Radiofrequency vs. Cryoballoon vs. Thoracoscopic Surgical Ablation for Atrial Fibrillation: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Catheter Ablation: Radiofrequency and Cryoballoon
2.2.1. Catheter Ablation with Radiofrequency
2.2.2. Cryoballoon Catheter Ablation
2.3. Thoracoscopic Surgical Ablation
2.4. Post-Procedural Care and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural Characteristics
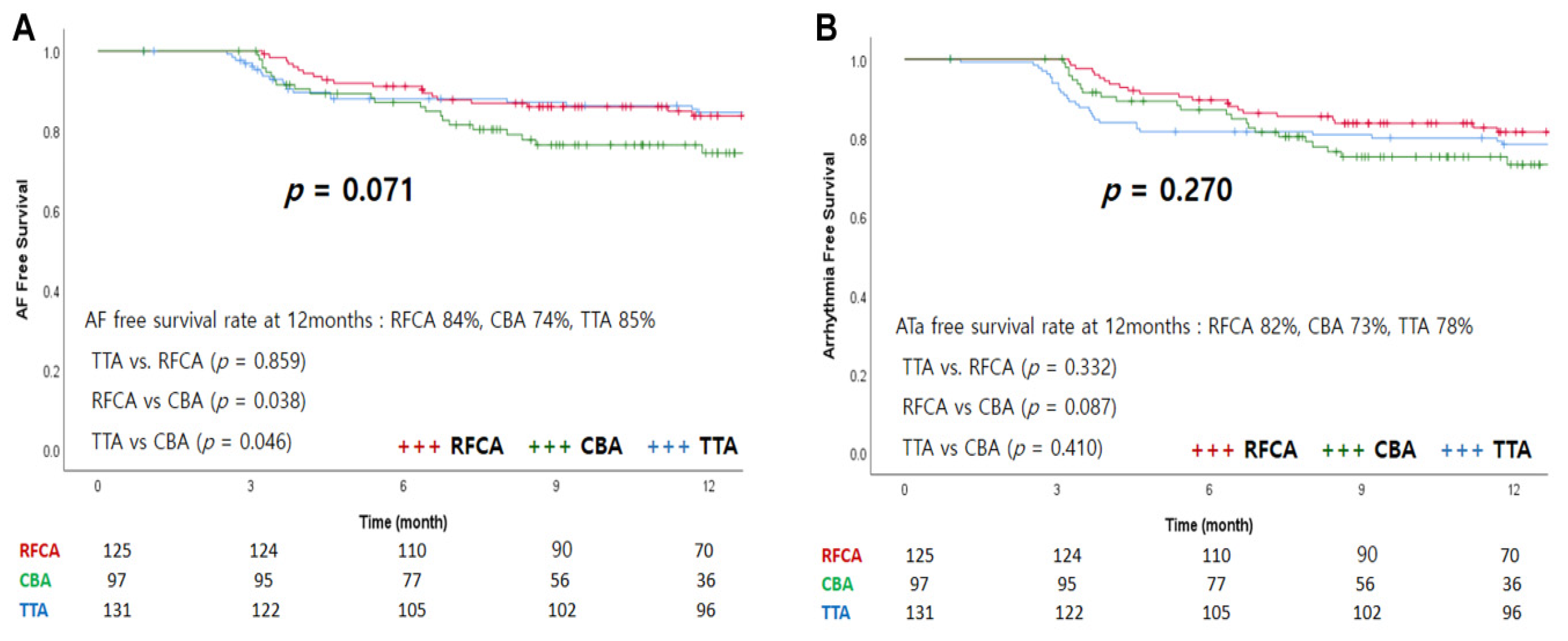
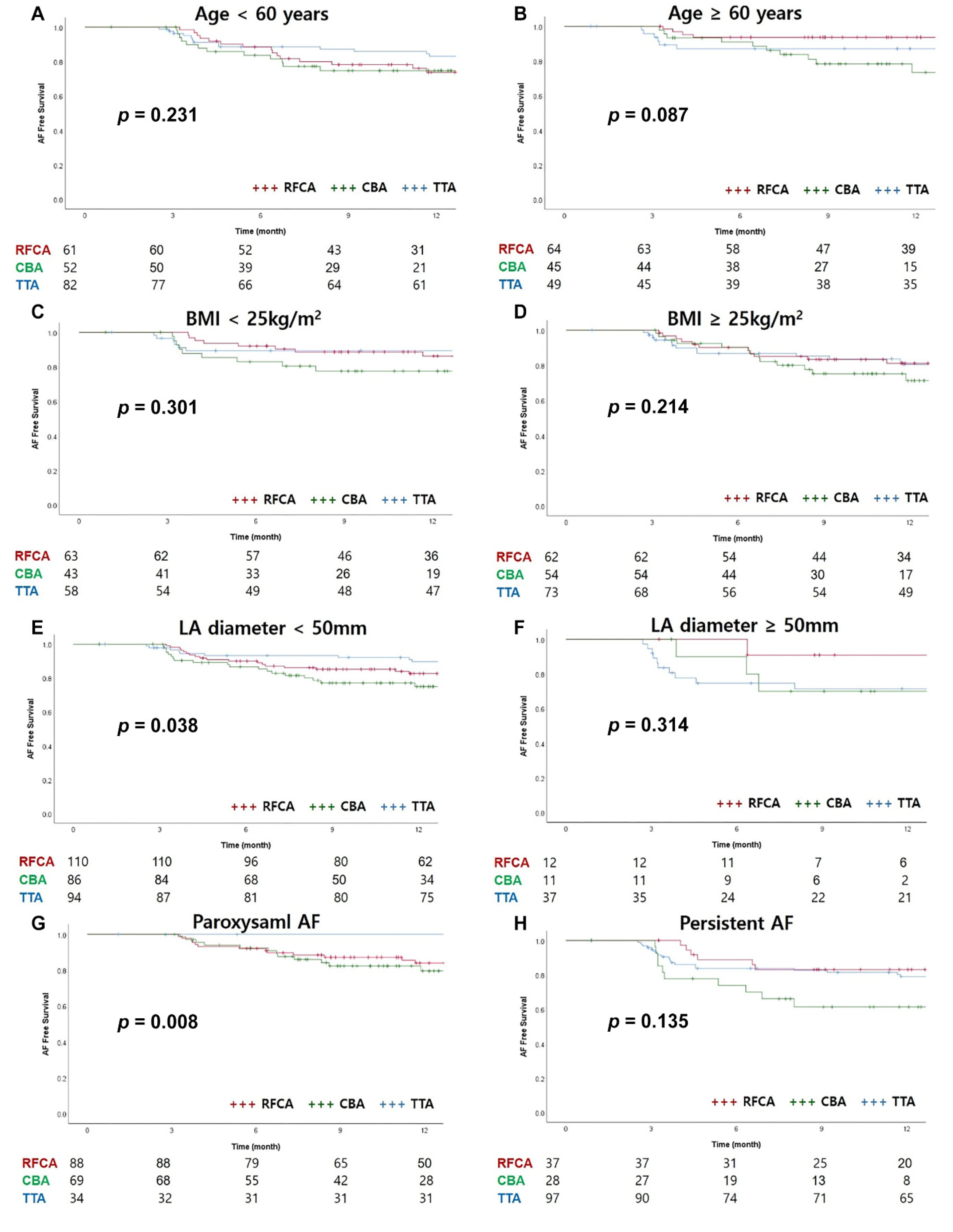
3.3. Follow-Up
3.4. Procedure-Related Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frost, L.; Vestergaard, P.; Mosekilde, L. Hyperthyroidism and risk of atrial fibrillation or flutter: A population-based study. ACC Curr. J. Rev. 2004, 164, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Mont, L.; Elosua, R.; Brugada, J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace 2008, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Duval, S.; Soliman, E.Z.; Ambrose, M.; Eberly, L.; Alonso, A. Smoking and incidence of atrial fibrillation: Results from the atherosclerosis risk in communities (ARIC) study. Heart Rhythm 2011, 8, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, K.; Mascia, G.; Martin, C.A.; Bazoukis, G.; Frontera, A.; Cheniti, G.; Letsas, K.P.; Efremidis, M.; Georgopoulos, S.; Gkalapis, C.; et al. Atrial fibrillation in Brugada syndrome: Current perspectives. J. Cardiovasc. Electrophysiol. 2020, 31, 975–984. [Google Scholar] [CrossRef]
- Platonov, P.G.; McNitt, S.; Polonsky, B.; Rosero, S.Z.; Zareba, W. Atrial fibrillation in long QT syndrome by genotype. Circ. Arrhythmia Electrophysiol. 2019, 12, e007213. [Google Scholar] [CrossRef] [PubMed]
- Mascia, G.; Arbelo, E.; Solimene, F.; Giaccardi, M.; Brugada, R.; Brugada, J. The long-QT syndrome and exercise practice: The never-ending debate. J. Cardiovasc. Electrophysiol. 2018, 29, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Hart, C.L.; Hole, D.J.; McMurray, J.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am. J. Med. 2002, 113, 359–364. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham heart study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Nyong, J.; Amit, G.; Adler, A.J.; Owolabi, O.O.; Perel, P.; Prieto-Merino, D.; Lambiase, P.; Casas, J.P.; Morillo, C.A. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst. Rev. 2016, 11, CD012088. [Google Scholar] [CrossRef]
