Chronological Appearance of Endocrine and Metabolic Dysfunctions Induced by an Unhealthy Diet in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Animals
2.3. Serum Measurements
2.4. Protein Carbonyl Groups and Reduced Glutathione (GSH)
2.5. Liver Triglyceride Content
2.6. Liver Glycogen Content
2.7. Glucokinase (EC 2.7.1.2) Activity
2.8. Glucose-6-Phosphatase (G6Pase) Activity
2.9. Glucose-6-Phosphate Dehydrogenase (G6P-DH) Activity
2.10. Islet Isolation
2.11. Insulin Secretion
2.12. Total RNA
2.13. Analysis of Gene Expression by Real-Time Polymerase Chain Reaction (qPCR)
2.14. Statistical Analysis
3. Results
3.1. Body Weight and Water Intake
3.2. Serum Glucose, Insulin, and Triglyceride Levels
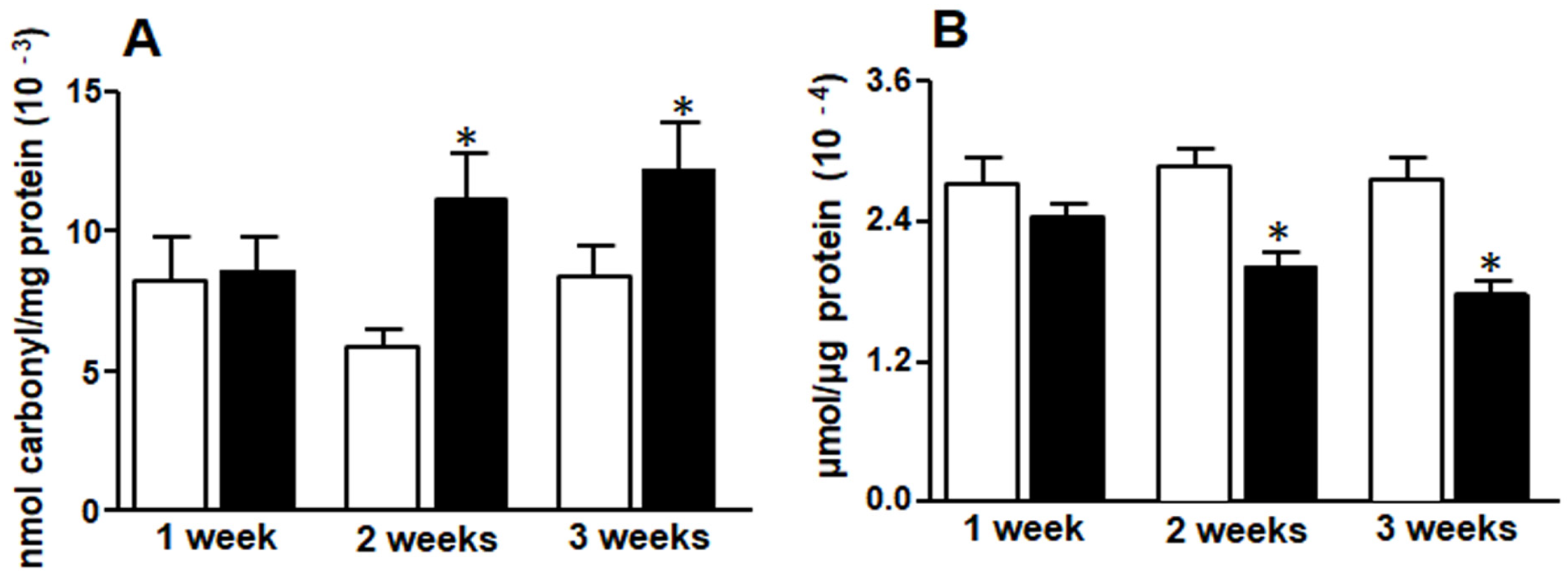
3.3. Liver Protein Carbonyl Groups and Reduced Glutathione (GSH)
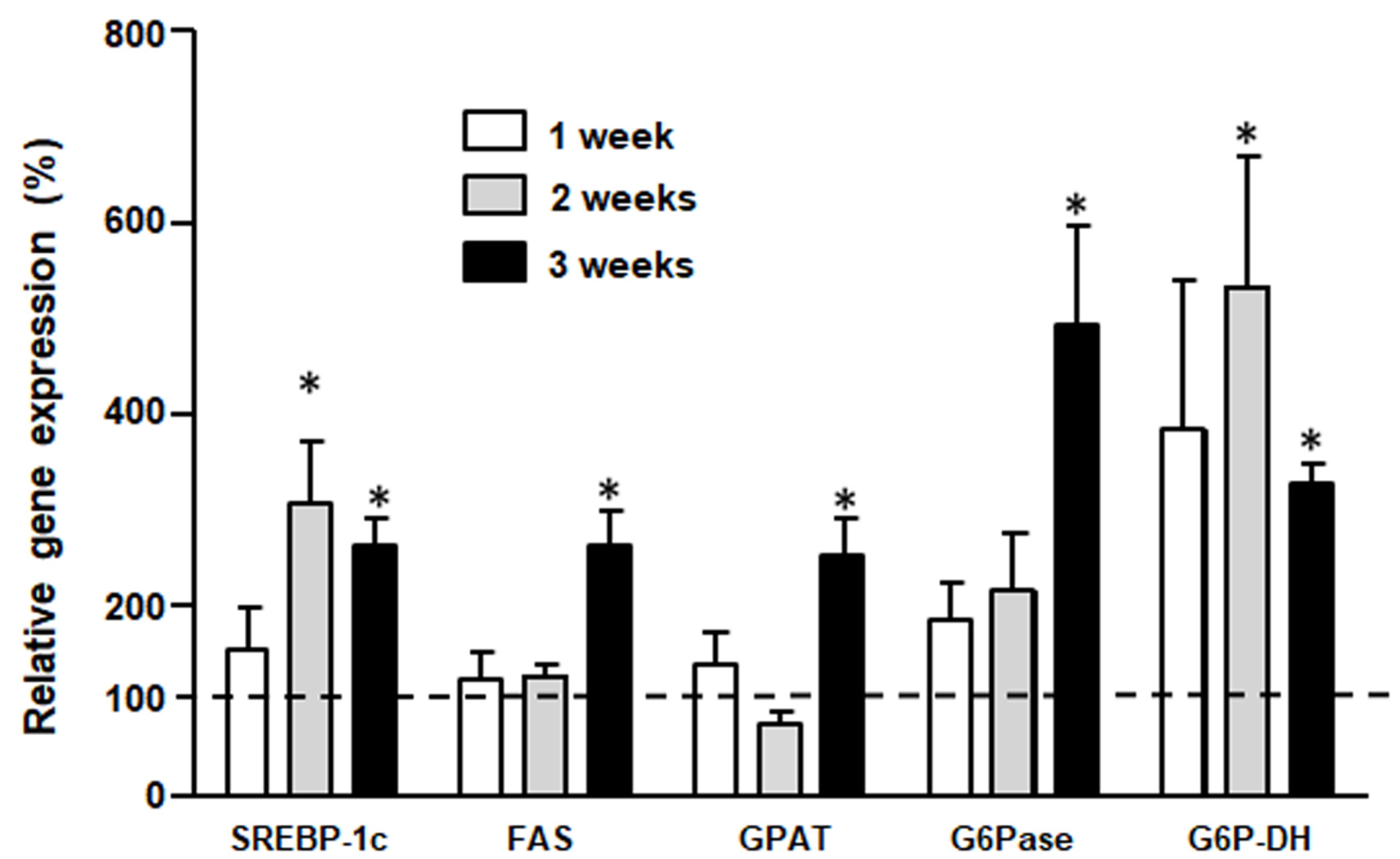
3.4. Liver Gene Expression (by qPCR)
3.5. Content of Liver Triglyceride and Glycogen
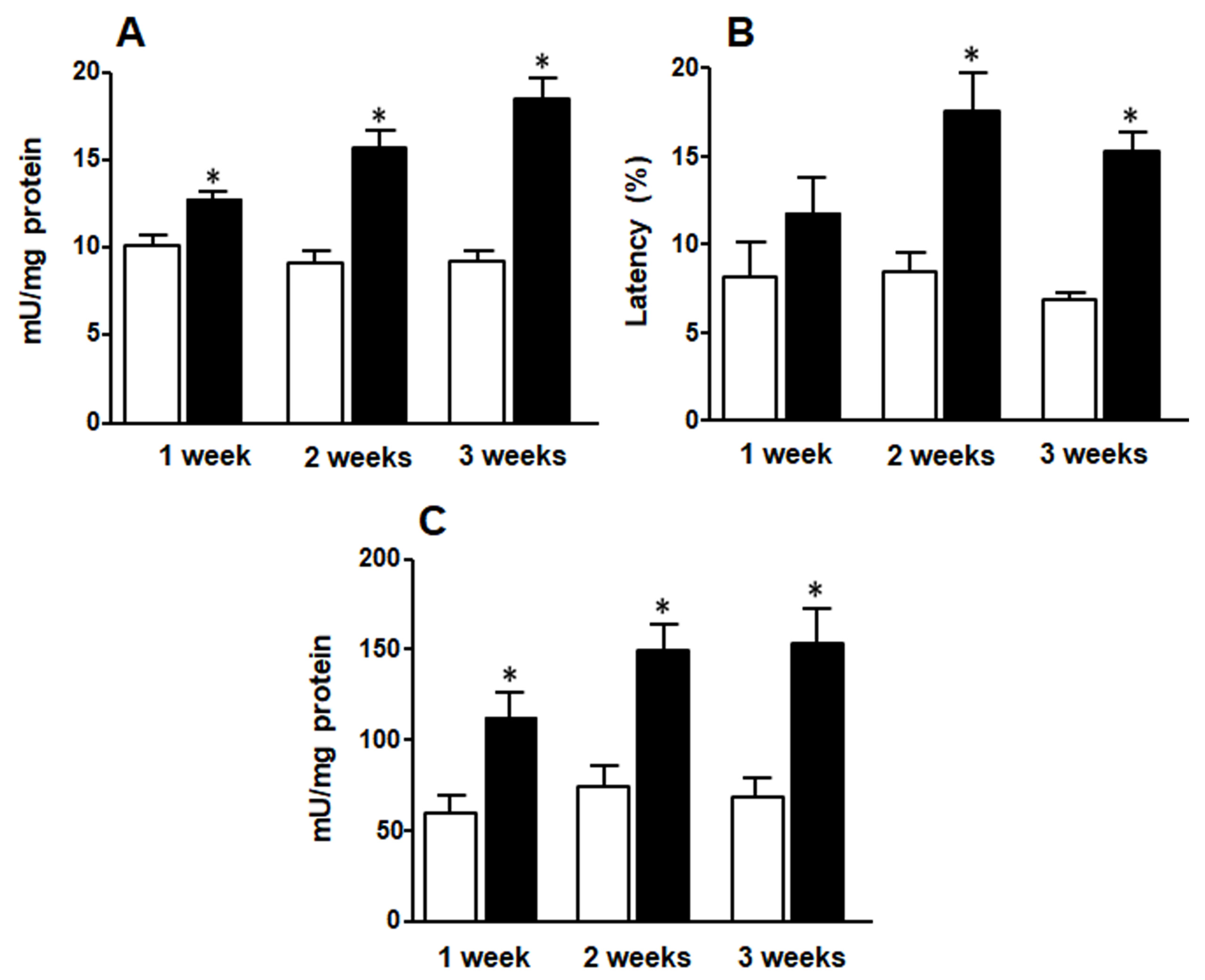
3.6. Liver Glucokinase, G6P-DH, and G6Pase Activities
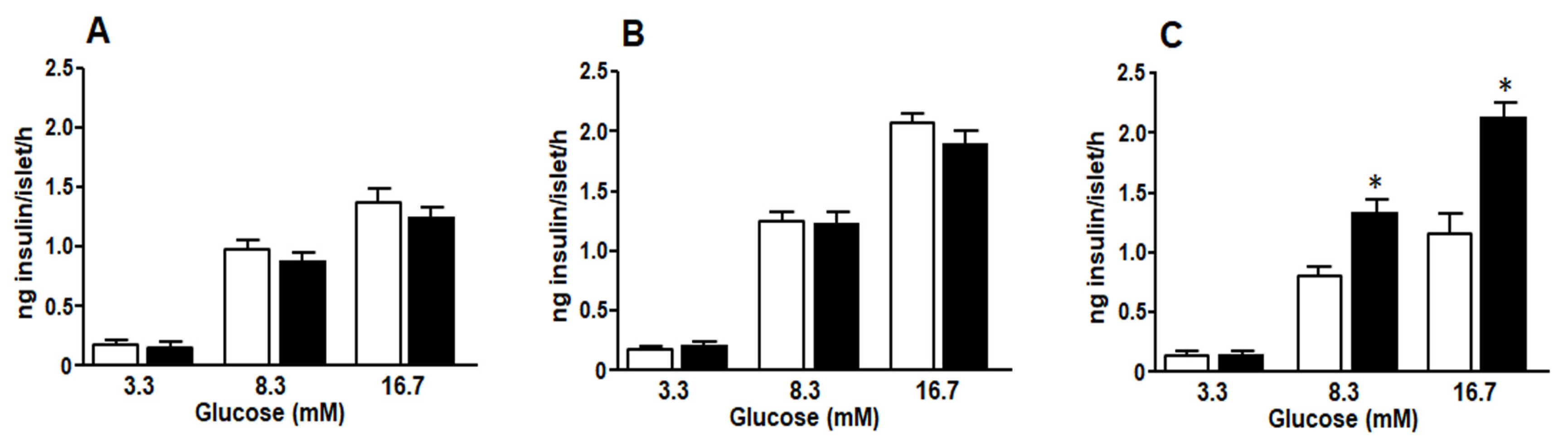
3.7. Glucose Stimulated Insulin Secretion
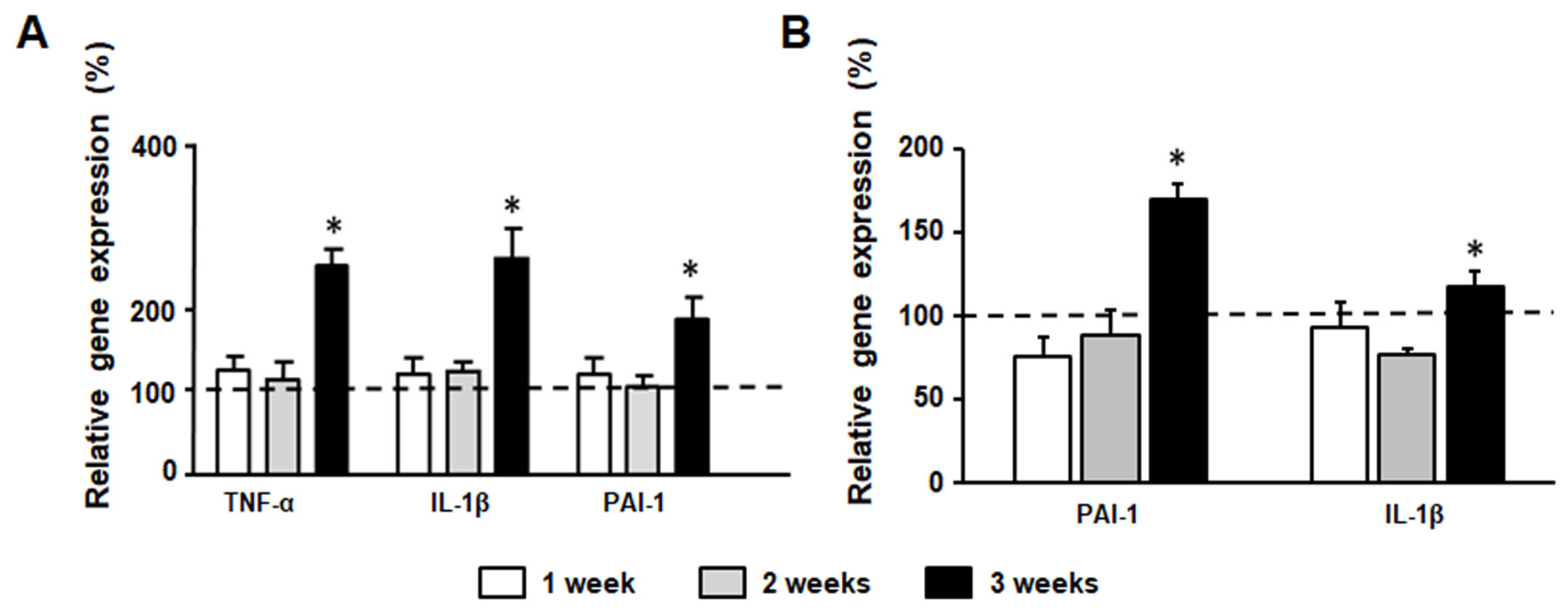
3.8. Inflammation Marker Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Rosinger, A.; Herrick, K.; Gahche, J.; Park, S. Sugar-sweetened beverage consumption among U.S. adults, 2011–2014. NCHS Data Brief 2017, 270, 1–8. [Google Scholar]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metabol. 2011, 22, 60–65. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Huang, R.C.; Mori, T.A.; Beilin, L.J.; Jebb, S.A. Prospective associations between sugar-sweetened beverage intake and cardiometabolic risk factors in adolescents. Am. J. Clin. Nutr. 2013, 98, 327–334. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Pan, A.; Hu, F.B. Effects of carbohydrates on satiety: Differences between liquid and solid food. Curr. Opin Clin. Nutr. Metab. Care 2011, 14, 385–390. [Google Scholar] [CrossRef]
- Cassady, B.A.; Considine, R.V.; Mattes, R.D. Beverage consumption, appetite and energy intake: What did you expect? Am. J. Clin. Nutr. 2012, 95, 587–593. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef]
- Clifton, P.M.; Chan, L.; Moss, C.L.; Miller, M.D.; Cobiac, L. Beverage intake and obesity in Australian children. Nutr. Metab. 2011, 8, 87. [Google Scholar] [CrossRef]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef]
- Francini, F.; Castro, M.C.; Gagliardino, J.J.; Massa, M.L. Regulation of liver glucokinase activity in rats with fructose-induced insulin resistance and impaired glucose and lipid metabolism. Can. J. Physiol. Pharmacol. 2009, 87, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Francini, F.; Castro, C.; Schinella, G.; García, M.E.; Maiztegui, B.; Raschia, M.A.; Gagliardino, J.J.; Massa, M.L. Changes induced by a FRD on the hepatic metabolism and the antioxidant system. Life Sci. 2010, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Román, C.L.; Flores, L.E.; Maiztegui, B.; Raschia, M.A.; Del Zotto, H.; Gagliardino, J.J. Islet NADPH oxidase activity modulates β-cell mass and endocrine function in rats with fructose-induced oxidative stress. Biochim. Biophys. Acta 2014, 1840, 3475–3482. [Google Scholar] [CrossRef]
