The Relation of Body Mass Index to Muscular Viscoelastic Properties in Normal and Overweight Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
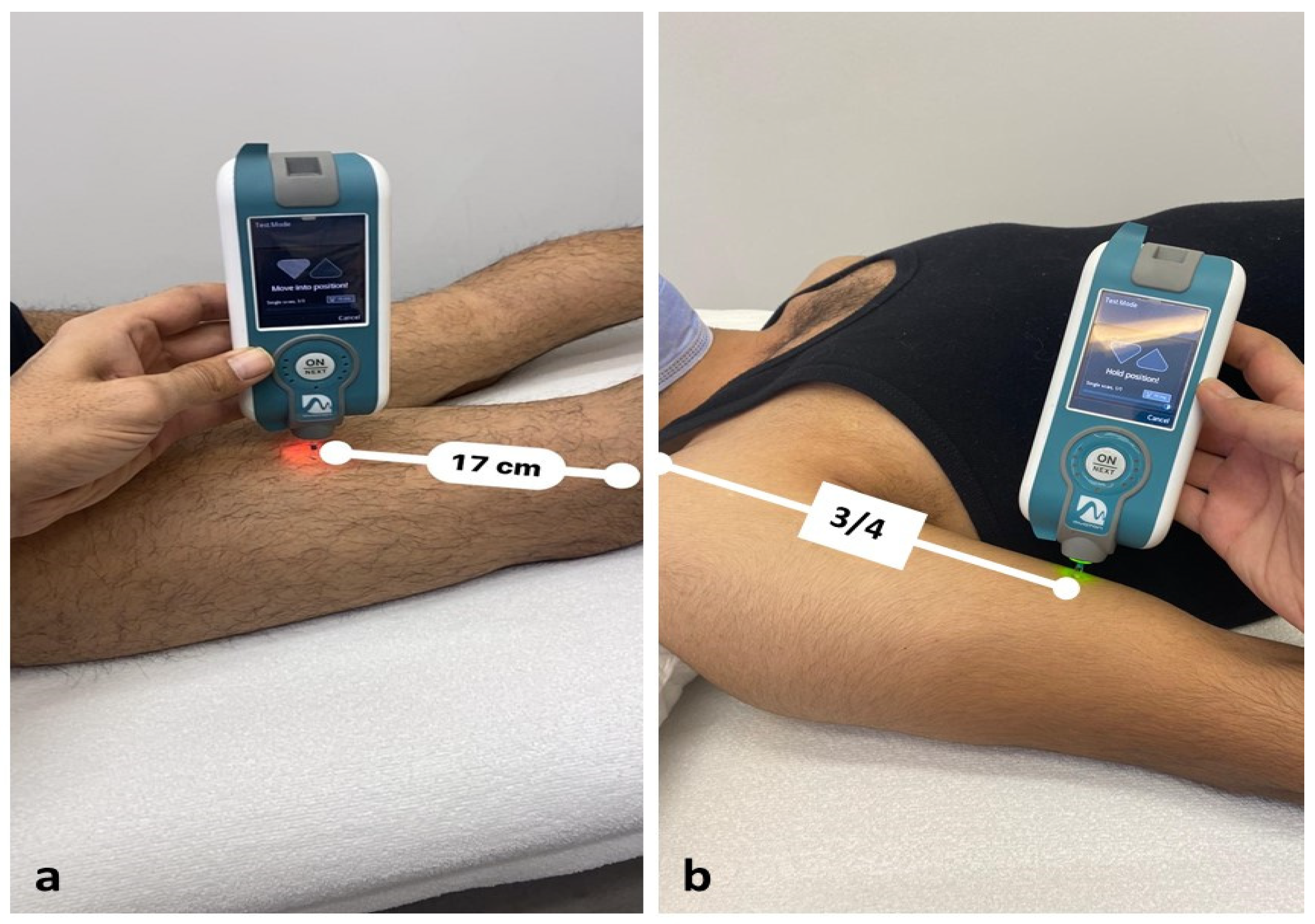
2.2. Procedures
2.3. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Intra-Observer Reliability
3.3. Correlation between the BB and BF Viscoelastic Properties and BMI
3.3.1. Study Sample
3.3.2. Females
3.3.3. Males
3.4. Comparison of BB Viscoelastic
3.5. Comparison of BF Viscoelastic Properties
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef]
- Wang, F.; McDonald, T.; Champagne, L.J.; Edington, D.W. Relationship of body mass index and physical activity to health care costs among employees. J. Occup. Med. 2004, 46, 428–436. [Google Scholar] [CrossRef][Green Version]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yagi, S.; Naoko, K.; Hideaki, O.; Shinji, U. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Šarabon, N.; Kozinc, Ž.; Podrekar, N. Using shear-wave elastography in skeletal muscle: A repeatability and reproducibility study on biceps femoris muscle. PLoS ONE 2019, 14, e0222008. [Google Scholar]
- Feng, Y.; Li, Y.; Liu, C.; Zhang, Z. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 1–9. [Google Scholar]
- Gapeyeva, H.; Vain, A. Methodical Guide: Principles of Applying Myoton in Physical Medicine and Rehabilitation; Müomeetria Ltd.: Tartu, Estonia, 2008. [Google Scholar]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Practical considerations for standardized recording of muscle mechanical properties using a myometric device: Recording site, muscle length, state of contraction and prior activity. J. Musculoskelet Res. 2018, 21, 1850010. [Google Scholar] [CrossRef]
- Faria, A.; Gabriel, R.; Abrantes, J.; Brás, R.; Moreira, H. Triceps-surae musculotendinous stiffness: Relative differences between obese and non-obese postmenopausal women. Clin. Biomech. 2009, 24, 866–871. [Google Scholar] [CrossRef]
- Kuo, W.H.; Jian, D.W.; Wang, T.G.; Wang, Y.C. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med. Biol. 2013, 39, 1356–1361. [Google Scholar] [CrossRef]
- Seo, A.; Lee, J.H.; Kusaka, Y. Estimation of trunk muscle parameters for a biomechanical model by age, height and weight. J. Occup. Health 2003, 45, 197–201. [Google Scholar] [CrossRef]
- Wood, S.; Pearsall, D.; Ross, R.; Reid, J. Trunk muscle parameters determined from MRI for lean to obese males. Clin. Biomech. 1996, 11, 139–144. [Google Scholar] [CrossRef]