- Jais, P.; Cauchemez, B.; Macle, L.; Daoud, E.; Khairy, P.; Subbiah, R.; Hocini, M.; Extramiana, F.; Sacher, F.; Bordachar, P.; et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The A4 study. Circulation 2008, 118, 2498–2505. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.K. Mechanisms of tissue destruction following cryosurgery. Ann. R. Coll. Surg. Engl. 1984, 66, 313–318. [Google Scholar] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Ep Europace 2018, 20, e1–e160. [Google Scholar]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: A randomized clinical trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Pak, H.-N.; Park, J.-W.; Yang, S.-Y.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Yu, H.T.; CRAFT Investigators. Cryoballoon versus high-power, short-duration radiofrequency ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: A single-center, prospective, randomized study. Circ. Arrhythmia Electrophysiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Niebauer, M.; Zurick, A.; Barnard, J.; Gillinov, A.M.; Chung, M.K.; van Wagoner, D.R. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ. Arrhythmia Electrophysiol. 2010, 3, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; Castella, M.; van Boven, W.; Berruezo, A.; Yilmaz, A.; Nadal, M.; Sandoval, E.; Calvo, N.; Brugada, J.; Kelder, J.; et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): A 2-center randomized clinical trial. Circulation 2012, 125, 23–30. [Google Scholar] [CrossRef]
- De Maat, G.E.; van Gelder, I.C.; Rienstra, M.; Quast, A.-F.B.; Tan, E.S.; Wiesfeld, A.C.; Pozzoli, A.; Mariani, M.A. Surgical vs. transcatheter pulmonary vein isolation as first invasive treatment in patients with atrial fibrillation: A matched group comparison. Europace 2013, 16, 33–39. [Google Scholar] [CrossRef][Green Version]
- Haldar, S.; Khan, H.R.; Boyalla, V.; Kralj-Hans, I.; Jones, S.; Lord, J.; Onyimadu, O.; Satishkumar, A.; Bahrami, T.; de Souza, A.; et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibril-lation: CASA-AF randomized controlled trial. Eur. Heart J. 2020, 41, 4471–4480. [Google Scholar] [CrossRef]