- Maiztegui, B.; Borelli, M.I.; Madrid, V.G.; Del Zotto, H.; Raschia, M.A.; Francini, F.; Massa, M.L.; Flores, L.E.; Rebolledo, O.R.; Gagliardino, J.J. sitagliptin prevents the development of metabolic and hormonal disturbances, increased β-cell apoptosis and liver steatosis induced by a fructose-rich diet in normal rats. Clin. Sci. 2011, 120, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Maiztegui, B.; Borelli, M.I.; Raschia, M.A.; Del Zotto, H.; Gagliardino, J.J. Islet adaptive changes to fructose-induced insulin resistance: Beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J. Endocrinol. 2009, 200, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Maiztegui, B.; Boggio, V.; Román, C.L.; Flores, L.E.; Zotto, H.D.; Ropolo, A.; Grasso, D.; Vaccaro, M.I.; Gagliardino, J.J. VMP1-related autophagy induced by a fructose-rich diet in β-cells: Its prevention by incretins. Clin. Sci. 2017, 131, 673–687. [Google Scholar] [CrossRef][Green Version]
- Maiztegui, B.; Román, C.L.; Gagliardino, J.J.; Flores, L.E. Impaired endocrine-metabolic homeostasis: Underlying mechanism of its induction by unbalanced diet. Clin. Sci. 2018, 132, 869–881. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indexes obtained from oral glucose tolerance testing. Diabetes Care 1999, 22, 1462–1479. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Wollins, N.E. A simple and rapid method to assay triacylglycerol in cells and tissues. J. Lipid Res. 2007, 48, 2514–2520. [Google Scholar] [CrossRef]