- Bailey, L.; Samuel, D.; Warner, M.; Stokes, M. Parameters representing muscle tone, elasticity and stiffness of biceps brachii in healthy older males: Symmetry and within-session reliability using the MyotonPRO. J. Neurol. Disord. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Leonard, C.T.; Deshner, W.P.; Romo, J.W.; Suoja, E.S.; Fehrer, S.C.; Mikhailenok, E.L. Myotonometer intra-and interrater reliabilities. Arch. Phys. Med. Rehabil. 2003, 84, 928–932. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Aird, L.; Bailey, L.; Mooney, K.; Mullix, J.; Warner, M.; Samuel, D.; Stokes, M. Interrater reliability of muscle tone, stiffness and elasticity measurements of rectus femoris and biceps brachii in healthy young and older males. Work Pap. Health Sci. 2013, 4, 1–11. [Google Scholar]
- Chuang, L.L.; Wu, C.Y.; Lin, K.C. Reliability, validity, and responsiveness of myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; van der Schans, C.; Hobbelen, H. Psychometric properties of the MyotonPRO in dementia patients with paratonia. Gerontology 2018, 64, 401–412. [Google Scholar] [CrossRef]
- Lidström, Å.; Ahlsten, G.; Hirchfeld, H.; Norrlin, S. Intrarater and interrater reliability of myotonometer measurements of muscle tone in children. J. Child. Neurol. 2009, 24, 267–274. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Saris, W.; Blair, S.; Van Baak, M.; Eaton, S.; Davies p Di Pietro, L.; Fogelholm, M.; Rissanen, A.; Schoeller, D.; Swinburn, B.; Tremblay, A.; et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes. Rev. 2003, 4, 101–114. [Google Scholar] [CrossRef]
- Gervasi, M.; Sisti, D.; Amatori, S.; Andreazza, M.; Benelli, P.; Sestili, P.; Rocchi, M.B.L.; Calavalle, A.R. Muscular viscoelastic characteristics of athletes participating in the European Master Indoor Athletics Championship. Eur. J. Appl. Physiol. 2017, 117, 1739–1746. [Google Scholar] [CrossRef]
- Saglam, M.; Arikan, H.; Savci, S.; Inal, I.D.; Bosnak, G.M.; Karabulut, E.; Tokgozoglu, L. International physical activity questionnaire: Reliability and validity of the Turkish version. Percept. Mot. Skills. 2010, 111, 278–284. [Google Scholar] [CrossRef]
- Zinder, S.M.; Padua, D.A. Reliability, validity, and precision of a handheld myometer for assessing in vivo muscle stiffness. J. Sport Rehabil. 2011, 20. [Google Scholar] [CrossRef]
- Schneebeli, A.; Falla, D.; Clijsen, R.; Barbero, M. Myotonometry for the evaluation of Achilles tendon mechanical properties: A reliability and construct validity study. BMJ Open Sport Exerc. Med. 2020, 6, e000726. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Li, Y.P.; Wang, X.Q.; Zhang, Z.J. Quantifying the stiffness of Achilles tendon: Intra-and inter-operator reliability and the effect of ankle joint motion. Med. Sci. Monit. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018, 24, 4876. [Google Scholar] [CrossRef] [PubMed]
- Gavronski, G.; Veraksitš, A.; Vasar, E.; Maaroos, J. Evaluation of viscoelastic parameters of the skeletal muscles in junior triathletes. Physiol. Meas. 2007, 28, 625. [Google Scholar] [CrossRef] [PubMed]
- Myoton. [updated 23 July 2021]. Available online: https://www.myoton.com/technology/ (accessed on 12 August 2021).
