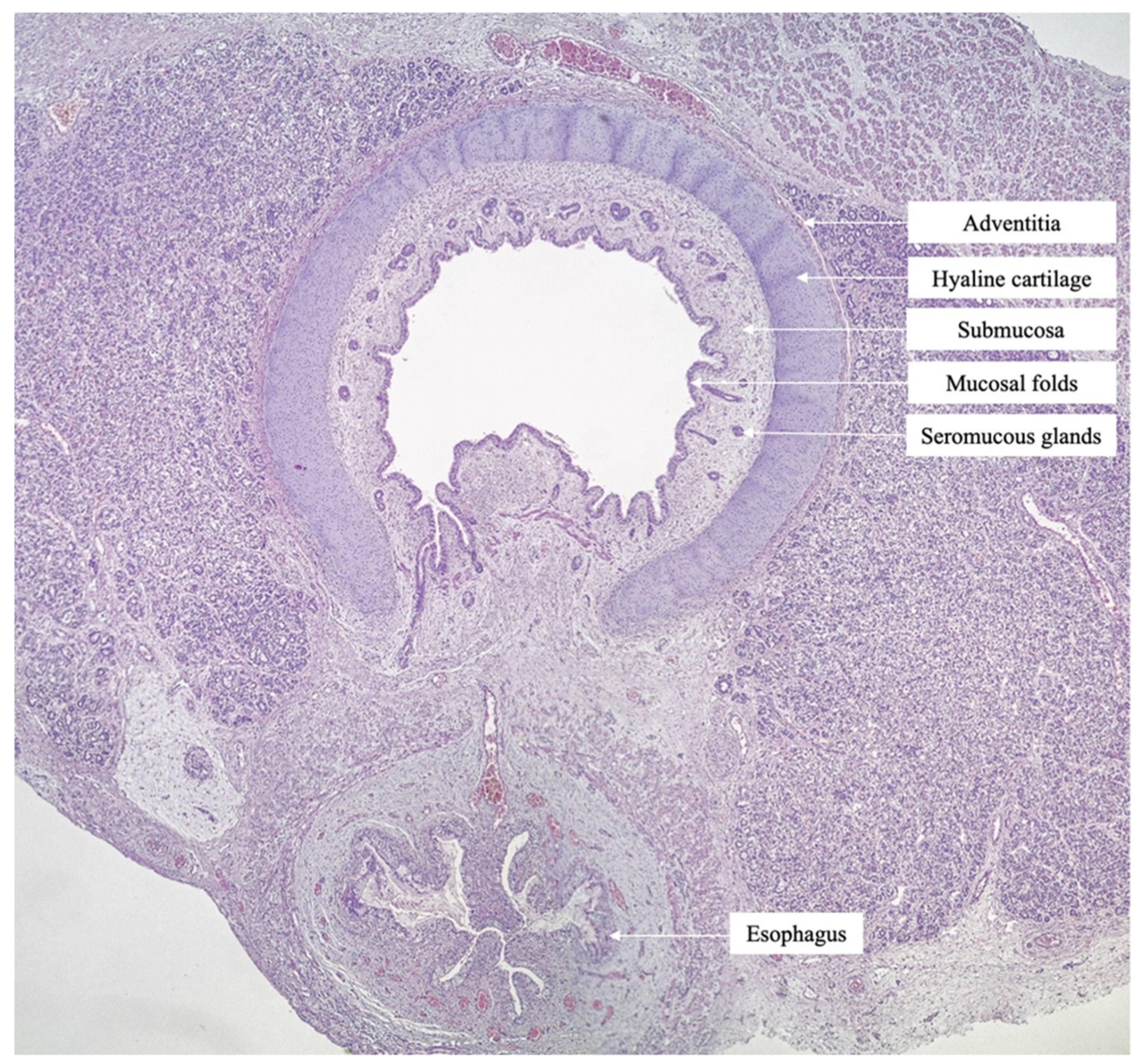
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartnick, C.J.; Rutter, M.; Lang, F.; Willging, J.P.; Cotton, R.T. Congenital high airway obstruction syndrome and airway reconstruction: An evolving paradigm. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Witters, I.; Fryns, J.-P.; De Catte, L.; Moerman, P. Prenatal diagnosis and pulmonary pathology in congenital high airway obstruction sequence. Prenat. Diagn. 2009, 29, 1081–1084. [Google Scholar] [CrossRef]
- Nicolas, C.T.; Lynch-Salamon, D.; Bendel-Stenzel, E.; Tibesar, R.; Luks, F.; Eyerly-Webb, S.; Lillegard, J.B. Fetoscopy-Assisted Percutaneous Decompression of the Distal Trachea and Lungs Reverses Hydrops Fetalis and Fetal Distress in a Fetus with Laryngeal Atresia. Fetal Diagn. Ther. 2019, 46, 75–80. [Google Scholar] [CrossRef]
- Dighe, M.K.; Peterson, S.E.; Dubinsky, T.J.; Perkins, J.; Cheng, E. EXIT procedure: Technique and indications with prenatal imaging parameters for assessment of airway patency. Radiographics 2011, 31, 511–526. [Google Scholar] [CrossRef]
- Mesens, T.; Witters, I.; Van Robaeys, J.; Peeters, H.; Fryns, J.P. Congenital High Airway Obstruction Syndrome (CHAOS) as part of Fraser syndrome: Ultrasound and autopsy findings. Genet. Couns. 2013, 24, 367–371. [Google Scholar] [PubMed]