- Sarkozy, A.; de Potter, T.; Heidbuchel, H.; Ernst, S.; Kosiuk, J.; Vano, E.; Picano, E.; Arbelo, E.; Tedrow, U.; Potpara, T.; et al. Occupational radiation exposure in the electrophysiology laboratory with a focus on personnel with reproductive potential and during pregnancy: A European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS). Ep Europace 2017, 19, 1909–1922. [Google Scholar] [CrossRef]
- Giaccardi, M.; Anselmino, M.; del Greco, M.; Mascia, G.; Perini, A.P.; Mascia, P.; de Ferrari, G.M.; Picano, E. Radiation awareness in an Italian multispecialist sample assessed with a web-based survey. Acta Cardiol. 2020, 76, 1–5. [Google Scholar] [CrossRef]
- Mascia, G.; Giaccardi, M. A new era in zero X-ray ablation. Arrhythmia Electrophysiol. Rev. 2020, 9, 121–127. [Google Scholar] [CrossRef]
- Pani, A.; Giuseppina, B.; Bonanno, C.; Grazia Bongiorni, M.; Bottoni, N.; Brambilla, R.; Ceglia, S.D.; Della Bella, P.; Vito, G.D.; Malaspina, D.; et al. Predictors of zero X-ray ablation for supraventricular tachycardias in a nationwide multicenter experience. Circ. Arrhythmia Electrophysiol. 2018, 11, e005592. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, G.; Chen, X.; Chen, G.; Yang, S.; Guo, P.; Wang, Y.; Wang, D.W. Meta-analysis of zero or near-zero fluoroscopy use during ablation of cardiac arrhythmias. Am. J. Cardiol. 2016, 118, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Holl, M.J.; Bhagwandien, R.E.; Firouzi, M.; de Ruiter, W.A.; Szili-Torok, T.; Yap, S.-C. Reducing radiation exposure in second-generation cryoballoon ablation without compromising clinical outcome. J. Interv. Card. Electrophysiol. 2021, 60, 287–294. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 353) | RFCA (n = 125) | CBA (n = 97) | TTA (n = 131) | p-Value | |
|---|---|---|---|---|---|
| Gender (Female) | 60 (17%) | 22 (18%) | 21 (22%) | 17 (13%) | 0.222 |
| Age (years) | 56.9 ± 9.8 | 56.9 ± 10.5 | 57.3 ± 9.7 | 56.6 ± 9.2 | 0.875 |
| Body mass index | 25.5 ± 3.1 | 25.1 ± 2.2 | 25.6 ± 3.2 | 25.8 ± 3.7 | 0.156 |
| Comorbidities | |||||
| Hypertension | 140 (40%) | 53 (42%) | 43 (44%) | 45 (34%) | 0.256 |
| Diabetes mellitus | 41 (12%) | 18 (14%) | 9 (9%) | 14 (11%) | 0.471 |