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Measurement of ageing effects on muscle tone and mechanical properties of rectus femoris and biceps brachii in healthy males and females using a novel hand-held myometric device. Arch. Gerontol. Geriatr. 2016, 62, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rihvk, I.; Clough, A.; Clough, P. Investigation to compare static stretching and proprioceptive neuromuscular facilitation contract–relax stretching effects on the visco-elastic parameters of the biceps femoris muscle. Int. Musculoskelet. Med. 2010, 32, 157–162. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Lee, H. The measurement of stiffness for major muscles with shear wave elastography and myoton: A quantitative analysis study. Diagnostics 2021, 11, 524. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Hoffman, L.R.; Koppenhaver, S.L.; MacDonald, C.W.; Herrera, J.M.; Streuli, J.; Visco, Z.L.; Wildermuth, N.; Albin, S.R. Normative Parameters of Gastrocnemius Muscle Stiffness and Associations with Patient Characteristics and Function. Int. J. Sports Phys. Ther. 2021, 16, 41. [Google Scholar]
- White, A.; Abbott, H.; Masi, A.T.; Henderson, J.; Nair, K. Biomechanical properties of low back myofascial tissue in younger adult ankylosing spondylitis patients and matched healthy control subjects. Clin. Biomech. 2018, 57, 67–73. [Google Scholar] [CrossRef]
- Kawai, T.; Takamoto, K.; Bito, I. Previous hamstring muscle strain injury alters passive tissue stiffness and vibration sense. J. Bodyw. Mov. Ther. 2021, 27, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.T.; Padua, D.A.; Weinhold, P.S.; Guskiewicz, K.M. Comparison of triceps surae structural stiffness and material modulus across sex. Clin. Biomech. 2006, 21, 159–167. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Xiao, S.; Wang, B.; Fu, W. Gender Difference in Architectural and Mechanical Properties of Medial Gastrocnemius–Achilles Tendon Unit In Vivo. Life 2021, 11, 569. [Google Scholar] [CrossRef]
- Morse, C.I. Gender differences in the passive stiffness of the human gastrocnemius muscle during stretch. Eur. J. Appl. Physiol. 2011, 111, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Chino, K.; Takahashi, H. Association of gastrocnemius muscle stiffness with passive ankle joint stiffness and sex-related difference in the joint stiffness. J. Appl. Biomech. 2018, 34, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Saeki, J.; Ikezoe, T.; Yoshimi, S.; Nakamura, M.; Ichihashi, N. Menstrual cycle variation and gender difference in muscle stiffness of triceps surae. Clin. Biomech. 2019, 61, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K.-N. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clinl. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef]
