Beneficial Effects of Robot-Assisted Gait Training on Functional Recovery in Women after Stroke: A Cohort Study
Abstract
1. Introduction
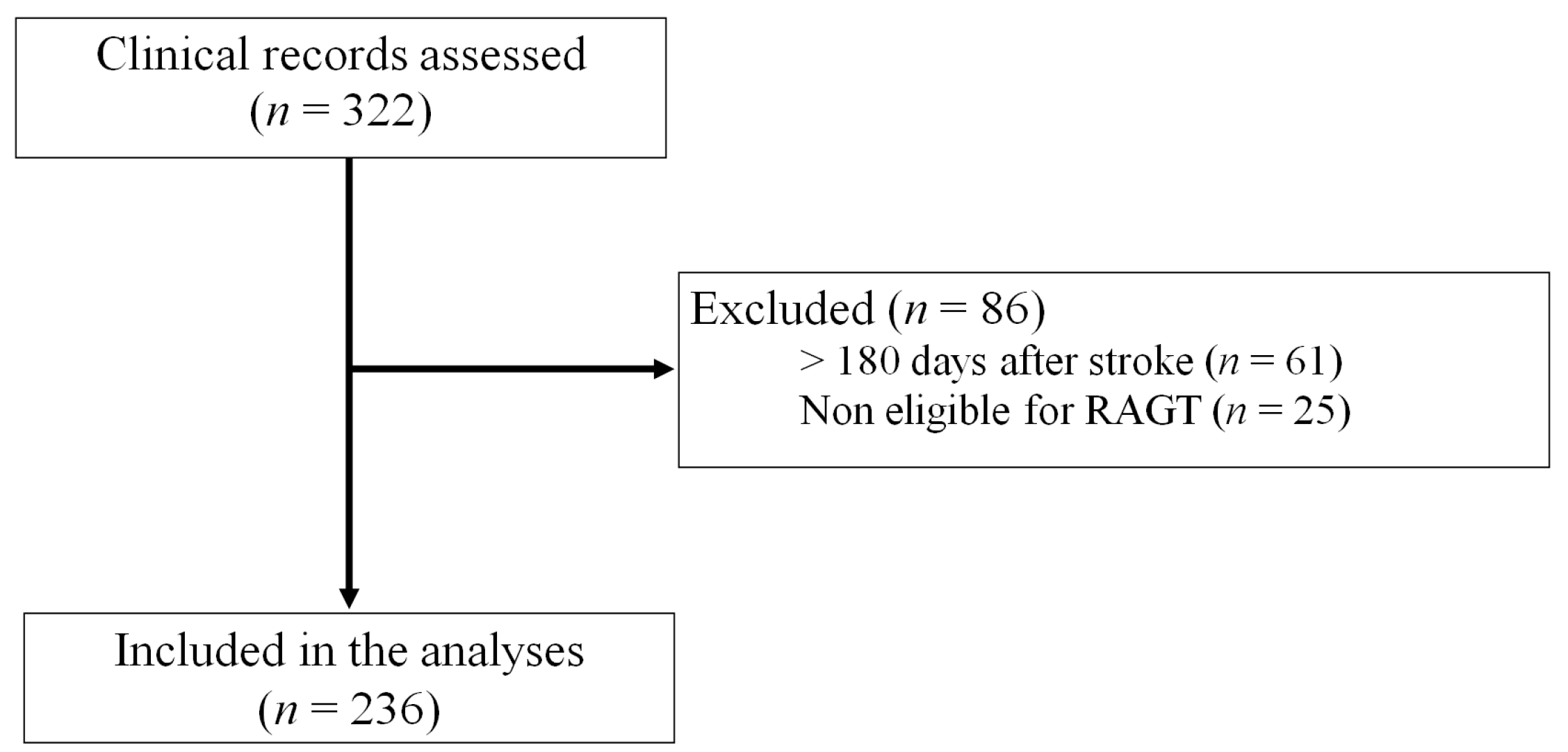
2. Materials and Methods
2.1. Subjects
2.2. Interventions
2.3. Outcome Parameters
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics at the Admission of Rehabilitation
3.2. Differences in Rehabilitation Treatments
3.3. Comparison of Outcomes
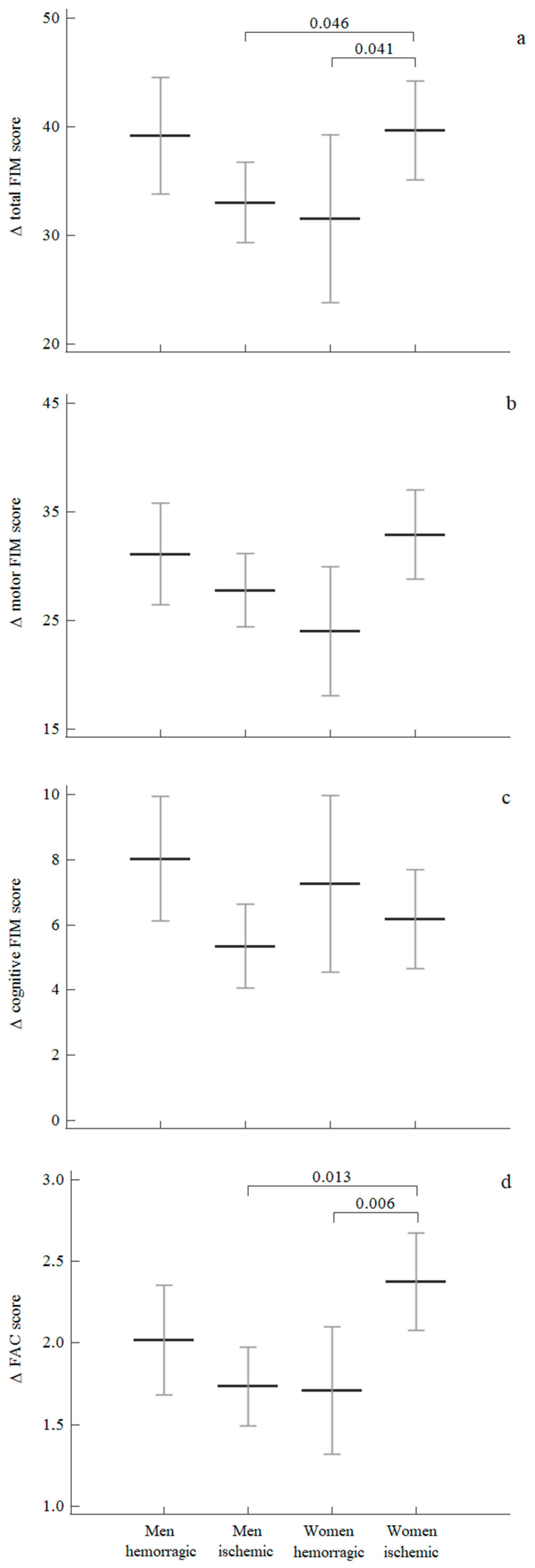
3.4. Sex Differences According to the Type of Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Gall, S.; Blizzard, C.L.; Lannin, N.A.; Thrift, A.G.; Anderson, C.S.; Kim, J.; Grimley, R.; Castley, H.C.; Kilkenny, M.F.; et al. Sex Differences in Causes of Death After Stroke: Evidence from a National, Prospective Registry. J. Women Health 2021, 30, 314–323. [Google Scholar] [CrossRef]
- Carcel, C.; Wang, X.; Sandset, E.C.; Delcourt, C.; Arima, H.; Lindley, R.; Hackett, M.L.; Lavados, P.; Robinson, T.G.; Venturelli, P.M.; et al. Sex differences in treatment and outcome after stroke. Neurology 2019, 93, e2170–e2180. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Reeves, M.J. Sex Differences in Stroke Recovery and Stroke-Specific Quality of Life. Stroke 2007, 38, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.L.; Tran, P.L.; Martin, K.; Blizzard, L.; Srikanth, V. Sex Differences in Long-Term Outcomes After Stroke. Stroke 2012, 43, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Asplund, K.; Glader, E.-L.; Norrving, B.; Stegmayr, B.; Terént, A.; Åsberg, K.H.; Wester, P.-O. Self-Reported Depression and Use of Antidepressants after Stroke: A National Survey. Stroke 2004, 35, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Glader, E.-L.; Stegmayr, B.; Asplund, K. Poststroke Fatigue. Stroke 2002, 33, 1327–1333. [Google Scholar] [CrossRef]
- Taveggia, G.; Borboni, A.; Mulé, C.; Villafañe, J.H.; Negrini, S. Conflicting results of robot-assisted versus usual gait training during postacute rehabilitation of stroke patients. Int. J. Rehabil. Res. 2016, 39, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.J.; Tang, P.-F. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Morton, M.G.; Wikholm, J.B. Importance of four variables of walking to patients with stroke. Int. J. Rehabil. Res. 1991, 14, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Warraich, Z.; Kleim, J.A. Neural Plasticity: The Biological Substrate for Neurorehabilitation. PM R 2010, 2, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 2017, CD002840. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Wagenaar, R.C.; Twisk, J.W.; Lankhorst, G.J.; Koetsier, J.C. Intensity of leg and arm training after primary middle-cerebral-artery stroke: A randomised trial. Lancet 1999, 354, 191–196. [Google Scholar] [CrossRef]