- Chun, Y.; Yin, Z.D. Glycogen assay for diagnosis of female genital Chlamydia trachomatis infection. J. Clin. Microbiol. 1998, 36, 1081–1082. [Google Scholar] [CrossRef]
- Massa, L.; Baltrusch, S.; Okar, D.A.; Lange, A.J.; Lenzen, S.; Tiedge, M. Interaction of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBPase-2) with glucokinase activates glucose phosphorylation and glucose metabolism in insulin-producing cells. Diabetes 2004, 53, 1020–1029. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Arion, W.J. Glucose-6-phosphatase. In Methods in Enzymology; Wood, W.A., Ed.; Academic Press: New York, NY, USA, 1966; Volume IX, pp. 619–625. [Google Scholar]
- Lange, A.J.; Arion, W.J.; Beaudet, A.L. Type Ib glycogen storage disease is caused by a defect in the flucose-6-phosphate translocase of the microsomal glucose-6-phosphatase system. J. Biol. Chem. 1980, 255, 8381–8384. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Muller, P.Y.; Janovjak, H.; Miserez, A.R.; Dobbie, Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Bio Tech. 2002, 32, 1372–1379. [Google Scholar]
- Bizeau, M.E.; Pagliassotti, M.J. Hepatic adaptations to sucrose and fructose. Metabolism 2005, 54, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, K.W.; Serlie, M.J. Fructose consumption, lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Thirunavukkarasu, V.; Anuradha, C.V. Influence of α-lipoic acid on lipid peroxidation and antioxidant defense system in blood of insulin-resistant rats. Diabetes Obes. Metab. 