- Miller, A.E.J.; MacDougall, J.; Tarnopolsky, M.; Sale, D. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. 1993, 66, 254–262. [Google Scholar] [CrossRef]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef]
- Shorten, M.R. Muscle elasticity and human performance. Med. Sport Sci. 1987, 25, 1–18. [Google Scholar]
- Abe, T.; Brechue, W.F.; Fujita, S.; Brown, J.B. Gender differences in FFM accumulation and architectural characteristics of muscle. Med. Sci. Sports Exerc. 1998, 30, 1066–1070. [Google Scholar] [CrossRef]
- Gajdosik, R.; Giuliani, C.; Bohannon, R. Passive compliance and length of the hamstring muscles of healthy men anc women. Clin. Biomech. 1990, 5, 23–29. [Google Scholar] [CrossRef]
- Chleboun, G.S.; Howell, J.N.; Conatser, R.R.; Giesey, J.J. The relationship between elbow flexor volume and angular stiffness at the elbow. Clin. Biomech. 1997, 12, 383–392. [Google Scholar] [CrossRef]
- Komi, P.V. Physiological and biomechanical correlates of muscle function: Effects of muscle structure and stretch—Shortening cycle on force and speed. Exerc. Sport Sci. Rev. 1984, 12, 81–122. [Google Scholar] [CrossRef]
- Garaulet, M.; Perez-Llamas, F.; Fuente, T.; Zamora, S.; Tebar, F.J. Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur. J. Endocrinol. 2000, 143, 657–666. [Google Scholar] [CrossRef]
- Fröhlich-Zwahlen, A.; Casartelli, N.; Item-Glatthorn, J.; Maffiuletti, N. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: A preliminary study. J. Electromyogr. Kinesiol. 2014, 24, 762–769. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef]
- Kvist, H.; Chowdhury, B.; Grangård, U.; Tylen, U.; Sjöström, L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: Predictive equations. Am. J. Clin. Nutr. 1988, 48, 1351–1361. [Google Scholar] [CrossRef]
- Bell, D.R.; Blackburn, J.T.; Ondrak, K.S.; Hackney, A.C.; Hudson, J.D.; Norcross, M.F.; Padua, D.A. The effects of oral contraceptive use on muscle stiffness across the menstrual cycle. Clin. J. Sport Med. 2011, 21, 467–473. [Google Scholar] [CrossRef]
- Bell, D.R.; Blackburn, J.T.; Norcorss, M.F.; Ondrak, K.S.; Hudson, J.D.; Hackney, A.; Padua, D.A. Estrogen and muscle stiffness have a negative relationship in females. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 361–367. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Ratel, S.; Sartorio, A.; Martin, V. The impact of obesity on in vivo human skeletal muscle function. Curr. Obes. Rep. 2013, 2, 251–260. [Google Scholar] [CrossRef]
- Brady, A.O.; Straight, C.; Schmidt, M.; Evans, E. Impact of body mass index on the relationship between muscle quality and physical function in older women. J. Nutr. Health Aging. 2014, 18, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Mullix, J.; Warner, M.; Stokes, M. Testing muscle tone and mechanical properties of rectus femoris and biceps femoris using a novel hand held MyotonPRO device: Relative ratios and reliability. Work. Pap. Health Sci. 2012, 1, 1–8. [Google Scholar]