- Van Peppen, R.P.S.; Kwakkel, G.; Wood-Dauphinee, S.; Hendriks, H.J.M.; Van Der Wees, P.J.; Dekker, J. The impact of physical therapy on functional outcomes after stroke: What’s the evidence? Clin. Rehabil. 2004, 18, 833–862. [Google Scholar] [CrossRef]
- Laffont, I.; Bakhti, K.; Coroian, F.; van Dokkum, L.; Mottet, D.; Schweighofer, N.; Froger, J. Innovative technologies applied to sensorimotor rehabilitation after stroke. Ann. Phys. Rehabil. Med. 2014, 57, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.; van Wegen, E.; Van Peppen, R.; Van Der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What Is the Evidence for Physical Therapy Poststroke? A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-Weight–Supported Treadmill Rehabilitation after Stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef]
- Cho, J.-E.; Yoo, J.S.; Kim, K.E.; Cho, S.T.; Jang, W.S.; Cho, K.H.; Lee, W.-H. Systematic Review of Appropriate Robotic Intervention for Gait Function in Subacute Stroke Patients. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Diedrichsen, J.; Flanagan, J.R. Principles of sensorimotor learning. Nat. Rev. Neurosci. 2011, 12, 739–751. [Google Scholar] [CrossRef]
- Dierick, F.; Dehas, M.; Isambert, J.-L.; Injeyan, S.; Bouché, A.-F.; Bleyenheuft, Y.; Portnoy, S. Hemorrhagic versus ischemic stroke: Who can best benefit from blended conventional physiotherapy with robotic-assisted gait therapy? PLoS ONE 2017, 12, e0178636. [Google Scholar] [CrossRef]
- Iosa, M.; Morone, G.; Bragoni, M.; De Angelis, D.; Venturiero, V.; Coiro, P.; Pratesi, L.; Paolucci, S. Driving electromechanically assisted Gait Trainer for people with stroke. J. Rehabil. Res. Dev. 2011, 48, 135. [Google Scholar] [CrossRef]
- Straudi, S.; Manfredini, F.; Lamberti, N.; Martinuzzi, C.; Maietti, E.; Basaglia, N. Robot-assisted gait training is not superior to intensive overground walking in multiple sclerosis with severe disability (the RAGTIME study): A randomized controlled trial. Mult. Scler. J. 2019, 26, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Wall, A.; Borg, J.; Palmcrantz, S. Self-perceived functioning and disability after randomized conventional and electromechanically-assisted gait training in subacute stroke: A 6 months follow-up. NeuroRehabilitation 2019, 45, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Cheng, Y.