Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management
Abstract
1. Introduction
2. Epidemiology
3. Pathogenesis
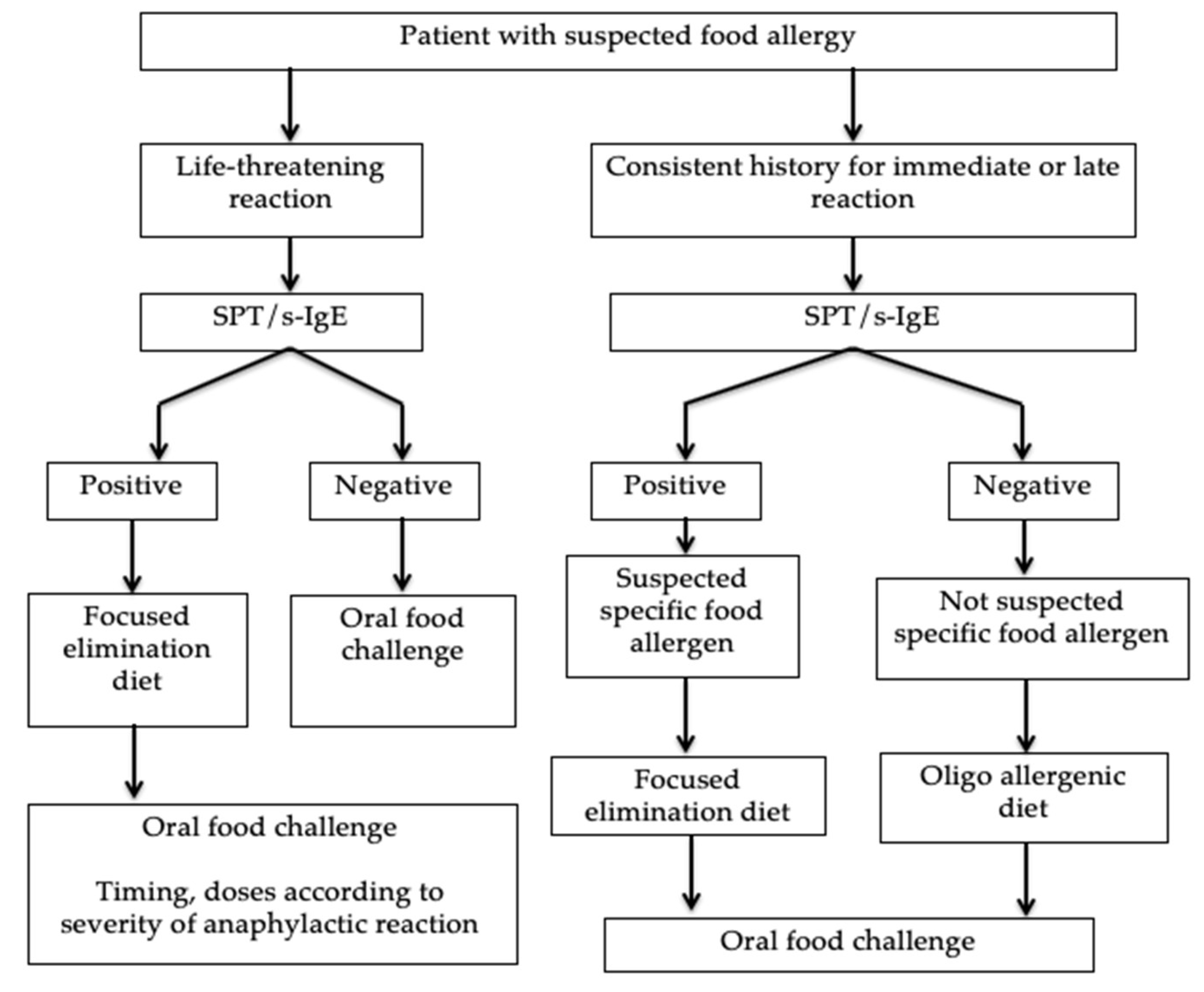
4. Diagnosis
5. Prevention
Timing the Introduction of Complementary Foods and the Risk of Developing Food Allergies
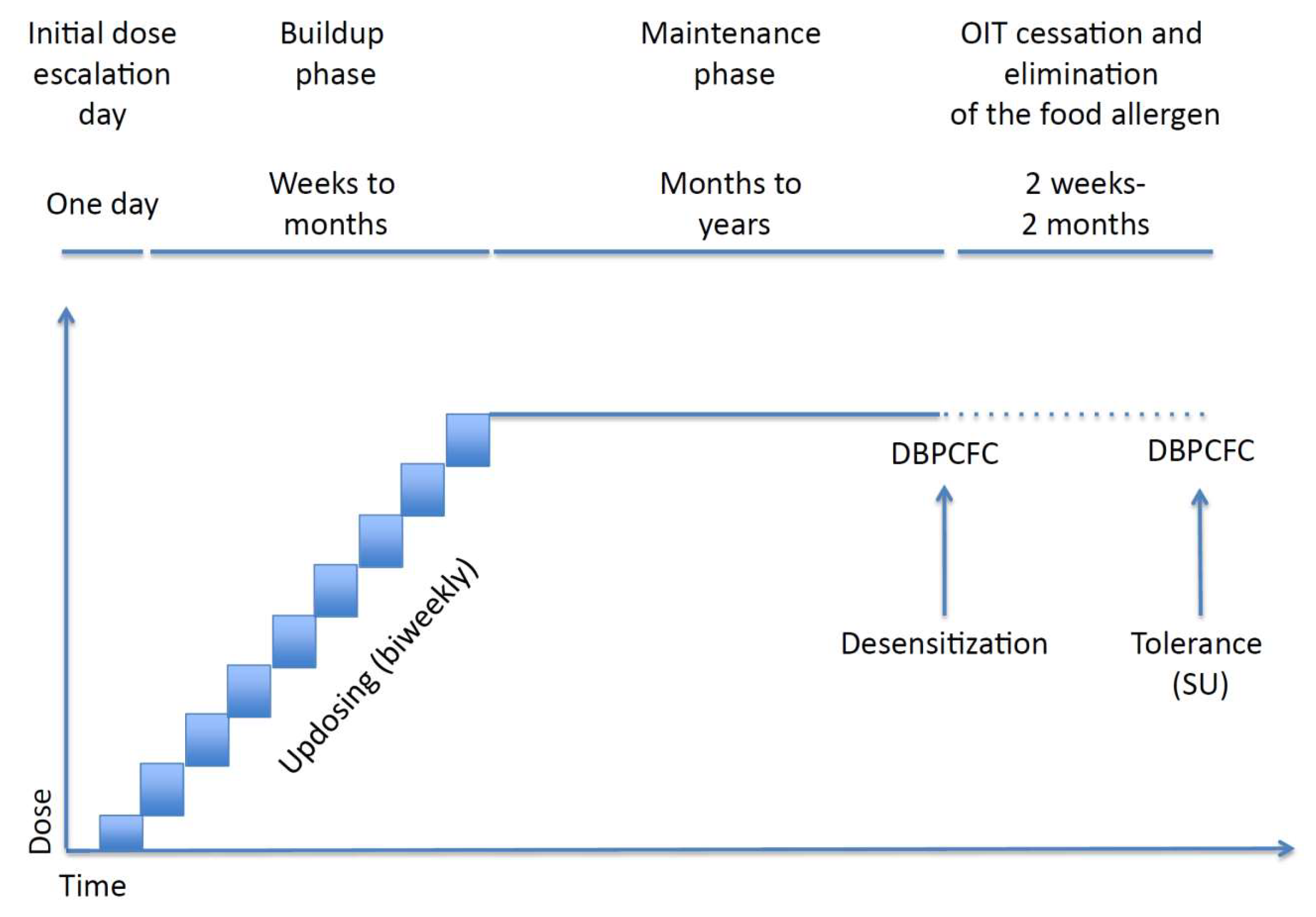
6. Management
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-sponsored expert panel report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011, 128, 9–17. [Google Scholar] [CrossRef]
- Winberg, A.; West, C.E.; Strinnholm, Å.; Nordström, L.; Hedman, L.; Rönmark, E. Assessment of Allergy to Milk, Egg, Cod, and Wheat in Swedish Schoolchildren: A Population Based Cohort Study. PLoS ONE 2015, 10, e0131804. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.C.; Matsui, E.C.; Peng, R.; Salo, P.M.; Zeldin, D.C.; Keet, C.A. Racial/ethnic and socioeconomic differences in self-reported food allergy among food-sensitized children in National Health and Nutrition Examination Survey III. Ann. Allergy Asthma Immunol. 2016, 117, 570–572. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [Google Scholar] [CrossRef]
- Mills, E.N.; Mackie, A.R.; Burney, P.; Beyer, K.; Frewer, L.; Madsen, C.; Botjes, E.; Crevel, R.W.; van Ree, R. The prevalence, cost and basis of food allergy across Europe. Allergy 2007, 62, 717–722. [Google Scholar] [CrossRef]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.; Dharmage, S.C.; et al. HealthNuts Investigators. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668–676. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Fiocchi, A.; Grabenhenrich, L.; Roberts, G.; Grimshaw, K.E.; Fiandor, A.; Larco, J.I.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Incidence and natural history of hen’s egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy 2016, 71, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.L.; Koplin, J.J.; Gurrin, L.C.; Dharmage, S.C.; Wake, M.; Ponsonby, A.L.; Tang, M.L.K.; Lowe, A.J.; Matheson, M.; Dwyer, T.; et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J. Allergy Clin. Immunol. 2017, 140, 145–153. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Moonesinghe, H.; Mackenzie, H.; Venter, C.; Kilburn, S.; Turner, P.; Weir, K.; Dean, T. Prevalence of fish and shellfish allergy: A systematic review. Ann. Allergy Asthma Immunol. 2016, 117, 264–272. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef]
- Schülke, S. Induction of Interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, W.; Zhu, H.J. The immune suppressive function of transforming growth factor-β (TGF-β) in human diseases. Growth 2015, 33, 92–101. [Google Scholar] [CrossRef]