- Gaál, V.; Vida, L.; Adamovich, K.; Mosdósi, B.; Tárnok, A. Tracheal agenesis: A rare cause of inability to secure the airways in a newborn. Otolaryngol. Case Rep. 2020, 17, 100243. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, M.Y.; Kang, O.J.; Kim, R.; Chung, J.H.; Won, H.S.; Lee, P.R.; Jung, E.; Lee, B.S.; Choi, W.J. Perinatal outcome of fetuses with congenital high airway obstruction syndrome: A single-center experience. Obstet. Gynecol. Sci. 2021, 64, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Mudaliyar, U.S.; Sreedhar, S. Chaos syndrome. BJR Case Rep. 2017, 3, 20160046. [Google Scholar] [CrossRef]
- Kornacki, J.; Szydłowski, J.; Skrzypczak, J.; Szczepańska, M.; Rajewski, M.; Koziołek, A.; Gaca, M.; Wender-Ożegowska, E. Use of ex utero intrapartum treatment procedure in fetal neck and high airway anomalies–report of four clinical cases. J. Matern. Fetal Neonatal Med. 2019, 32, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Simetka, O.; Petros, M.; Dolezalkova, E.; Kovacova, H.; Matura, D.; Dvořáčková, J. Prenatal diagnosis of tracheal atresia in a twin pregnancy. Ultrasound Obstet. Gynecol. 2014, 43, 717–718. [Google Scholar] [CrossRef] [Green Version]