-H.; Lai, C.-H.; Lin, Y.-N. Clinical non-superiority of technology-assisted gait training with body weight support in patients with subacute stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2020, 63, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of robotic gait training after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2020, 63, 518–534. [Google Scholar] [CrossRef]
- Arthur, H.M.; Blanchard, C.; Gunn, E.; Kodis, J.; Walker, S.; Toner, B. Exercise Trajectories of Women from Entry to a 6-Month Cardiac Rehabilitation Program to One Year after Discharge. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Foy, C.G.; Rejeski, W.J.; Berry, M.J.; Zaccaro, D.; Woodard, C.M. Gender Moderates the Effects of Exercise Therapy on Health-Related Quality of Life Among COPD Patients. Chest 2001, 119, 70–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dörenkamp, S.; Mesters, E.; De Bie, R.; Teijink, J.A.W.; Van Breukelen, G. Patient Characteristics and Comorbidities Influence Walking Distances in Symptomatic Peripheral Arterial Disease: A Large One-Year Physiotherapy Cohort Study. PLoS ONE 2016, 11, e0146828. [Google Scholar] [CrossRef]
- Gommans, L.N.; Scheltinga, M.R.; van Sambeek, M.R.; Maas, A.H.; Bendermacher, B.L.; Teijink, J.A. Gender differences following supervised exercise therapy in patients with intermittent claudication. J. Vasc. Surg. 2015, 62, 681–688. [Google Scholar] [CrossRef]
- Manfredini, R.; Lamberti, N.; Manfredini, F.; Straudi, S.; Fabbian, F.; Borrego, M.A.R.; Basaglia, N.; Torres, J.M.C.; Soto, P.J.L. Gender Differences in Outcomes Following a Pain-Free, Home-Based Exercise Program for Claudication. J. Women Health 2019, 28, 1313–1321. [Google Scholar] [CrossRef]
- Hyun, K.; Negrone, A.; Redfern, J.; Atkins, E.; Chow, C.; Kilian, J.; Rajaratnam, R.; Brieger, D. Gender Difference in Secondary Prevention of Cardiovascular Disease and Outcomes Following the Survival of Acute Coronary Syndrome. Hear. Lung Circ. 2021, 30, 121–127. [Google Scholar] [CrossRef]
- Thilarajah, S.; Mentiplay, B.F.; Bower, K.J.; Tan, D.; Pua, Y.H.; Williams, G.; Koh, G.; Clark, R.A. Factors Associated With Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1876–1889. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.-F.; Huang, Y.-C.; Chiu, H.-Y.; Chang, H.-J.; Huang, H.-C. Systematic Review and Meta-Analysis of Home-Based Rehabilitation on Improving Physical Function among Home-Dwelling Patients with a Stroke. Arch. Phys. Med. Rehabil. 2020, 101, 359–373. [Google Scholar] [CrossRef]
