Automated Medical Care: Bradycardia Detection and Cardiac Monitoring of Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Baseline Drift Removal
2.2. Denoising
2.3. Preterm Infant ECG Bradycardia Detection
2.4. Data Compression
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sbrollini, A.; Mancinelli, M.; Marcantoni, I.; Morettini, M.; Burattini, L. Bradycardia Assessment in Preterm Infants. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing—MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; pp. 100–107. [Google Scholar]
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menonf, R.; van Look, P.F.A. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World. Health. Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef] [PubMed]
- European Perinatal Health Report. Available online: https://www.europeristat.com/index.php/reports/european-perinatal-health-report-2015.html (accessed on 29 August 2021).
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; and Siahanidou, T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: A systematic review and meta-analysis. J. Pediatr. 2019, 210, e65. [Google Scholar] [CrossRef]
- Saavedra, L.P.J.; Prates, K.V.; Gonçalves, G.D.; Piovan, S.; Matafome, P.; Mathias, P.C.F. COVID-19 During Development: A Matter of Concern. Front. Cell Dev. Biol. 2021, 9, 659032. [Google Scholar] [CrossRef]
- Liao, L.; Deng, Y.; and Zhao, D. Association of low birth weight and premature birth with the risk of metabolic syndrome: A meta-analysis. Front. Pediatr. 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, W.N.; de Moraes, C.L.; dos Santos Rodrigues, A.P.; Noll, M.; Arruda, J.T.; Mendonça, C.R. Maternal Coronavirus Infections and Neonates Born to Mothers with SARS-CoV-2: A Systematic Review. Healthcare 2020, 8, 511. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Afshar, Y.; Boscardin, W.J.; Keller, R.L.; Mardy, A.H.; Prahl, M.K.; Phillips, C.T.; Asiodu, I.V.; Berghella, V.; Chambers, B.D.; et al. Infant Outcomes Following Maternal Infection with SARS-CoV-2: First Report from the PRIORITY Study. Clin. Infect. Dis. 2021, 73, e2810–e2813. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.Y.; Ho, L.C.; To, W.W.K.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef]
- Schwartz, A.D.; Graham, A.L. Potential Maternal and Infant Outcomes from Coronavirus 2019-nCoV (SARS-CoV-2) Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Suliman, K.; Peng, L.; Siddique, R.; Nabi, G.; Nawsherwan; Xue, M.; Liu, J.; Han, G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Cavicchiolo, M.E.; Lolli, E.; Trevisanuto, D.; Baraldi, E. Managing a tertiary-level NICU in the time of COVID-19: Lessons learned from a high-risk zone. Pediatric Pulmonol. 2020, 55, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ctvnews.ca/health/coronavirus/more-kids-hospitalized-with-covid-19-in-u-s-states-with-lower-vaccination-rates-cdc-report-finds-1.5573431 (accessed on 19 October 2021).
- Available online: https://www.mai.gov.ro/informare-covid-19-grupul-de-comunicare-strategica-19-octombrie-ora-13-00-2/ (accessed on 19 October 2021).