- Artunc Ulkumen, B.; Pala, H.G.; Nese, N.; Tarhan, S.; Baytur, Y. Prenatal diagnosis of congenital high airway obstruction syndrome: Report of two cases and brief review of the literature. Case Rep. Obstet. Gynecol. 2013, 2013, 728974. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, S.K.; Goudy, S.; Prickett, K.; Ellis, J. EXIT (ex utero intrapartum treatment) in a growth restricted fetus with tracheal atresia. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Vallera, C.; Heitmiller, E.S.; Isaac, G.; Lee, M.; Crino, J.; Boss, E.F.; Ishman, S.L. Ex utero intrapartum treatment procedure for management of congenital high airway obstruction syndrome in a vertex/breech twin gestation. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Saadai, P.; Jelin, E.B.; Nijagal, A.; Schecter, S.C.; Hirose, S.; MacKenzie, T.C.; Rand, L.; Goldstein, R.; Farrell, J.; Harrison, M.; et al. Long-term outcomes after fetal therapy for congenital high airway obstructive syndrome. J. Pediatr. Surg. 2012, 47, 1095–1100. [Google Scholar] [CrossRef]
- Roybal, J.L.; Liechty, K.W.; Hedrick, H.L.; Bebbington, M.W.; Johnson, M.P.; Coleman, B.G.; Adzick, N.S.; Flake, A.W. Predicting the severity of congenital high airway obstruction syndrome. J. Pediatr. Surg. 2010, 45, 1633–1639. [Google Scholar] [CrossRef]
- Oepkes, D.; Teunissen, A.K.K.; van de Velde, M.; Devlieger, H.; Delaere, P.; Deprest, J. Congenital high airway obstruction syndrome successfully managed with ex-utero intrapartum treatment. Ultrasound Obstet. Gynecol. 2003, 22, 437–439. [Google Scholar] [CrossRef]