2004, 6, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Madani, S.; El Boustani, E.S.; Belleville, J.; Prost, J. Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats fed a diet enriched with fructose and saturated fatty acids. Nutrition 2005, 21, 240–248. [Google Scholar] [CrossRef]
- Cummings, B.P.; Stanhope, K.L.; Graham, J.L.; Evans, J.L.; Baskin, D.G.; Griffen, S.C.; Havel, P.J. Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: Amelioration by the antioxidant, α-lipoic acid. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1343–R1350. [Google Scholar] [CrossRef]
- Castro, M.C.; Francini, F.; Schinella, G.; Caldiz, C.I.; Zubiría, M.G.; Gagliardino, J.J.; Massa, M.L. Apocynin administration prevents the changes induced by a fructose-rich diet on rat liver metabolism and the antioxidant system. Clin. Sci. 2012, 123, 681–692. [Google Scholar] [CrossRef]
- Castro, M.C.; Massa, M.L.; Schinella, G.; Gagliardino, J.J.; Francini, F. Lipoic acid prevents liver metabolic changes induced by administration of a fructose-rich diet. BBA—GEN Subj. 2013, 1830, 2226–2232. [Google Scholar] [CrossRef]
- Castro, M.C.; Massa, M.L.; Arbeláez, L.G.; Schinella, G.; Gagliardino, J.J.; Francini, F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015, 137, 1–6. [Google Scholar] [CrossRef]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—What isotopic tracer studies tell us. Nutr. Metab. 2012, 9, 89. [Google Scholar] [CrossRef]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [CrossRef]
- McGuinness, O.P.; Cherrington, A.D. Effects of fructose on hepatic glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 441–448. [Google Scholar] [CrossRef]