- Chuang, L.L.; Wu, C.Y.; Lin, K.C.; Lur, S.Y. Quantitative mechanical properties of the relaxed biceps and triceps brachii muscles in patients with subacute stroke: A reliability study of the myoton-3 myometer. Stroke Res. Treat. 2012, 2012, 617694. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.L.; Lin, K.C.; Wu, C.Y.; Chang, C.W.; Chen, H.C.; Yin, H.P.; Wang, L. Relative and absolute reliabilities of the myotonometric measurements of hemiparetic arms in patients with stroke. Arch. Phys. Med. Rehabil. 2013, 94, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, M.; Mannion, A.F. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef]
- Rahemi, H.; Nigam, N.; Wakeling, J.M. The effect of intramuscular fat on skeletal muscle mechanics: Implications for the elderly and obese. J. R. Soc. Interface. 2015, 12, 20150365. [Google Scholar] [CrossRef]
- Ryu, M.; Jo, J.; Lee, Y.; Chung, Y.S.; Kim, K.M.; Baek, W.C. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013, 42, 734–740. [Google Scholar] [CrossRef]
- Garcia-Vicencio, S.; Coudeyre, E.; Kluka, V.; Cardenoux, C.; Jegu, A.; Fourot, A.V.; Ratel, S.; Martin, V. The bigger, the stronger? Insights from muscle architecture and nervous characteristics in obese adolescent girls. Int. J. Obesity. 2016, 40, 245–251. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.; Winwood, K.; Morse, C.; Onambélé, G. The impact of obesity on skeletal muscle architecture in untrained young vs. old women. J. Anat. 2014, 225, 675–684. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Jubeau, M.; Agosti, F.; De Col, A.; Sartorio, A. Quadriceps muscle function characteristics in severely obese and nonobese adolescents. Eur. J. Appl. Physiol. 2008, 103, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Barbat-Artigas, S.; Pion, C.H.; Leduc-Gaudet, J.P.; Rolland, Y.; Aubertin-Leheudre, M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J. Am. Med. Dir. Assoc. 2014, 15, 303.e13–303.e20. [Google Scholar] [CrossRef] [PubMed]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J. Validity and reliability of three methods of stiffness assessment. J. Sport Health Sci. 2016, 5, 476–483. [Google Scholar] [CrossRef] [PubMed]
| Normal Weight | Overweight | |||||
|---|---|---|---|---|---|---|
| Total (n = 86) | Male (n = 43) | Female (n = 43) | Total (n = 86) | Male (n = 43) | Female (n = 43) | |
| Age (year) | 25.20 ± 4.92 | 24.67 ± 4.89 | 25.72 ± 4.96 | 26.80 ± 5.83 | 26.14 ± 5.95 | 27.47 ± 5.71 |
| Body Weight (kg) | 64.18 ± 9.33 | 69.49 ± 9.00 | 58.86 ± 6.13 | 77.58 ± 8.04 | 80.23 ± 7.83 | 74.94 ± 7.42 |
| Height (m) | 1.70 ± 0.08 | 1.76 ± 0.08 | 1.64 ± 0.53 | 1.68 ± 0.08 | 1.71 ± 0.07 | 1.65 ± 0.06 |