- Lim, F.-Y.; Crombleholme, T.M.; Hedrick, H.L.; Flake, A.W.; Johnson, M.P.; Howell, L.J.; Adzick, N. Congenital high airway obstruction syndrome: Natural history and management. J. Pediatr. Surg. 2003, 38, 940–945. [Google Scholar] [CrossRef]
- Vidaeff, A.C.; Szmuk, P.; Mastrobattista, J.M.; Rowe, T.F.; Ghelber, O. More or less CHAOS: Case report and literature review suggesting the existence of a distinct subtype of congenital high airway obstruction syndrome. Ultrasound Obstet. Gynecol. 2007, 30, 114–117. [Google Scholar] [CrossRef]
- Syngelaki, A.; Hammami, A.; Bower, S.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of fetal non-chromosomal abnormalities on routine ultrasound examination at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2019, 54, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Suciu, I.D.; Toader, O.D.; Galeva, S.; Pop, L.G. Non-Invasive Prenatal Testing beyond Trisomies. J. Med. Life 2019, 12, 221–224. [Google Scholar] [CrossRef]
- Van der Meij, K.R.M.; Sistermans, E.A.; Macville, M.V.E.; Stevens, S.J.C.; Bax, C.J.; Bekker, M.N.; Bilardo, C.M.; Boon, E.M.J.; Boter, M.; Diderich, K.E.M.; et al. TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am. J. Hum. Genet. 2019, 105, 1091–1101. [Google Scholar] [CrossRef]