- Sulas, E.; Urru, M.; Tumbarello, R.; Raffo, L.; Pani, D. Automatic detection of complete and measurable cardiac cycles in antenatal pulsed-wave Doppler signals. Comput. Methods Programs Biomed. 2020, 190, 105336. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, X.; Chen, X.; Guo, J. Automatic Premature Ventricular Contraction Detection Using Deep Metric Learning and KNN. Biosensors 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Semenova, O.; Carra, G.; Lightbody, G.; Boylan, G.; Dempsey, E.; Temko, A. Prediction of short-term health outcomes in preterm neonates from heart-rate variability and blood pressure using boosted decision trees. Comput. Methods Programs Biomed. 2019, 180, 104996. [Google Scholar] [CrossRef]
- Davidson, E.; Simpson, C.R.; Demiris, G.; Sheikh, A.; McKinstry, B. Integrating Telehealth Care-Generated Data with the Family Practice Electronic Medical Record: Qualitative Exploration of the Views of Primary Care Staff. Interact. J. Med. Res. 2013, 2, e29. [Google Scholar] [CrossRef] [PubMed]
- Roelle, L.; Dalal, A.S.; Miller, N.; Orr, W.B.; van Hare, G.; Avari Silva, J.N. The impact of direct-to-consumer wearables in pediatric electrophysiology telehealth clinics: A real-world case series. Cardiovasc. Digit. Health J. 2020, 3, 169–171. [Google Scholar] [CrossRef]
- Anton, O.; Fernandez, R.; Rendon-Morales, E.; Aviles-Espinosa, R.; Jordan, H.; Rabe, H. Heart Rate Monitoring in Newborn Babies: A Systematic Review. Neonatology 2019, 116, 199–210. [Google Scholar] [CrossRef]
- Dima, M.; Enatescu, I.; Craina, M.; Petre, I.; Iacob, E.R.; Iacob, D. First neonates with severe acute respiratory syndrome coronavirus 2 infection in Romania: Three case reports. Medicine 2020, 99, e21284. [Google Scholar] [CrossRef]
- Fyfe, K.; Yiallourou, S.R.; Horne, R.S.C. Cardiovascular Consequences of Preterm Birth in the First Year of Life. In Preterm Birth—Mother and Child; Morrison, J., Ed.; Intech Open: Rijeka, Croatia, 2012; pp. 319–340. ISBN 978-953-307-828-1. [Google Scholar] [CrossRef]
- Collada, A.; Mayer, C.A.; MacFarlane, P.M. Blood and urine biomarkers associated with long-term respiratory dysfunction following neonatal hyperoxia exposure: Implications for prematurity and risk of SIDS. Respir. Physiol. Neurobiol. 2020, 279, 103465. [Google Scholar] [CrossRef] [PubMed]
- Durankus, F.; Ciftdemir, N.A.; Ozbek, U.V.; Duran, R.; Acunas, B. Comparison of Sleep Problems Between Term and Preterm Born Preschool Children. Sleep Med. 2020, 75, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Cailleau, L.; Weber, R.; Cabon, S.; Flamant, C.; Roué, J.M.; Favrais, G.; Gascoin, G.; Thollot, A.; Esvan, M.; Porée, F.; et al. Quiet Sleep Organization of Very Preterm Infants Is Correlated with Postnatal Maturation. Front. Pediatr. 2020, 8, 559658. [Google Scholar] [CrossRef]
- Qiu, C.; Ma, C.; Fan, N.; Zhang, X.; Zheng, G. Comparative efficacy of pulmonary surfactant in respiratory distress syndrome in preterm infants: A Bayesian network meta-analysis. Arch. Med. Sci. 2020. Manuscript accepted. [Google Scholar] [CrossRef]
- Altuve, M.; Carrault, G.; Beuchee, A.; Pladys, P.; Hernandez, A.I. On-line apnea-bradycardia detection using hidden semi-Markov models. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August—3 September 2011; pp. 4374–4377. [Google Scholar] [CrossRef]
- Handoka, N.M.; Azzam, M.; Gobarah, A. Predictors of early synchronized non-invasive ventilation failure for infants < 32 weeks of gestational age with respiratory distress syndrome. Arch. Med. Sci. 2019, 15, 680–687. [Google Scholar]
- Gee, A.H.; Barbieri, R.; Paydarfar, D.; Indic, P. Predicting Bradycardia in Preterm Infants Using Point Process Analysis of Heart Rate. IEEE Trans. Biomed. Eng. 2017, 64, 2300–2308. [Google Scholar] [CrossRef]
- Rotariu, C.; Pasarica, A.; Costin, H.; Nemescu, D. Spectral analysis of fetal heart rate variability associated with fetal acidosis and base deficit values. In Proceedings of the 2014 International Conference on Development and Application Systems (DAS), Suceava, Romania, 15–17 May 2014; pp. 210–213. [Google Scholar] [CrossRef]
- Chiera, M.; Cerritelli, F.; Casini, A.; Barsotti, N.; Boschiero, D.; Cavigioli, F.; Corti, C.G.; Manzotti, A. Heart Rate Variability in the Perinatal Period: A Critical and Conceptual Review. Front. Neurosci. 2020, 14, 561186. [Google Scholar] [CrossRef] [PubMed]
- Thiriez, G.; Mougey, C.; Vermeylen, D.; Wermenbol, V.; Lanquart, J.P.; Lin, J.S.; Franco, P. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin. Auton. Res. 2015, 25, 233–242. [Google Scholar] [CrossRef]