- Petersen, K.F.; Laurent, D.; Yu, C.; Cline, G.W.; Shulman, G.I. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes 2001, 50, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Watford, M. Small amounts of dietary fructose dramatically increase hepatic glucose uptake through a novel mechanism of glucokinase activation. Nutr. Rev. 2002, 60, 253–257. [Google Scholar]
- Khan, A.; Efendic, S. Evidence that increased glucose cycling in islets of diabetic ob/ob mice is a primary feature of the disease. Am. J. Physiol. 1995, 269, E623–E626. [Google Scholar] [CrossRef]
- Ostenson, C.G.; Khan, A.; Abdel-Halim, S.M.; Guenifi, A.; Suzuki, K.; Goto, Y.; Efendic, S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rats. Diabetologia 1993, 36, 3–8. [Google Scholar] [CrossRef]
- Robertson, R.; Zhou, H.; Zhang, T.; Harmon, J.S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 2007, 48, 139–146. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Minireview: Secondary cell failure in type 2 diabetes a convergence of glucotoxicity and lipotoxicity. Endocrinology 2002, 143, 339–342. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Sauter, N.S.; Schulthess, F.T.; Galasso, R.; Castellani, L.W.; Maedler, K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 2008, 149, 2208–2218. [Google Scholar] [CrossRef]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar] [CrossRef]

| Gene | GeneBank Accession Number | Sequences |
|---|---|---|