- Suciu, I.; Galeva, S.; Azim, S.A.; Pop, L.; Toader, O. First-trimester screening-biomarkers and cell-free DNA. J. Matern. Fetal Neonatal Med. 2021, 34, 3983–3989. [Google Scholar] [CrossRef]
- Minnella, G.P.; Crupano, F.M.; Syngelaki, A.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of major heart defects by routine first-trimester ultrasound examination: Association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound Obstet. Gynecol. 2020, 55, 637–644. [Google Scholar] [CrossRef]
- Pop, L.G.; Radoi, V.; Sipos, P. Demonstration of holoprosencephaly, proboscis and cyclopia in fetus without aneuploidy, but high level of homozygosity. Prenat. Diagn. 2021, 41, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Cariati, F.; Sarno, L.; Conforti, A.; Bagnulo, F.; Strina, I.; Pastore, L.; Maruotti, G.M.; Alviggi, C. Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges. Genes 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.; Chitty, L.S. Beyond screening for chromosomal abnormalities: Advances in non-invasive diagnosis of single gene disorders and fetal exome sequencing. Semin. Fetal Neonatal Med. 2018, 23, 94–101. [Google Scholar] [CrossRef] [PubMed]
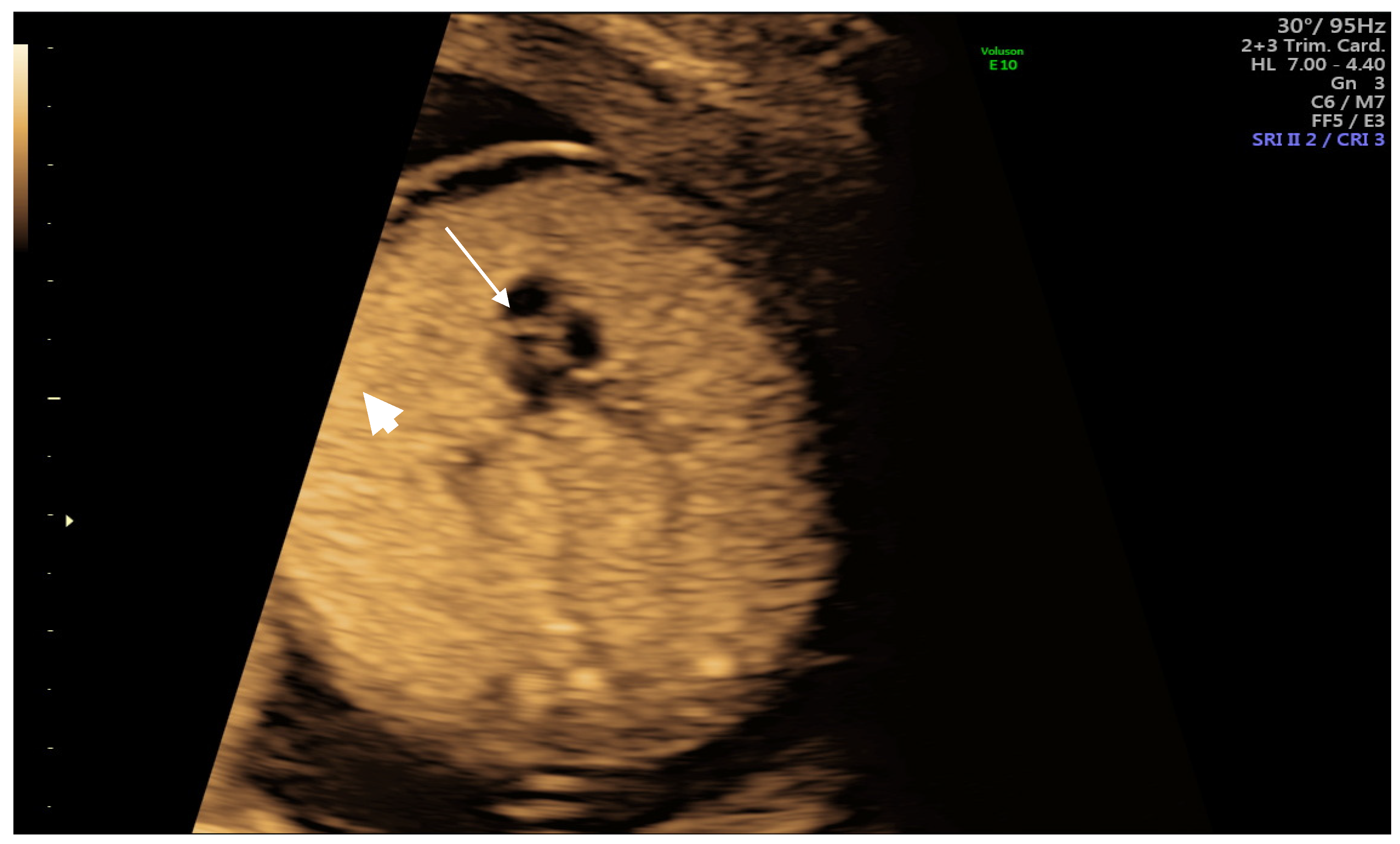
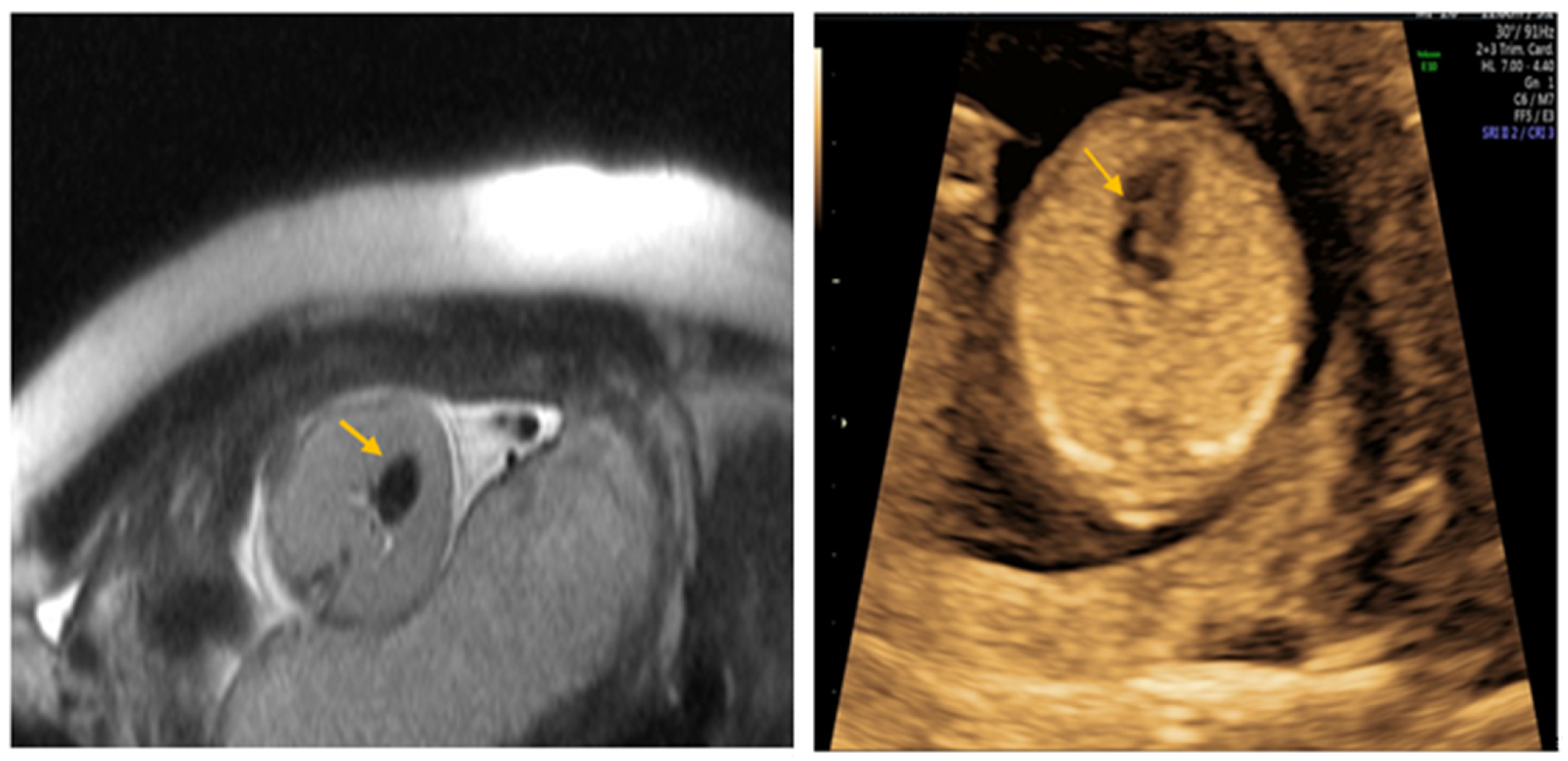