- Dimitrijević, L.; Bjelakovic, B.; Colovic, H.; Mikov, A.; Vesna, Z.; Kocic, M.; Lukic, S. Assessment of general movements and heart rate variability in prediction of neurodevelopmental outcome in preterm infants. Early Hum. Dev. 2016, 99, 7–12. [Google Scholar] [CrossRef]
- Frasch, M.G. Saving the brain one heartbeat at a time. J. Physiol. 2018, 596, 5503–5504. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Lear, C.; Beacom, M.; Ikeda, T.; Gunn, A.; Bennet, L. Evolving changes in fetal heart rate variability and brain injury after hypoxia-ischemia in preterm fetal sheep. J. Physiol. 2018, 596, 6093–6104. [Google Scholar] [CrossRef]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart Rate Variability for Risk Assessment of Myocardial Ischemia in Patients Without Known Coronary Artery Disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef] [PubMed]
- Fyfe-Johnson, A.; Muller, C.; Alonso, A.; Folsom, A.; Gottesman, R.; Rosamond, W.; Whitsel, E.; Agarwal, S.; MacLehose, R. Heart Rate Variability and Incident Stroke: The Atherosclerosis Risk in Communities Study. Stroke 2016, 47, 1452–1458. [Google Scholar] [CrossRef]
- Kleiger, R.; Stein, P.; Bigger, J. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Dos Santos Beozzo, G.P.N.; de Carvalho, W.B.; Krebs, V.L.J.; Gibelli, M.A.B.C.; Zacharias, R.S.B.; Rossetto, L.E.S.; Francisco, R.P.V. Neonatal manifestations in COVID-19 patients at a Brazilian tertiary center. Clinics 2020, 75, e2407. [Google Scholar] [CrossRef] [PubMed]
- Sanna, G.; Serrau, G.; Bassareo, P.P.; Neroni, P.; Fanos, V.; Marcialis, M.A. Children’s heart and COVID-19: [3] Up-to-date evidence in the form of a systematic review. Eur. J. Pediatr. 2020, 179, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Amaratunga, E.A.; Corwin, D.S.; Moran, L.; Snyder, R. Bradycardia in Patients with COVID-19: A Calm before the Storm? Cureus 2020, 12, e8599. [Google Scholar] [CrossRef] [PubMed]
- Barkas, F.; Styla, C.P.; Bechlioulis, A.; Milionis, H.; Liberopoulos, E. Sinus Bradycardia Associated with Remdesivir Treatment in COVID-19: A Case Report and Literature Review. J. Cardiovasc. Dev. Dis. 2021, 8, 18. [Google Scholar] [CrossRef]
- Zhou, M.; Wong, C.K.; Un, K.C.; Lau, Y.M.; Lee, J.C.Y.; Tam, F.C.C.; Lua, Y.M.; Lai, W.H.; Tam, A.R.; Lam, Y.Y.; et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS ONE 2021, 16, e0246732. [Google Scholar]
- Chinitz, J.S.; Goyal, R.; Harding, M.; Veseli, G.; Gruberg, L.; Jadonath, R.; Maccaro, P.; Gandotra, P.; Ong, L.; Epstein, L.M. Bradyarrhythmias in patients with COVID-19: Marker of poor prognosis? Pacing Clin. Electrophysiol. 2020, 43, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Capoferri, G.; Osthoff, M.; Egli, A.; Stoeckle, M.; Bassetti, S. Relative bradycardia in patients with coronavirus disease 2019 (COVID-19). Clin. Microbiol. Infect. 2021, 27, 295–296. [Google Scholar] [CrossRef]
- Douedi, S.; Mararenko, A.; Alshami, A.; Al-Azzawi, M.; Ajam, F.; Patel, S.; Douedi, H.; Calderon, D. COVID-19 Induced Bradyarrhythmia and Relative Bradycardia: An Overview. J. Arrhythm. 2021, 37, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Andrew, L.; Dailey-Schwartz, M.D.; Jameson, A.; Dyal, M.D.; William, T.; Mahle, M.D.; Matthew, E.; Oster, M.D. MPH Implementation of a Practice Plan for the Out-patient Cardiac Evaluation of Children after Acute SARS-CoV-2 Infection and a Report of Outcomes. Am. Heart J. 2021, 241, 83–86. [Google Scholar] [CrossRef]
- Kilicaslan, O.; Isancli, D.K.; Ulutas, O.Y.; Ergin, S.O.; Karbuz, A. A case of bradycardia during SARS CoV-2 infection in a 14-year-old child. Infect. Dis. 2021, 53, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.G.; Cohen, M.I.; Levorson, R.E.; Tzeng, M.B. COVID-19 associated multisystem inflammatory syndrome in children presenting uniquely with sinus node dysfunction in the setting of shock. Cardiol. Young 2021, 31, 1202–1204. [Google Scholar] [CrossRef]
- Ikeuchi, K.; Saito, M.; Yamamoto, S.; Nagai, H.; Adachi, E. Relative Bradycardia in Patients with Mild-to-Moderate Coronavirus Disease, Japan. Emerg. Infect. Dis. 2020, 26, 2504–2506. [Google Scholar] [CrossRef]
- Hallberg, T.C.; Bjorklund, A.R.; Slusher, T.M.; Rodgers, N. Sinus bradycardia in a toddler with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19. BMJ Case Rep. 2021, 14, e242058. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Available online: https://archive.physionet.org/physiobank/database/picsdb/ (accessed on 15 April 2021).