| BMI (kg/m2) | 22.01 ± 1.70 | 22.35 ± 1.65 | 21.67 ± 1.70 | 27.23 ± 1.36 | 27.26 ± 1.25 | 27.19 ± 1.47 |
| All (n = 172) | Normal Weight (n = 86) | Overweight (n = 86) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ICC2,1 (95% CI) | SEM95 | MDC | ICC2,1 (95% CI) | SEM95 | MDC | ICC2,1 (95% CI) | SEM95 | MDC | |
| Right | |||||||||
| BB Tone (Hz) | 0.930 (0.703–0.982) | 0.56 | 1.56 | 0.937 (0.687–0.985) | 0.43 | 1.19 | 0.928 (0.718–0.962) | 0.67 | 1.85 |
| BB Stiffness (N/m) | 0.915 (0.777–0.958) | 13.84 | 38.38 | 0.941 (0.824–0.963) | 10.57 | 29.32 | 0.916 (0.741–0.954) | 14.2 | 39.36 |
| BB Elasticity (log) | 0.959 (0.878–0.969) | 0.04 | 0.13 | 0.970 (0.876–0.984) | 0.03 | 0.11 | 0.963 (0.851–0.982) | 0.04 | 0.12 |
| BF Tone (Hz) | 0.919 (0.548–0.957) | 0.64 | 1.77 | 0.913 (0.797–0.960) | 0.64 | 1.78 | 0.904 (0.762–0.951) | 0.72 | 1.99 |
| BF Stiffness (N/m) | 0.889 (0.662–0.925) | 18.80 | 52.12 | 0.925 (0.835–0.946) | 14.76 | 40.92 | 0.898 (0.684–0.934) | 19.5 | 54.06 |
| BF Elasticity (log) | 0.931 (0.740–0.968) | 0.07 | 0.18 | 0.958 (0.855–0.982) | 0.05 | 0.14 | 0.911 (0.805–0.966) | 0.09 | 0.25 |
| Left | |||||||||
| BB Tone (Hz) | 0.938 (0.689–0.977) | 0.44 | 1.21 | 0.923 (0.850–0.992) | 0.51 | 1.42 | 0.944 (0.828–0.965) | 0.38 | 1.06 |
| BB Stiffness (N/m) | 0.922 (0.766–0.948) | 12.31 | 34.14 | 0.931 (0.823–0.972) | 12.37 | 34.29 | 0.918 (0.799–0.943) | 11.81 | 32.75 |
| BB Elasticity (log) | 0.901 (0.752–0.943) | 0.07 | 0.21 | 0.967 (0.789–0.988) | 0.04 | 0.11 | 0.928 (0.866–0.982) | 0.09 | 0.26 |
| BF Tone (Hz) | 0.919 (0.760–0.961) | 0.58 | 1.60 | 0.937 (0.794–0.975) | 0.51 | 1.39 | 0.909 (0.688–0.957) | 0.59 | 1.64 |
| BF Stiffness (N/m) | 0.931 (0.789–0.958) | 13.37 | 37.06 | 0.943 (0.777–0.967) | 12.04 | 33.39 | 0.918 (0.823–0.949) | 13.88 | 38.49 |
| BF Elasticity (log) | 0.902 (0.833–0.986) | 0.08 | 0.22 | 0.918 (0.704–0.970) | 0.07 | 0.19 | 0.915 (0.841–0.930) | 0.08 | 0.22 |
| Overall (n = 172) | Males (n = 86) | Females (n = 86) | ||
|---|---|---|---|---|
| Right BB Tone (Hz) | r | 0.02 | 0.09 | −0.11 |
| Right BB Stiffness (N/m) | r | 0.27 ** | 0.37 ** | 0.18 |
| Right BB Elasticity (log) | r | 0.28 ** | 0.35 ** | 0.25 * |
| Left BB Tone (Hz) | r | −0.06 | −0.11 | −0.07 |
| Left BB Stiffness (N/m) | r | 0.14 | 0.22 * | 0.06 |
| Left BB Elasticity (log) | r | 0.13 | 0.16 | 0.12 |
| Right BF Tone (Hz) | r | 0.17 * | 0.22 * | 0.15 |
| Right BF Stiffness (N/m) | r | 0.19 * | 0.26 * | 0.16 |
| Right BF Elasticity (log) | r | 0.04 | 0.04 | 0.04 |
| Left BF Tone (Hz) | r | 0.19 * | 0.27 * | 0.15 |
| Left BF Stiffness (N/m) | r | 0.21 ** | 0.30 ** | 0.18 |
| Left BF Elasticity (log) | r | −0.03 | −0.17 | 0.02 |