- Wambre, E.; Bajzik, V.; DeLong, J.H.; O’Brien, K.; Nguyen, Q.A.; Speake, C.; Gersuk, V.H.; DeBerg, H.A.; Whalen, E.; Ni, C.; et al. A phenotypically and functionally distinct human T(H)2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017, 9, eaam9171c. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Iweala, O.I.; Burks, A.W. Food Allergy: Our Evolving Understanding of Its Pathogenesis, Prevention, and Treatment. Curr. Allergy Asthma Rep. 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy. 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update-2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Lack, G. Diagnosing peanut allergy with skin prick and specific IgE testing. J. Allergy Clin. Immunol. 2005, 115, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Sporik, R.; Hill, D.J.; Hosking, C.S. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin. Exp. Allergy 2000, 30, 1540–1546. [Google Scholar] [CrossRef]
- Sampson, H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J. Allergy Clin. Immunol. 2001, 107, 891–896. [Google Scholar] [CrossRef]
- Knight, A.K.; Shreffler, W.G.; Sampson, H.A.; Sicherer, S.H.; Noone, S.; Mofidi, S.; Nowak-Wegrzyn, A. Skin prick test to egg white provides additional diagnostic utility to serum egg white- specific IgE antibody concentration in children. J. Allergy Clin. Immunol. 2006, 117, 842–847. [Google Scholar] [CrossRef]
- Sampson, H.A.; van Wijk, R.G.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar]
- Gomes-Belo, J.; Hannachi, F.; Swan, K.; Santos, A.F. Advances in Food Allergy Diagnosis. Curr. Pediatr. Rev. 2018, 14, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B. Hazelnut allergens: Molecular characterization, detection, and clinical relevance. Crit. Rev. Food Sci. Nutr. 2016, 56, 2579–2605. [Google Scholar] [CrossRef] [PubMed]
- Masthoff, L.J.; Mattsson, L.; Zuidmeer-Jongejan, L.; Lidholm, J.; Andersson, K.; Akkerdaas, J.H.; Versteeg, S.A.; Garino, C.; Meijer, Y.; Kentie, P.; et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J. Allergy Clin. Immunol. 2013, 132, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef]
- Santos, A.F.; Lack, G. Basophil activation test: Food challenge in a test tube or specialist research tool? Clin. Transl. Allergy 2016, 6, 10. [Google Scholar] [CrossRef]
- Glaumann, S.; Nopp, A.; Johansson, S.G.; Rudengren, M.; Borres, M.P.; Nilsson, C. Basophil allergen threshold sensitivity, CD-sens, IgE- sensitization and DBPCFC in peanut-sensitized children. Allergy 2012, 67, 242–247. [Google Scholar] [CrossRef]
- Ocmant, A.; Mulier, S.; Hanssens, L.; Goldman, M.; Casimir, G.L.; Mascart, F.; Schandené, L. Basophil activation tests for the diagnosis of food allergy in children. Clin. Exp. Allergy 2009, 39, 1234–1245. [Google Scholar] [CrossRef]
- Sato, S.; Tachimoto, H.; Shukuya, A.; Kurosaka, N.; Yanagida, N.; Utsunomiya, T.; Iguchi, M.; Komata, T.; Imai, T.; Tomikawa, M.; et al. Basophil activation marker CD203c is useful in the diagnosis of hen’s egg and cow’s milk allergies in children. Int. Arch. Allergy Immunol. 2010, 152 (Suppl. 1), 54–61. [Google Scholar] [CrossRef]