- Zhu, Z.; Liu, T.; Li, G.; Li, T.; Inoue, Y. Wearable sensor systems for infants. Sensors 2015, 15, 3721–3749. [Google Scholar] [CrossRef] [PubMed]
- Cresi, F.; Cocchi, E.; Maggiora, E.; Pirra, A.; Logrippo, F.; Ariotti, M.C.; Peila, C.; Bertino, E.; Coscia, A. Pre-discharge Cardiorespiratory Monitoring in Preterm Infants. The CORE Study. Front. Pediatr. 2020, 8, 234. [Google Scholar] [CrossRef]
- Vollmer, M. Robust, Simple and Reliable Measure of Heart Rate Variability using Relative RR Intervals. In Proceedings of the Computing in Cardiology Conference, Nice, France, 6–9 September 2015; pp. 609–612. [Google Scholar] [CrossRef]
- Mix, D.F.; Olejniczak, K.J. Elements of Wavelets for Engineers and Scientists; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Graps, A. An Introduction to Wavelets. IEEE Comput. Sci. Eng. 1995, 2, 50–61. [Google Scholar] [CrossRef]
- Arvinti, B.; Costache, M. Adaptive Thresholding Algorithm for Noisy Electrocardiograms using Reverse Biorthogonal Mother Wavelets. In Proceedings of the 13th International IEEE Symposium on Electronics and Telecommunications (ISETC), Timisoara, Romania, 8–9 November 2018; pp. 277–280. [Google Scholar] [CrossRef]