| Prior stroke/TIA | 52 (15%) | 9 (7%) | 7 (6%) | 36 (28%) | <0.001 |
| Heart failure | 18 (5%) | 4 (3%) | 4 (4%) | 10 (8%) | 0.234 |
| C-V score | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.346 |
| C-V score ≥2 | 128 (36%) | 40 (32%) | 31 (32%) | 57 (44%) | 0.088 |
| Type of AF | <0.001 | ||||
| Paroxysmal | 191 (54%) | 89 (71%) | 69 (71%) | 34 (26%) | |
| Persistent | 162 (46%) | 37 (29%) | 28 (29%) | 91 (74%) | |
| Echocardiographic parameter | |||||
| LV EF (%) | 61.5 ± 7.3 | 62.3 ± 6.2 | 60.9 ± 8.5 | 61.2 ± 7.2 | 0.286 |
| LA diameter (mm) | 43.3 ± 6.5 | 41.4 ± 5.8 | 41.7 ± 6.3 | 46.2 ± 6.4 | <0.001 |
| LA volume index | 43.3 ± 14.9 | 40.1 ± 13.3 | 40.6 ± 12.9 | 48.4 ± 16.3 | <0.001 |
| LA > 50 mm | 60 (17%) | 12 (10%) | 11 (11%) | 37 (28%) | <0.001 |
| Medication | |||||
| AAD * at discharge | 301 (85%) | 108 (86%) | 83 (86%) | 110 (84%) | 0.856 |
| AAD * at 6 months | 164 (47%) | 50 (40%) | 43 (44%) | 75 (57%) | 0.017 |
| RFCA (n = 125) | CBA (n = 97) | TTA (n = 131) | p-Value | |
|---|---|---|---|---|
| Procedure duration (min) | 139 ± 34 | 90 ± 20 | 157 ± 39 | <0.001 |
| Fluoroscopy time (min) | 25 ± 11 | 29 ± 10 | - | 0.010 |
| Length of hospital stay (days) | 3.7 ± 0.6 | 3.8 ± 0.9 | 10.2 ± 4.7 | <0.001 |
| Pulmonary vein isolation | 125 (100%) | 97 (100%) | 131 (100%) | |
| Roof line | 51 (42%) | 0 (0%) | 120 (92%) | |
| Posterior line | 46 (37%) | 0 (0%) | 129 (99%) | |
| Mitral isthmus line | 33 (32%) | 0 (0%) | 0 (0%) | |
| Endocardial CS | 17 (17%) | 0 (0%) | 0 (0%) | |
| SVC isolation | 18 (18%) | 0 (0%) | 52 (40%) | |
| CTI line | 84 (68%) | 7 (8%) | 0 (0%) | |
| Ganglionic plexi | 0 (0%) | 0 (0%) | 118 (90%) | |
| LAA exclusion | 0 (0%) | 0 (0%) | 128 (98%) |
| RFCA (n = 125) | CBA (n = 97) | TTA (n = 131) | |
|---|---|---|---|
| Any atrial tachyarrhythmia | 24 (19%) | 25 (26%) | 41 (31%) |
| Atrial fibrillation | 20 (16%) | 24 (25%) | 29 (22%) |
| Atrial flutter | 5 (4%) | 1 (1%) | 13 (10%) |
| Atrial tachycardia | 0 (0%) | 0 (0%) | 1 (1%) |
| Univariate Analysis | Multivariate Analysis * | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 0.998 (0.978–1.109) | 0.868 | 0.993 (0.970–1.017) | 0.993 |
| Gender (Female) | 0.873 (0.495–1.542) | 0.641 | 0.900 (0501–1.617) | 0.725 |