- Tokuda, R.; Nagao, M.; Hiraguchi, Y.; Hosoki, K.; Matsuda, T.; Kouno, K.; Morita, E.; Fujisawa, T. Antigen-induced expression of CD203c on basophils predicts IgE-mediated wheat allergy. Allergol. Int. 2009, 58, 193–199. [Google Scholar] [CrossRef]
- Santos, A.F.; Douiri, A.; Becares, N.; Wu, S.Y.; Stephens, A.; Radulovic, S.; Chan, S.M.; Fox, A.T.; Du Toit, G.; Turcanu, V.; et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014, 134, 645–652. [Google Scholar] [CrossRef]
- Gamboa, P.M.; Cáceres, O.; Antepara, I.; Sánchez-Monge, R.; Ahrazem, O.; Salcedo, G.; Barber, D.; Lombardero, M.; Sanz, M.L. Two different profiles of peach allergy in the north of Spain. Allergy 2007, 62, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Assa’ad, A.H.; Bahna, S.L.; Bock, S.A.; Sicherer, S.H.; Teuber, S.S.; Adverse Reactions to Food Committee of American Academy of Allergy; Asthma & Immunology. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology. Work Group report: Oral food challenge testing. J. Allergy Clin. Immunol. 2009, 123 (Suppl. 6), S365–S383. [Google Scholar] [PubMed]
- Wood, R.A. Diagnostic elimination diets and oral food provocation. Chem. Immunol. Allergy 2015, 101, 87–95. [Google Scholar] [PubMed]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Litonjua, A.A. Childhood asthma is a fat- soluble vitamin deficiency disease. Clin. Exp. Allergy 2008, 38, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Horwood, L.J.; Shannon, F.T. Asthma and infant diet. Arch. Dis. Child. 1983, 58, 48–51. [Google Scholar] [CrossRef]
- Kajosaari, M.; Saarinen, U.M. Prophylaxis of atopic disease by six months’ total solid food elimination. Evaluation of 135 exclusively breast-fed infants of atopic families. Acta Paediatr. Scand. 1983, 72, 411–414. [Google Scholar] [CrossRef]
- Forsyth, J.S.; Ogston, S.A.; Clark, A.; Florey, C.D.; Howie, P.W. Relation between early introduction of solid food to infants and their weight and illnesses during the first two years of life. BMJ 1993, 306, 1572–1576. [Google Scholar] [CrossRef]
- Kleinman, R.E. American Academy of Pediatrics recommendations for complementary feeding. Pediatrics 2000, 106, 1274. [Google Scholar]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Sayre, P.H.; Roberts, G.; Sever, M.L.; Lawson, K.; Bahnson, H.T.; Brough, H.A.; Santos, A.F.; Harris, K.M.; Radulovic, S.; et al. Effect of avoidance on peanut allergy after early peanut consumption. N. Engl. J. Med. 2016, 374, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.J.; Metcalf, J.; Makrides, M.; Gold, M.S.; Quinn, P.; West, C.E.; Loh, R.; Prescott, S.L. Early regular egg exposure in infants with eczema: A randomized controlled trial. J. Allergy Clin. Immunol. 2013, 132, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.J.; Sullivan, T.R.; Gold, M.S.; Prescott, S.L.; Makrides, M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1600–1607. [Google Scholar] [CrossRef]