| β-actin | NM_031144.2 | FW 5′-AGAGGGAAATCGTGCGTGAC-3′ RV 5′-CGATAGTGATGACCTGACCGT-3′ |
| SREBP-1c | XM_213329.5 | FW 5′-TTTCTTCGTGGATGGGGACT-3′ RV 5′-CTGTAGATATCCAAGAGCATC-3′ |
| FAS | NM_017332.1 | FW 5′-GTCTGCAGCTACCCACCCGTG-3′ RV 5′-CTTCTCCAGGGTGGGGACCAG -3′ |
| GPAT | NM_017274.1 | FW 5′-GACGAAGCCTTCCGAAGGA-3′ RV 5′-GACTTGCTGGCGGTGAAGAG-3′ |
| G6Pase | NM_013098.2 | FW 5′-GATCGCTGACCTCAGGAACGC-3′ RV 5′-AGAGGCACGGAGCTGTTGCTG-3′ |
| G6P-DH | NM_017006.2 | FW 5′-TTCCGGGATGGCCTTCTAC-3′ RV 5′-TTTGCGGATGTCATCCACTGT-3′ |
| TNF-α | NM_012675.3 | FW 5′-GGCATGGATCTCAAAGACAACC-3′ RV 5′-CAAATCGGCTGACGGTGTG-3′ |
| IL-1β | NM_031512.2 | FW 5′-ACAAGGAGAGACAAGCAACGAC-3′ RV 5′-TCTTCTTTGGGTATTGTTTGGG-3′ |
| PAI-1 | NM_012620.1 | FW 5′-CCACGGTGAAGCAGGTGGACT-3′ RV 5′-TGCTGGCCTCTAAGAAGGGG-3′ |
| C1 | F1 | C2 | F2 | C3 | F3 | |
|---|---|---|---|---|---|---|
| Body weight gain (g) | 33.6 ± 3.3 | 29.1 ± 2.9 | 61.0 ± 6.6 | 77.4 ± 3.3 | 95.5 ± 8.1 | 94.25 ± 10.9 |
| Food intake (g/rat/day) | 18.6 ± 0.4 | 12.4 ± 0.6 * | 18.7 ± 0.9 | 14.2 ± 0.8 * | 19.6 ± 0.6 | 15.3 ± 1.0 * |
| Drink intake (ml/rat/day) | 22.0 ± 0.1 | 51.3 ± 7.9 * | 23.6 ± 1.3 | 64.7 ± 9.3 * | 25.3 ± 0.6 | 58.1 ± 0.2 * |
| Calories Kcal/day | 53.8 ± 3.9 | 56.6 ± 4.1 | 57.5 ± 2.5 | 63.5 ± 5.5 | 60.1 ± 5.9 | 65.1 ± 6.1 |
| C1 | F1 | C2 | F2 | C3 | F3 | |
|---|---|---|---|---|---|---|
| Glycemia (mg/dL) | 115 ± 3 | 118 ± 4 | 103 ± 3 | 121 ± 6 | 117 ± 2 | 117 ± 3 |
| Insulinemia (ng/mL) | 0.5 ± 0.07 | 0.6 ± 0.06 | 0.6 ± 0.06 | 0.7 ± 0.06 | 0.6 ± 0.04 | 1.1 ± 0.20 * |
| Triglyceridemia (mg/dL) | 103 ± 6 | 134 ± 11 * | 112 ± 15 | 172 ± 15 * | 102 ± 11 | 226 ± 31 * |