| BMI | 1.099 (1.038–1.164) | 0.001 | 1.054 (0990–1.121) | 0.098 |
| Hypertension | 1.230 (0.815–1.855) | 0.324 | - | |
| LA diameter | 1.073 (1.039–1.108) | <0.001 | 1.082 (1.046–1.119) | <0.001 |
| Type of AF | - | |||
| Paroxysmal | Reference | |||
| Persistent | 1.942 (1.279–2.947) | 0.002 | ||
| Ablation method | ||||
| TTA | Reference | Reference | ||
| RFCA | 0.854 (0.511–1.430) | 0.273 | 1.333 (0.722–2.301) | 0.302 |
| CRYO | 1.289 (0.765–2.171) | 0.341 | 1.773 (1.029–3.055) | 0.039 |
| RFCA (n = 125) | CBA (n = 97) | TTA (n = 131) | |
|---|---|---|---|
| Procedural complication | 3 (2%) | 4 (4%) | 5 (4%) |
| Death | 0 (0%) | 0 (0%) | 1 (1%) |
| Stroke/TIA | 0 (0%) | 0 (0%) | 1 (1%) |
| Atrial-esophageal fistula | 0 (0%) | 0 (0%) | 0 (0%) |
| Pericardial effusion | 0 (0%) | 1 (1%) | 0 (0%) |
| Pericarditis | 3 (2%) | 0 (0%) | 2 (2%) |
| Sternal wound infection | 0 (0%) | 0(0%) | 1 (1%) |
| Vascular access complication | 0 (0%) | 1 (1%) | 0 (0%) |
| Phrenic nerve injury | 0 (0%) | 2 (2%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-J.; Choi, J.H.; Kim, H.R.; Park, S.-J.; Jeong, D.S.; On, Y.K.; Kim, J.S.; Park, K.-M. Radiofrequency vs. Cryoballoon vs. Thoracoscopic Surgical Ablation for Atrial Fibrillation: A Single-Center Experience. Medicina 2021, 57, 1023. https://doi.org/10.3390/medicina57101023
Kwon H-J, Choi JH, Kim HR, Park S-J, Jeong DS, On YK, Kim JS, Park K-M. Radiofrequency vs. Cryoballoon vs. Thoracoscopic Surgical Ablation for Atrial Fibrillation: A Single-Center Experience. Medicina. 2021; 57(10):1023. https://doi.org/10.3390/medicina57101023
Chicago/Turabian StyleKwon, Hee-Jin, Ji Hoon Choi, Hye Ree Kim, Seung-Jung Park, Dong Seop Jeong, Young Keun On, June Soo Kim, and Kyoung-Min Park. 2021. "Radiofrequency vs. Cryoballoon vs. Thoracoscopic Surgical Ablation for Atrial Fibrillation: A Single-Center Experience" Medicina 57, no. 10: 1023. https://doi.org/10.3390/medicina57101023
APA StyleKwon, H.-J., Choi, J. H., Kim, H. R., Park, S.-J., Jeong, D. S., On, Y. K., Kim, J. S., & Park, K.-M. (2021). Radiofrequency vs. Cryoballoon vs. Thoracoscopic Surgical Ablation for Atrial Fibrillation: A Single-Center Experience. Medicina, 57(10), 1023. https://doi.org/10.3390/medicina57101023