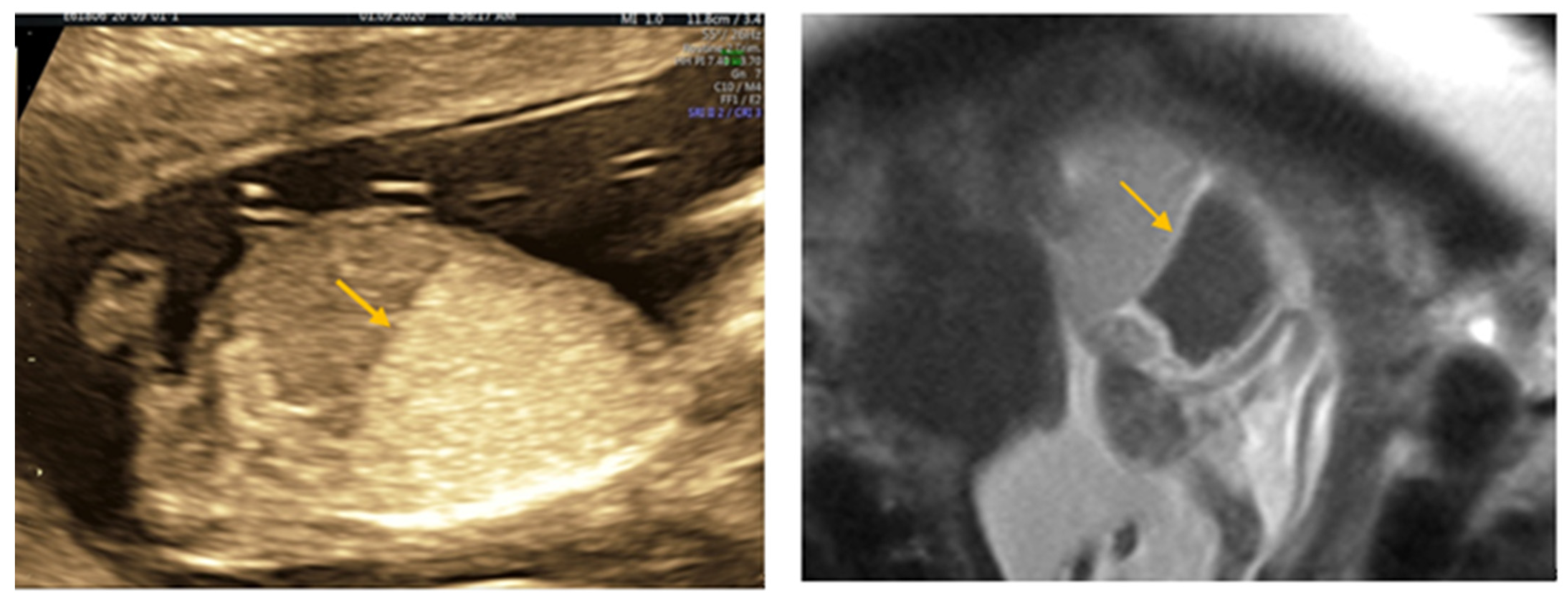

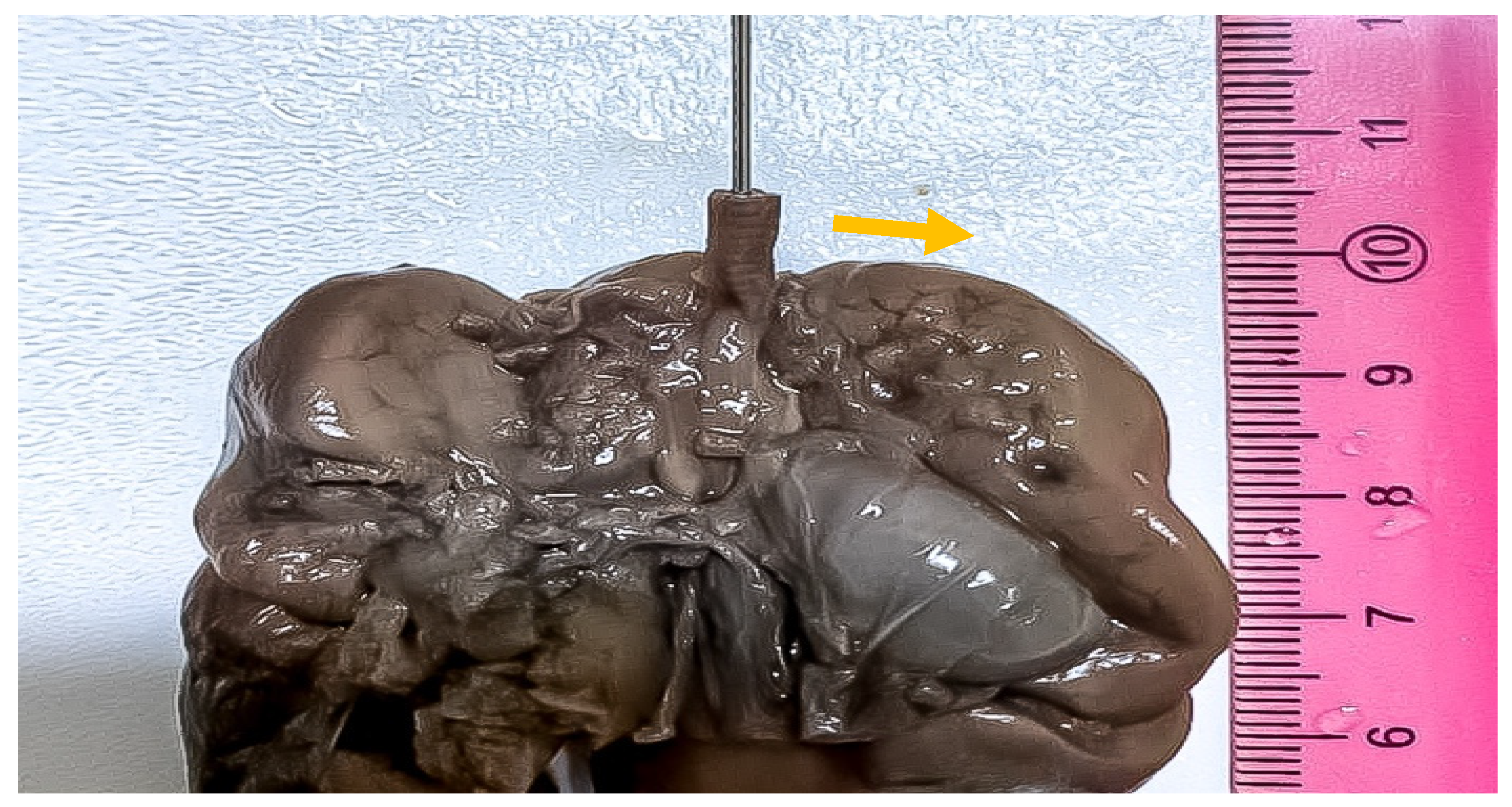
| Author | Year | Age | Weeks at Diagnosis | Weeks at Delivery | Outcome | |
|---|---|---|---|---|---|---|
| 1 | Jong [7] | 2020 | 31 | 23 | 37 | waiting surgery |
| 2 | Umesh [8] | 2017 | 27 | 24 | 24 | termination |
| 3 | Kornacki [9] | 2017 | 29 | 22 | 35 | failed tracheostomy, died |
| 4 | Simetka [10] | 2014 | 32 | 19 (DCDA) | 31 | exitus |
| 5 | Ulkumen [11] | 2013 | 31 | 17 | 18 | termination |
| 6 | Gonzales [12] | 2018 | 30 | 24 | 33 | laryngo tracheal reconstruction |
| 7 | Elliot [13] | 2013 | 44 | 22 (DCDA) | 36 | laryngo tracheal reconstruction |
| 8 | Saadai [14] | 2012 | 33 | 21 | 30 | Fetoscopy, EXIT, thrieving |
| 9 | 29 | 21 | 34 | EXIT, ventilator dependant at 18 months | ||
| 10 | 21 | 21 | termination | |||
| 11 | 21 | 21 | 35 | prune belly sdr. ventilator dependant | ||
| 12 | 19 | 19 | termination | |||
| 13 | Roybay [15] | 2010 | N/A | 20 | 31 | tracheal reconstruction at 16 months |
| 14 | 2010 | N/A | 20 | 31 | failed exit, exitus | |
| 15 | Oepkes [16] | 2003 | 33 | 26 | 37 | tracheoplasty, thrieving at 5 months |
| 16 | Lim [17] | 2003 | N/A | 19 | 31 | laryngo tracheal reconstruction, normal development at 5 years |