- Larson, D.R. Unitary Systems and wavelet sets. In Wavelet Analysis and Applications; Tao, Q., Mang, I.V., Yuesheng, X., Eds.; Birkhäuser: Basel, Switzerland, 2007; pp. 143–171. [Google Scholar]
- Arvinti, B.; Nafornita, C.; Isar, A.; Costache, M. ECG signal compression using wavelets. Preliminary results. In Proceedings of the ISSCS 2011—International Symposium on Signals, Circuits and Systems, Iasi, Romania, 30 June–1 July 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Holschneider, M.; Kronland-Martinet, R.; Morlet, J.; Tchamitchian, P. A real-time algorithm for signal analysis with the help of the wavelet transform. In Wavelets, Time-Frequency Methods and Phase Space; Springer-Verlag: New York, NY, USA, 1989; pp. 289–297. [Google Scholar]
- Chen, S.W.; Chen, Y.H. Hardware Design and Implementation of a Wavelet De-Noising Procedure for Medical Signal Preprocessing. Sensors 2015, 15, 26396–26414. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Jbari, A.; Bourouhou, A. ECG Signal Denoising by Discrete Wavelet Transform. Int. J. Online Eng. 2017, 13, 51–68. [Google Scholar] [CrossRef]
- Li, S.; Jiang, S.; Jiang, S.; Wu, J.; Xiong, W.; Diao, S. A Hybrid Wavelet-Based Method for the Peak Detection of Photoplethysmography Signals. Comput. Math. Methods. Med. 2017, 2017, 9468503. [Google Scholar] [CrossRef] [PubMed]


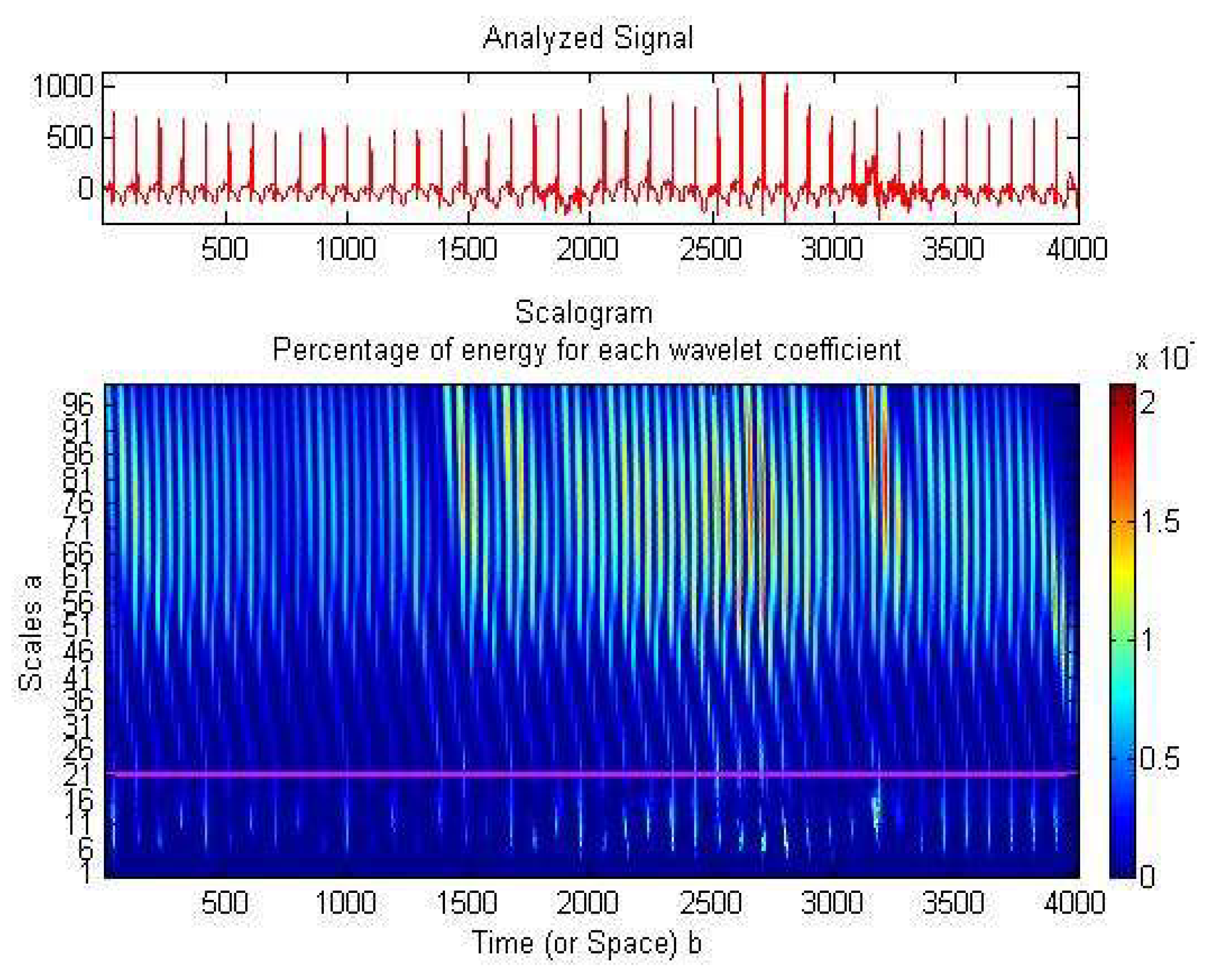
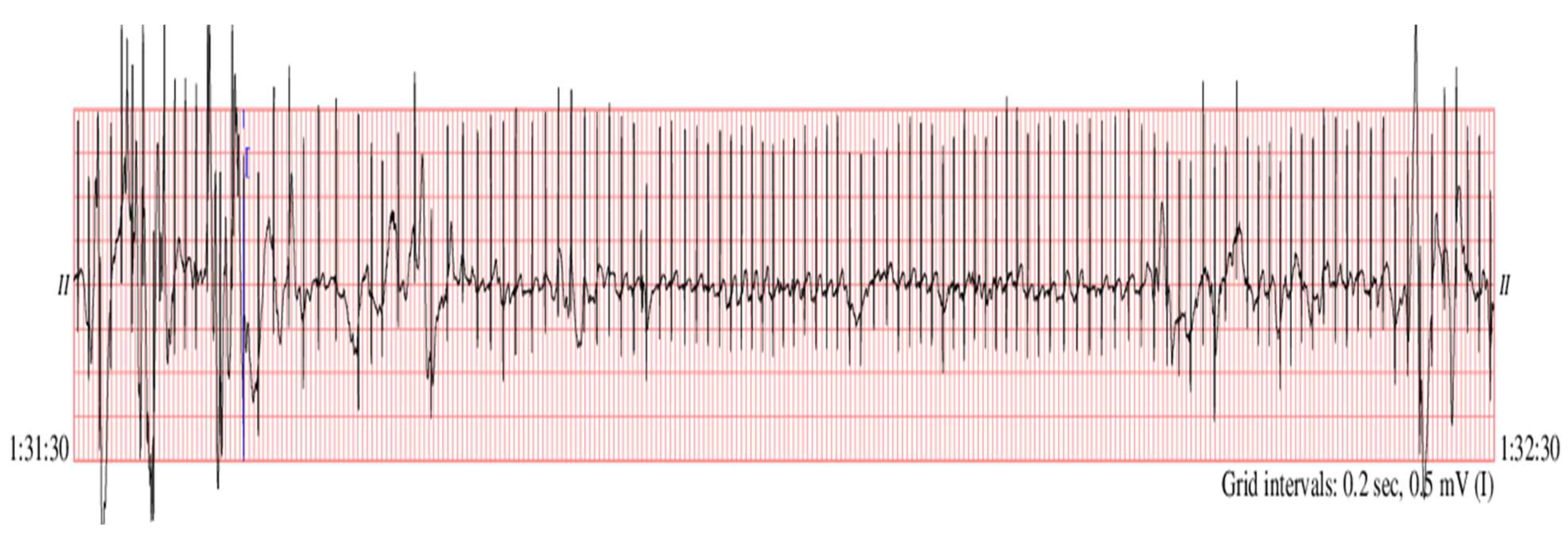
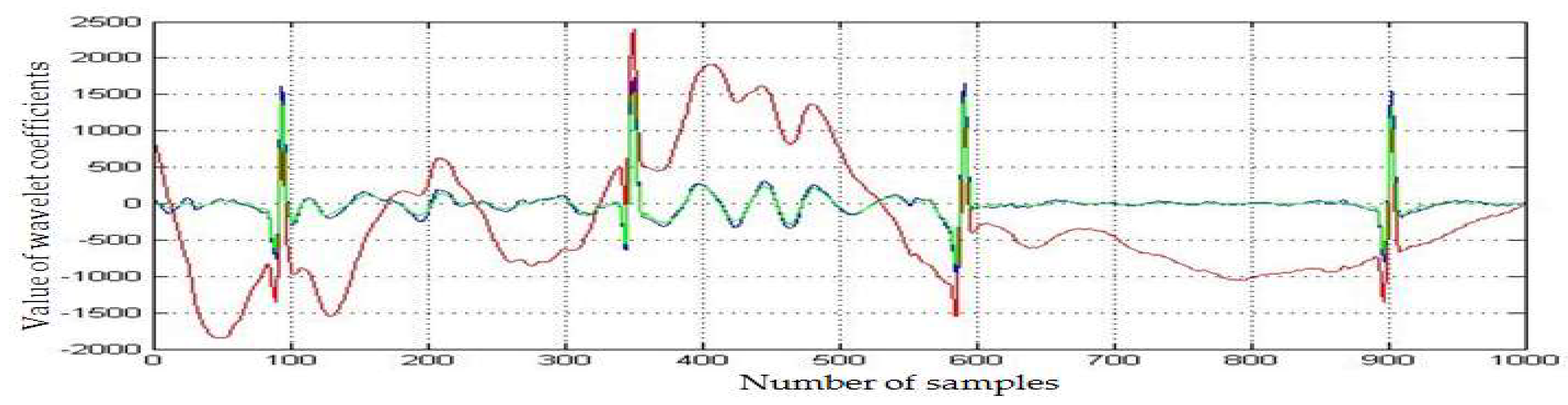
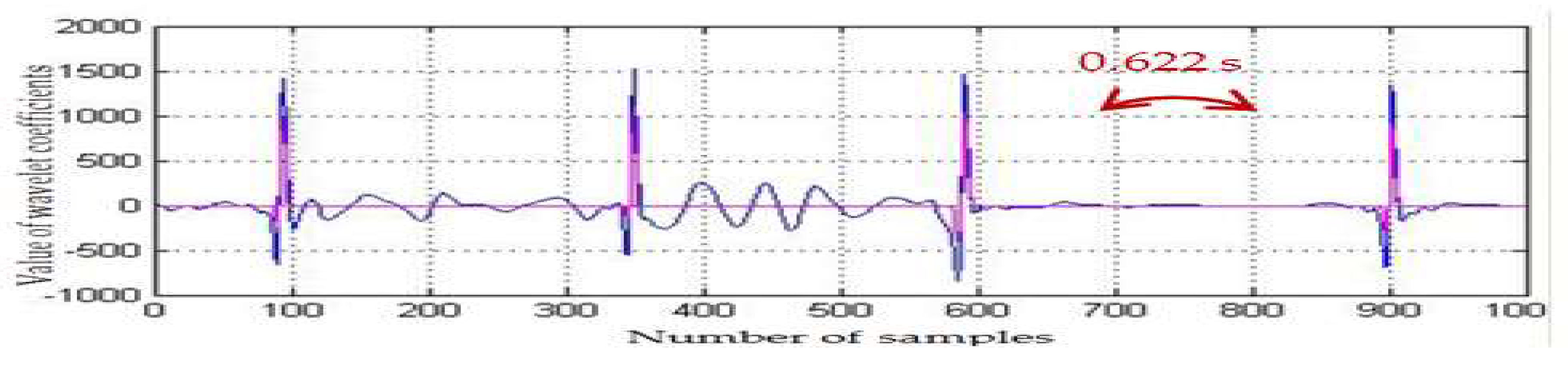


| ECG | Detected RR-Interval > 0.6 (s) (1000 Samples) | CR Values (1000 Samples) | Statistical Parameters | |
|---|---|---|---|---|
| SNR Improvement (dB) | RMSSD (ms) (1000 Samples) | |||
| infant1_ecgm | 0.67, 0.68, 0.67 | 2.57 | 4.07 | 76.48 |
| infant2_ecgm | 0.612 | 3.07 | 1.34 | 18.38 |
| infant3_ecgm | 0.614 | 6.59 | 4.95 | 42.58 |
| infant4_ecgm | 1.27 | 3.79 | 2.84 | 654.38 |
| infant5_ecgm | 0.624, 0.636 | 1.72 | 6.42 | 60.56 |
| infant6_ecgm | 0.622 | 7.42 | 10.18 | 82.67 |
| infant7_ecgm | 0.72 | 2.92 | 1.69 | 165.46 |
| infant8_ecgm | 0.74 | 2.99 | 1.86 | 249.89 |