- Hay, C.C.; Graham, J.E.; Pappadis, M.R.; Sander, A.M.; Hong, I.; Reistetter, T.A. The Impact of One’s Sex and Social Living Situation on Rehabilitation Outcomes After a Stroke. Am. J. Phys. Med. Rehabil. 2020, 99, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, A.; Insalata, G.; Papi, G.; Rinnoci, V.; Donnini, I.; Martini, M.; Falsini, C.; Hakiki, B.; Romoli, A.; Barbato, C.; et al. Gender differences in post-stroke functional outcome at discharge from an intensive rehabilitation hospital. Eur. J. Neurol. 2021, 28, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Iosa, M.; Marinozzi, F.; D’Antonio, E.; Poli, P.; Masiero, S.; Molinari, M.; Paolucci, S. Effectiveness of Robotic Assisted Walking Therapy: The Role of Age and Sex. In Biosystems Biorobotics; Springer: Amsterdam, The Netherlands, 2014; Volume 7, pp. 569–573. [Google Scholar]
- Sohrabji, F.; Park, M.J.; Mahnke, A.H. Sex differences in stroke therapies. J. Neurosci. Res. 2017, 95, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Van Hedel, H.J.A.; Network, F.T.A.; Severini, G.; Scarton, A.; O’Brien, A.; Reed, T.; Gaebler-Spira, D.; Egan, T.; Meyer-Heim, A.; Graser, J.; et al. Advanced Robotic Therapy Integrated Centers (ARTIC): An international collaboration facilitating the application of rehabilitation technologies. J. Neuroeng. Rehabil. 2018, 15, 30. [Google Scholar] [CrossRef]
- Lexell, J.; Brogårdh, C. The use of ICF in the neurorehabilitation process. Neurorehabilitation 2015, 36, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Beninato, M.; Gill-Body, K.M.; Salles, S.; Stark, P.C.; Black-Schaffer, R.M.; Stein, J. Determination of the Minimal Clinically Important Difference in the FIM Instrument in Patients With Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 32–39. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive Validity and Responsiveness of the Functional Ambulation Category in Hemiparetic Patients after Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Persky, R.W.; Turtzo, L.C.; McCullough, L.D. Stroke in Women: Disparities and Outcomes. Curr. Cardiol. Rep. 2010, 12, 6–13. [Google Scholar] [CrossRef]