- Bellach, J.; Schwarz, V.; Ahrens, B.; Trendelenburg, V.; Aksünger, Ö.; Kalb, B.; Niggemann, B.; Keil, T.; Beyer, K. Randomized placebo- controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 2017, 139, 1591–1599. [Google Scholar] [CrossRef]
- Wei-Liang Tan, J.; Valerio, C.; Barnes, E.H.; Turner, P.J.; Van Asperen, P.A.; Kakakios, A.M.; Campbell, D.E.; Beating Egg Allergy Trial (BEAT) Study Group. A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1621–1628. [Google Scholar] [CrossRef]
- Natsume, O.; Kabashima, S.; Nakasato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized trial of intro- duction of allergenic foods in breast fed infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef]
- Grimshaw, K.; Logan, K.; O’Donovan, S.; Kiely, M.; Patient, K.; van Bilsen, J.; Beyer, K.; Campbell, D.E.; Garcia-Larsen, V.; Grabenhenrich, L.; et al. Modifying the infant’s diet to prevent food allergy. Arch. Dis. Child. 2017, 102, 179–186. [Google Scholar] [CrossRef]
- Caffarelli, C.; Di Mauro, D.; Mastrorilli, C.; Bottau, P.; Cipriani, F.; Ricci, G. Solid Food Introduction and the Development of Food Allergies. Nutrients 2018, 10, E1790. [Google Scholar] [CrossRef]
- Campbell, D.; Vale, S.; Smith, J.; Roche, I.; Netting, M.; Allen, K. ASCIA-P5: ASCIA Guidelines for Infant Feeding and Allergy Prevention. Intern. Med. J. 2016, 46, 6. [Google Scholar] [CrossRef]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Allergy Clin. Immunol. 2017, 139, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.H.; Shek, L.P.-C.; Van Bever, H.P.; Vichyanond, P.; Ebisawa, M.; Wong, G.W.; Lee, B.W. Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI). Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective—An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr. Allergy Immunol. 2018, 29, 18–27. [Google Scholar]
- British Society for Allergy and Clinical Immunology/British Dietetic Association. Preventing Food Allergy in Higher Risk Infants: Guidance for Healthcare Professionals. 2018. Available online: https://www.bsaci.org/about/early-feeding-guidance (accessed on 30 July 2018).
- World Health Organization. Nutrition: Exclusive Breastfeeding. 2010. Available online: http://www.who.int/nutrition/topics/exclusive_breastfeeding/en/index.html (accessed on 30 July 2018).
- Worm, M.; Reese, I.; Ballmer-Weber, B.; Beyer, K.; Bischoff, S.C.; Classen, M.; Fischer, P.J.; Fuchs, T.; Huttegger, I.; Jappe, U.; et al. Guidelines on the management of IgE mediated food allergies. Guidelines of the German Society for Allergology and Clinical Immunology. Allergo J. Int. 2015, 24, 256–293. [Google Scholar] [CrossRef]