| 17 | present case | 2021 | 40 | 15 | 19 | exitus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, T.; Radoi, V.; Radulescu, M.; Ilian, A.; Toader, O.D.; Pop, L.G.; Bacalbasa, N. Prenatal Diagnosis and Outcome of Tracheal Agenesis as Part of Congenital High Airway Obstruction Syndrome. Case Presentation and Literature Review. Medicina 2021, 57, 1253. https://doi.org/10.3390/medicina57111253
Georgescu T, Radoi V, Radulescu M, Ilian A, Toader OD, Pop LG, Bacalbasa N. Prenatal Diagnosis and Outcome of Tracheal Agenesis as Part of Congenital High Airway Obstruction Syndrome. Case Presentation and Literature Review. Medicina. 2021; 57(11):1253. https://doi.org/10.3390/medicina57111253
Chicago/Turabian StyleGeorgescu, Tiberiu, Viorica Radoi, Micaela Radulescu, Aurora Ilian, Oana Daniela Toader, Lucian G. Pop, and Nicolae Bacalbasa. 2021. "Prenatal Diagnosis and Outcome of Tracheal Agenesis as Part of Congenital High Airway Obstruction Syndrome. Case Presentation and Literature Review" Medicina 57, no. 11: 1253. https://doi.org/10.3390/medicina57111253
APA StyleGeorgescu, T., Radoi, V., Radulescu, M., Ilian, A., Toader, O. D., Pop, L. G., & Bacalbasa, N. (2021). Prenatal Diagnosis and Outcome of Tracheal Agenesis as Part of Congenital High Airway Obstruction Syndrome. Case Presentation and Literature Review. Medicina, 57(11), 1253. https://doi.org/10.3390/medicina57111253