| Normal Weight | Overweight | 2 × 2 ANOVA (p-Values) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Right | Total (n = 86) | Male (n = 43) | Female (n = 43) | Total (n = 86) | Male (n = 43) | Female (n = 43) | BMI Effect | Sex Effect | Interaction |
| BB Tone (Hz) | 14.40 ± 1.72 | 14.68 ± 1.92 | 14.11 ± 1.48 | 14.53 ± 2.50 | 15.27 ± 2.98 | 13.79 ± 1.61 | 0.680 | 0.001 M | 0.150 |
| BB Stiffness (N/m) | 216.12 ± 43.55 | 215.70 ± 50.59 | 216.53 ± 35.76 | 237.92 ± 49.00 | 247.14 ± 57.19 | 228.70 ± 37.62 | 0.002 OW | 0.213 | 0.173 |
| BB Elasticity (log) | 1.00 ± 0.22 | 0.97 ± 0.20 | 1.02 ± 0.24 | 1.12 ± 0.24 | 1.08 ± 0.23 | 1.16 ± 0.25 | 0.001 OW | 0.078 | 0.671 |
| BF Tone (Hz) | 15.06 ± 2.18 | 15.97 ± 1.87 | 14.15 ± 2.10 | 15.71 ± 2.29 | 16.87 ± 2.32 | 14.56 ± 1.58 | 0.033 OW | 0.000 M | 0.415 |
| BF Stiffness (N/m) | 243.78 ± 53.90 | 261.95 ± 47.81 | 225.60 ± 54.01 | 262.83 ± 57.61 | 290.98 ± 61.07 | 234.67 ± 36.93 | 0.015 OW | 0.000 M | 0.199 |
| BF Elasticity (log) | 1.09 ± 0.25 | 1.20 ± 0.22 | 0.99 ± 0.25 | 1.10 ± 0.27 | 1.20 ± 0.31 | 1.01 ± 0.19 | 0.715 | 0.000 M | 0.800 |
| Left | |||||||||
| BF Tone (Hz) | 14.93 ± 2.00 | 15.51 ± 1.39 | 14.34 ± 2.33 | 15.60 ± 2.02 | 16.43 ± 1.97 | 14.77 ± 1.73 | 0.266 | 0.018 M | 0.665 |
| BF Stiffness (N/m) | 244.50 ± 50.47 | 258.74 ± 37.81 | 230.26 ± 57.53 | 265.52 ± 49.41 | 289.12 ± 48.15 | 241.93 ± 38.51 | 0.310 | 0.990 | 0.470 |
| BF Elasticity (log) | 1.14 ± 0.25 | 1.28 ± 0.24 | 1.01 ± 0.17 | 1.12 ± 0.26 | 1.21 ± 0.28 | 1.04 ± 0.20 | 0.019 OW | 0.253 | 0.390 |
| BB Tone (Hz) | 14.49 ± 1.85 | 14.86 ± 1.98 | 14.11 ± 1.66 | 14.19 ± 1.67 | 14.45 ± 1.63 | 13.93 ± 1.69 | 0.021 OW | 0.000 M | 0.390 |
| BB Stiffness (N/m) | 223.20 ± 47.10 | 220.79 ± 51.14 | 225.60 ± 43.16 | 230.07 ± 40.89 | 232.56 ± 41.27 | 227.58 ± 40.84 | 0.003 OW | 0.000 M | 0.186 |
| BB Elasticity (log) | 1.02 ± 0.23 | 0.98 ± 0.23 | 1.06 ± 0.22 | 1.11 ± 0.26 | 1.10 ± 0.33 | 1.11 ± 0.17 | 0.580 | 0.000 M | 0.187 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usgu, S.; Ramazanoğlu, E.; Yakut, Y. The Relation of Body Mass Index to Muscular Viscoelastic Properties in Normal and Overweight Individuals. Medicina 2021, 57, 1022. https://doi.org/10.3390/medicina57101022
Usgu S, Ramazanoğlu E, Yakut Y. The Relation of Body Mass Index to Muscular Viscoelastic Properties in Normal and Overweight Individuals. Medicina. 2021; 57(10):1022. https://doi.org/10.3390/medicina57101022
Chicago/Turabian StyleUsgu, Serkan, Engin Ramazanoğlu, and Yavuz Yakut. 2021. "The Relation of Body Mass Index to Muscular Viscoelastic Properties in Normal and Overweight Individuals" Medicina 57, no. 10: 1022. https://doi.org/10.3390/medicina57101022
APA StyleUsgu, S., Ramazanoğlu, E., & Yakut, Y. (2021). The Relation of Body Mass Index to Muscular Viscoelastic Properties in Normal and Overweight Individuals. Medicina, 57(10), 1022. https://doi.org/10.3390/medicina57101022