| HIS | 0.29 ± 0.05 | 0.23 ± 0.02 | 0.26 ± 0.04 | 0.19 ± 0.02 | 0.23 ± 0.01 | 0.13 ± 0.02 * |
| HOMA-IR | 3.5 ± 0.3 | 4.4 ± 0.5 | 3.8 ± 0.4 | 5.2 ± 0.5 | 4.3 ± 0.3 | 7.9 ± 0.6 * |
| HOMA-β | 35.6 ± 3.5 | 41.9 ± 4.8 | 48.9 ± 5.0 | 48.7 ± 4.5 | 42.6 ± 4.0 | 81.4 ± 6.8 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, M.C.; Villagarcía, H.G.; Román, C.L.; Maiztegui, B.; Flores, L.E.; Schinella, G.R.; Massa, M.L.; Francini, F. Chronological Appearance of Endocrine and Metabolic Dysfunctions Induced by an Unhealthy Diet in Rats. Medicina 2022, 58, 8. https://doi.org/10.3390/medicina58010008
Castro MC, Villagarcía HG, Román CL, Maiztegui B, Flores LE, Schinella GR, Massa ML, Francini F. Chronological Appearance of Endocrine and Metabolic Dysfunctions Induced by an Unhealthy Diet in Rats. Medicina. 2022; 58(1):8. https://doi.org/10.3390/medicina58010008
Chicago/Turabian StyleCastro, María Cecilia, Hernán Gonzalo Villagarcía, Carolina Lisi Román, Bárbara Maiztegui, Luis Emilio Flores, Guillermo Raúl Schinella, María Laura Massa, and Flavio Francini. 2022. "Chronological Appearance of Endocrine and Metabolic Dysfunctions Induced by an Unhealthy Diet in Rats" Medicina 58, no. 1: 8. https://doi.org/10.3390/medicina58010008
APA StyleCastro, M. C., Villagarcía, H. G., Román, C. L., Maiztegui, B., Flores, L. E., Schinella, G. R., Massa, M. L., & Francini, F. (2022). Chronological Appearance of Endocrine and Metabolic Dysfunctions Induced by an Unhealthy Diet in Rats. Medicina, 58(1), 8. https://doi.org/10.3390/medicina58010008