- White, B.M.; Magwood, G.S.; Burns, S.P.; Ellis, C., Jr. Sex Differences in Patient-Reported Poststroke Disability. J. Women Health 2018, 27, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Tanlaka, E.; King-Shier, K.; Green, T.; Seneviratne, C.; Dukelow, S. Sex Differences in Stroke Rehabilitation Care in Alberta. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2020, 47, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.; Eng, J.; Liu-Ambrose, T.; Richardson, J.; MacDermid, J.; Tang, A. Sex differences in the effects of exercise on cognition post-stroke: Secondary analysis of a randomized controlled trial. J. Rehabil. Med. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Battista, P.; Guida, P.; Lanzillo, B.; Tortelli, R. Sex Differences in Long-Term Mortality and Functional Outcome After Rehabilitation in Patients With Severe Stroke. Front. Neurol. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; DeRuyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Tedla, J.S.; Dixit, S.; Gular, K.; Abohashrh, M. Robotic-Assisted Gait Training Effect on Function and Gait Speed in Subacute and Chronic Stroke Population: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Neurol. 2019, 81, 103–111. [Google Scholar] [CrossRef]
- Perna, R.; Temple, J. Rehabilitation Outcomes: Ischemic versus Hemorrhagic Strokes. Behav. Neurol. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Furie, K.L.; Shafqat, S.; Rallis, N.; Chang, Y.; Stein, J. Functional recovery following rehabilitation after hemorrhagic and ischemic stroke. Arch. Phys. Med. Rehabil. 2003, 84, 968–972. [Google Scholar] [CrossRef]
- Paolucci, S.; Antonucci, G.; Grasso, M.G.; Bragoni, M.; Coiro, P.; De Angelis, D.; Fusco, F.R.; Morelli, D.; Venturiero, V.; Troisi, E.; et al. Functional Outcome of Ischemic and Hemorrhagic Stroke Patients After Inpatient Rehabilitation. Stroke 2003, 34, 2861–2865. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.M.; McCullough, M.L.D. Age and Sex Are Critical Factors in Ischemic Stroke Pathology. Endocrinology 2018, 159, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, N.; Straudi, S.; Malagoni, A.M.; Argirò, M.; Felisatti, M.; Nardini, E.; Zambon, C.; Basaglia, N.; Manfredini, F. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: A pilot randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Malagoni, A.M.; Cavazza, S.; Ferraresi, G.; Grassi, G.; Felisatti, M.; Lamberti, N.; Basaglia, N.; Manfredini, F. Effects of a “test in-train out” walking program versus supervised standard rehabilitation in chronic stroke patients: A feasibility and pilot randomised study. Eur. J. Phys. Rehabil. Med. 2016, 52, 279–287. [Google Scholar]
- Saunders, D.H.; Sanderson, M.; Hayes, S.; Johnson, L.; Kramer, S.; Carter, D.D.; Jarvis, H.; Brazzelli, M.; Mead, G.E. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2020, 2020, CD003316. [Google Scholar] [CrossRef]