| infant9_ecgm | 0.618, 0.802 | 4.44 | 3.71 | 130.107 |
| infant10_ecgm | 0.688 | 5.30 | 4.91 | 195.161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvinti, B.; Iacob, E.R.; Isar, A.; Iacob, D.; Costache, M. Automated Medical Care: Bradycardia Detection and Cardiac Monitoring of Preterm Infants. Medicina 2021, 57, 1199. https://doi.org/10.3390/medicina57111199
Arvinti B, Iacob ER, Isar A, Iacob D, Costache M. Automated Medical Care: Bradycardia Detection and Cardiac Monitoring of Preterm Infants. Medicina. 2021; 57(11):1199. https://doi.org/10.3390/medicina57111199
Chicago/Turabian StyleArvinti, Beatrice, Emil Radu Iacob, Alexandru Isar, Daniela Iacob, and Marius Costache. 2021. "Automated Medical Care: Bradycardia Detection and Cardiac Monitoring of Preterm Infants" Medicina 57, no. 11: 1199. https://doi.org/10.3390/medicina57111199
APA StyleArvinti, B., Iacob, E. R., Isar, A., Iacob, D., & Costache, M. (2021). Automated Medical Care: Bradycardia Detection and Cardiac Monitoring of Preterm Infants. Medicina, 57(11), 1199. https://doi.org/10.3390/medicina57111199