- Narisety, S.D.; Frischmeyer-Guerrerio, P.A.; Keet, C.A.; Gorelik, M.; Schroeder, J.; Hamilton, R.G.; Wood, R.A. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J. Allergy Clin. Immunol. 2015, 135, 1275–1282. [Google Scholar] [CrossRef]
- Nurmatov, U.; Dhami, S.; Arasi, S.; Pajno, G.B.; Fernandez-Rivas, M.; Muraro, A.; Roberts, G.; Akdis, C.; Alvaro-Lozano, M.; Beyer, K.; et al. Allergen immunotherapy for IgE-mediated food allergy: A systematic review and meta-analysis. Allergy 2017, 72, 1133–1147. [Google Scholar] [CrossRef]
- Gernez, Y.; Nowak-Węgrzyn, A. Immunotherapy for Food Allergy: Are We There Yet? J. Allergy Clin. Immunol. Pract. 2017, 5, 250–272. [Google Scholar] [CrossRef]
- Pajno, G.B.; Castagnoli, R.; Muraro, A.; Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Arasi, S. Allergen immunotherapy for IgE-mediated food allergy: There is a measure in everything to a proper proportion of therapy. Pediatr. Allergy Immunol. 2019, 30, 415–422. [Google Scholar] [CrossRef]
- Jones, S.M.; Burks, A.W.; Keet, C.; Vickery, B.P.; Scurlock, A.M.; Wood, R.A.; Liu, A.H.; Sicherer, S.H.; Henning, A.K.; Lindblad, R.W.; et al. Consortium of Food Allergy Research (CoFAR). Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J. Allergy Clin. Immunol. 2016, 137, 1117–1127. [Google Scholar] [CrossRef]
- Burks, A.W.; Wood, R.A.; Jones, S.M.; Sicherer, S.H.; Fleischer, D.M.; Scurlock, A.M.; Vickery, B.P.; Liu, A.H.; Henning, A.K.; Lindblad, R.; et al. Consortium of Food Allergy Research. Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. J. Allergy Clin. Immunol. 2015, 135, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Arias, A.; Tenias, J.M. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: A systematic review with meta-analysis. Ann. Allergy Asthma Immunol. 2014, 113, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Petroni, D.; Spergel, J.M. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann. Allergy Asthma Immunol. 2018, 120, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.R.; Nachshon, L.; Levy, M.B.; Elizur, A.; Katz, Y. Risk Factors and Treatment Outcomes for Oral Immunotherapy-Induced Gastrointestinal Symptoms and Eosinophilic Responses (OITIGER). J. Allergy Clin. Immunol. Pract. 2020, 8, 125–131. [Google Scholar] [CrossRef]
- Viaskin Epicutaneous Immunotherapy. Available online: https://www.dbv-technologies.com/viaskin-platform (accessed on 27 April 2016).
- Jones, S.M.; Sicherer, S.H.; Burks, A.W.; Leung, D.Y.; Lindblad, R.W.; Dawson, P.; Henning, A.K.; Berin, M.C.; Chiang, D.; Vickery, B.P.; et al. Consortium of Food Allergy Research. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J. Allergy Clin. Immunol. 2017, 139, 1242–1252. [Google Scholar] [CrossRef]