| Women (n = 91) | Men (n = 145) | Between-Group p | |
|---|---|---|---|
| Age, years | 64 (59–67) | 64 (61–66) | 0.74 |
| Ischemic stroke, n (%) | 56 (60) | 90 (62) | 0.68 |
| Haemorrhagic stroke, n (%) | 35 (40) | 55 (38) | 0.72 |
| Days since stroke | 35 (28–41) | 35 (30–43) | 0.69 |
| FIM, total score | 45 (35–51) | 46 (41–50) | 0.54 |
| FIM, motor component | 22 (19–25) | 20 (19–22) | 0.88 |
| FIM, cognitive component | 22 (19–25) | 24 (20–27) | 0.46 |
| FAC | 0.3 ± 0.7 | 0.4 ± 0.6 | 0.16 |
| Women (n = 91) | Men (n = 145) | Between-Group p in Variations Admission-Discharge | |||
|---|---|---|---|---|---|
| Admission | Discharge | Admission | Discharge | ||
| FIM, total | 45 (35–51) | 87 (79–92) ** | 46 (41–50) | 83 (78–91) ** | 0.75 |
| FIM, motor | 22 (19–25) | 56 (49–60) ** | 20 (19–22) | 54 (50–61) ** | 0.75 |
| FIM, cognitive | 22 (19–25) | 30 (29–31) ** | 24 (20–27) | 31 (30–21) ** | 0.97 |
| FAC | 0.3 ± 0.7 | 2.4 ± 1.3 ** | 0.4 ± 0.6 | 2.3 ± 1.4 ** | 0.11 |
| Women (n = 91) | Men (n = 145) | p | |
|---|---|---|---|
| ∆FIM, total score | 3.43 ± 2.87 | 2.89 ± 2.13 | 0.10 |
| ∆FIM, motor component | 2.80 ± 2.38 | 2.41 ± 1.92 | 0.15 |
| ∆FIM, cognitive component | 0.18 ± 0.27 | 0.14 ± 0.15 | 0.14 |
| ∆FAC | 0.20 ± 0.16 | 0.16 ± 0.14 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, N.; Manfredini, F.; Lissom, L.O.; Lavezzi, S.; Basaglia, N.; Straudi, S. Beneficial Effects of Robot-Assisted Gait Training on Functional Recovery in Women after Stroke: A Cohort Study. Medicina 2021, 57, 1200. https://doi.org/10.3390/medicina57111200
Lamberti N, Manfredini F, Lissom LO, Lavezzi S, Basaglia N, Straudi S. Beneficial Effects of Robot-Assisted Gait Training on Functional Recovery in Women after Stroke: A Cohort Study. Medicina. 2021; 57(11):1200. https://doi.org/10.3390/medicina57111200
Chicago/Turabian StyleLamberti, Nicola, Fabio Manfredini, Luc Oscar Lissom, Susanna Lavezzi, Nino Basaglia, and Sofia Straudi. 2021. "Beneficial Effects of Robot-Assisted Gait Training on Functional Recovery in Women after Stroke: A Cohort Study" Medicina 57, no. 11: 1200. https://doi.org/10.3390/medicina57111200
APA StyleLamberti, N., Manfredini, F., Lissom, L. O., Lavezzi, S., Basaglia, N., & Straudi, S. (2021). Beneficial Effects of Robot-Assisted Gait Training on Functional Recovery in Women after Stroke: A Cohort Study. Medicina, 57(11), 1200. https://doi.org/10.3390/medicina57111200