- Dupont, C.; Kalach, N.; Soulaines, P.; Legoué-Morillon, S.; Piloquet, H.; Benhamou, P.H. Cow’s milk epicutaneous immunotherapy in children: A pilot trial of safety, acceptability, and impact on allergic reactivity. J. Allergy Clin. Immunol. 2010, 125, 1165–1167. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, M.; Turner, P.J. Improving the safety of oral immunotherapy for food allergy. Pediatr. Allergy Immunol. 2016, 27, 117–125. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Lawson, K.; Masilamani, M.; Kattan, J.; Bahnson, H.T.; Sampson, H.A. Increased Tolerance to Less Extensively Heat-Denatured (Baked) Milk Products in Milk-Allergic Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 486–495. [Google Scholar] [CrossRef]
- Lambert, R.; Grimshaw, K.E.C.; Ellis, B.; Jaitly, J.; Roberts, G. Evidence that eating baked egg or milk influences egg or milk allergy resolution: A systematic review. Clin. Exp. Allergy 2017, 47, 829–837. [Google Scholar] [CrossRef]
- Esmaeilzadeh, H.; Alyasin, S.; Haghighat, M.; Nabavizadeh, H.; Esmaeilzadeh, E.; Mosavat, F. The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatr. Allergy Immunol. 2018, 29, 747–753. [Google Scholar] [CrossRef]
- Lin, C.; Lee, I.T.; Sampath, V.; Dinakar, C.; DeKruyff, R.H.; Schneider, L.C.; Nadeau, K.C. Combining anti-IgE with oral immunotherapy. Pediatr. Allergy Immunol. 2017, 28, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.C.; Rachid, R.; LeBovidge, J.; Blood, E.; Mittal, M.; Umetsu, D.T. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J. Allergy Clin. Immunol. 2013, 132, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Sampson, H.A.; Atkins, F.M.; Zeiger, R.S.; Lehrer, S.; Sachs, M.; Bush, R.K.; Metcalfe, D.D. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J. Allergy Clin. Immunol. 1988, 82, 986–997. [Google Scholar] [CrossRef]
- Lafuente, I.; Mazon, A.; Nieto, M.; Uixera, S.; Pina, R.; Nieto, A. Possible recurrence of symptoms after discontinuation of omalizumab in anti-IgE-assisted desensitization to egg. Pediatr. Allergy Immunol. 2014, 25, 717–719. [Google Scholar] [CrossRef]
- Wood, R.A.; Kim, J.S.; Lindblad, R.; Nadeau, K.; Henning, A.K.; Dawson, P.; Plaut, M.; Sampson, H.A. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J. Allergy Clin. Immunol. 2016, 137, 1103–1110. [Google Scholar] [CrossRef]
- Nadeau, K.C.; Schneider, L.C.; Hoyte, L.; Borras, I.; Umetsu, D.T. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J. Allergy Clin. Immunol. 2011, 127, 1622–1624. [Google Scholar] [CrossRef]
- Begin, P.; Dominguez, T.; Wilson, S.P.; Bacal, L.; Mehrotra, A.; Kausch, B.; Trela, A.; Tavassoli, M.; Hoyte, E.; O’Riordan, G.; et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin. Immunol. 2014, 10, 7. [Google Scholar] [CrossRef]
- El-Qutob, D. Off-Label Uses of Omalizumab. Clin. Rev. Allergy Immunol. 2016, 50, 84–96. [Google Scholar] [CrossRef]
- Tang, M.L.; Ponsonby, A.L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J. Allergy Clin. Immunol. 2015, 135, 737–744. [Google Scholar] [CrossRef]
- Hsiao, K.-C.; Ponsonby, A.-L.; Axelrad, C.; Pitkin, S.; Tang, M.L.K. Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomised, double-blind, placebo-controlled trial. Lancet Child Adolesc. Health 2017, 1, 97–105. [Google Scholar] [CrossRef]
| Food | >95% PPV | |
|---|---|---|
| SPT (mm) | s-IgE (KU/L) | |
| Egg white | ≥7 | ≥7 |
| Cow’s milk | ≥8 | ≥15 |
| Peanut | ≥8 | ≥14 |
| Fish | ≥20 | |
| Tolerance | Allergy | Ref. | Hypothesis |
|---|---|---|---|
Low rates of infant eczema:
| High rates of infant eczema:
| [44] | Dual allergen exposure |
High level of vitamin D:
| Vitamin D insufficiency:
| [45] | Vitamin D |
Dietary factors:
| Dietary factors:
| [44] | Dual allergen exposure |
High microbial exposure:
| Low microbial exposure:
| [46] | Hygiene |
| Scientific Society–Year (Reference) | ||||
|---|---|---|---|---|
| ASCIA, 2016 [61] | NIAID, 2017 [62] | ESPGHAN, 2017 [63] | APAPARI, 2018 [64] | BSACI 2018 [65] |
| Infants without eczema or food allergy
| Traditions and feeding patterns in the population on types of complementary foods should be considered.
| General population/at-risk infants (atopic family history, non-severe eczema)
| General population
|
| OIT | SLIT | EPIT | |
|---|---|---|---|
| Foods studied | Peanut, milk, egg, wheat | Peanut, milk, hazelnut, peach | Peanut, milk |
| Maintenance dose | 300–4000 mg | 2–7 mg | 50–500 μg (usually 250 μg) |
| Efficacy | More desirable Large effect on desensitisation | Less desirable Moderate effect | Currently being investigated |
| Safety | Less desirable | More desirable | More desirable |
| Adverse effects | Common during up-dosing Mostly gastrointestinal Can be systemic especially with co-factors; EoE < 8% | Mostly oro-pharyngeal Systemic reactions are rare | Local skin reactions |
| Adherence | Less good (especially due to GI symptoms) | Better than with OIT | Better than with OIT |
| Feasibility | Less good due to GI AE and changes to lifestyle | Easy | Easy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina 2020, 56, 111. https://doi.org/10.3390/medicina56030111
Barni S, Liccioli G, Sarti L, Giovannini M, Novembre E, Mori F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina. 2020; 56(3):111. https://doi.org/10.3390/medicina56030111
Chicago/Turabian StyleBarni, Simona, Giulia Liccioli, Lucrezia Sarti, Mattia Giovannini, Elio Novembre, and Francesca Mori. 2020. "Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management" Medicina 56, no. 3: 111. https://doi.org/10.3390/medicina56030111
APA StyleBarni, S., Liccioli, G., Sarti, L., Giovannini, M., Novembre, E., & Mori, F. (2020). Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina, 56(3), 111. https://doi.org/10.3390/medicina56030111






