Optimization and Rheological Study of an Exopolysaccharide Obtained from Fermented Mature Coconut Water with Lipomyces starkeyi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fermentation Conditions
2.3. Extraction of Crude LSEP
2.4. Single-Factor Experiment
2.5. Optimization of Crude LSEP Production
2.6. Rheological Measurement of Crude LSEP
2.7. Adsorption of Crude LSEP at MCT–Water Interface
3. Results
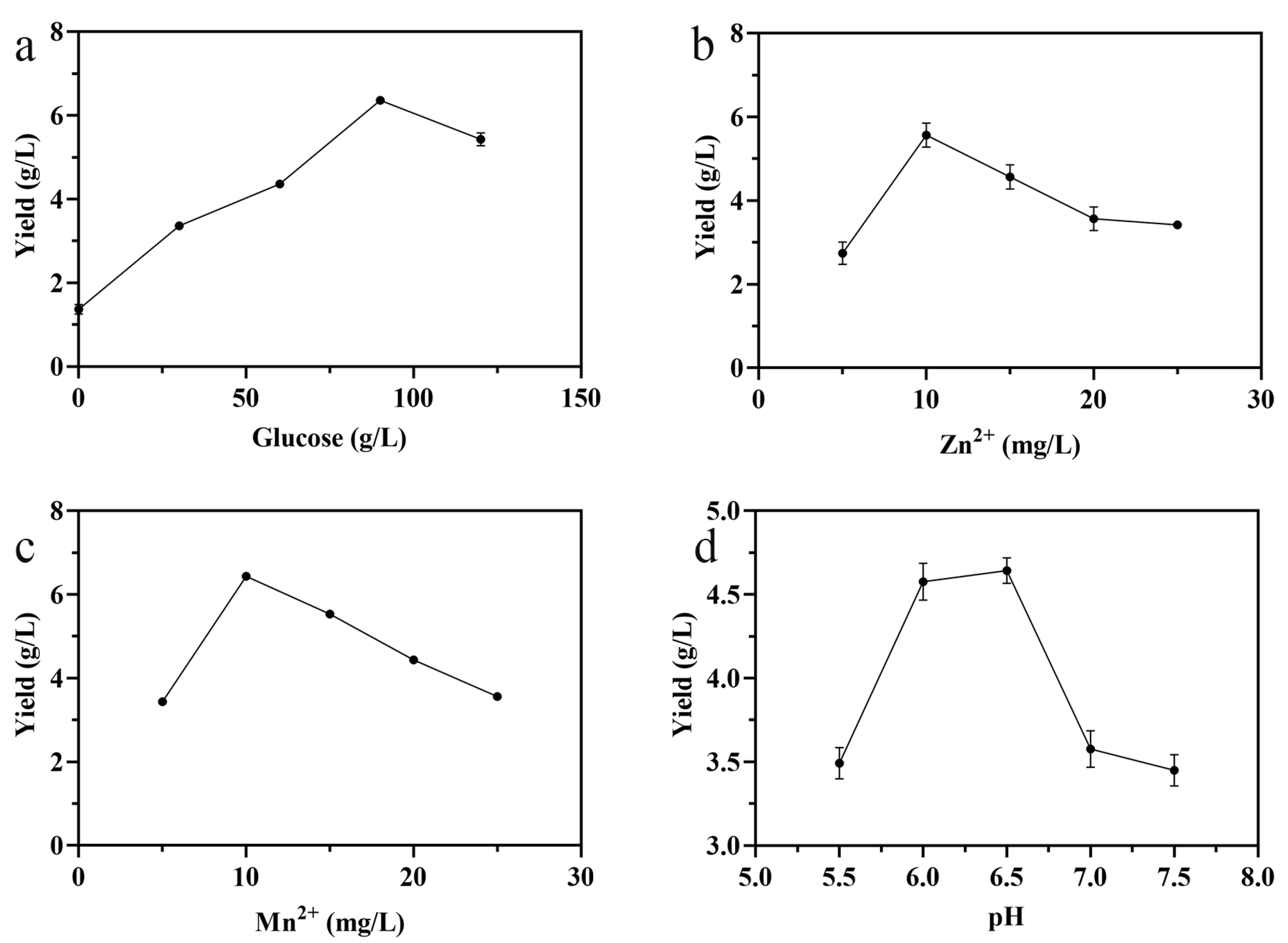
3.1. Single-Factor Experiment
3.2. Optimization of Crude LSEP Yield
3.2.1. Analysis of the Model
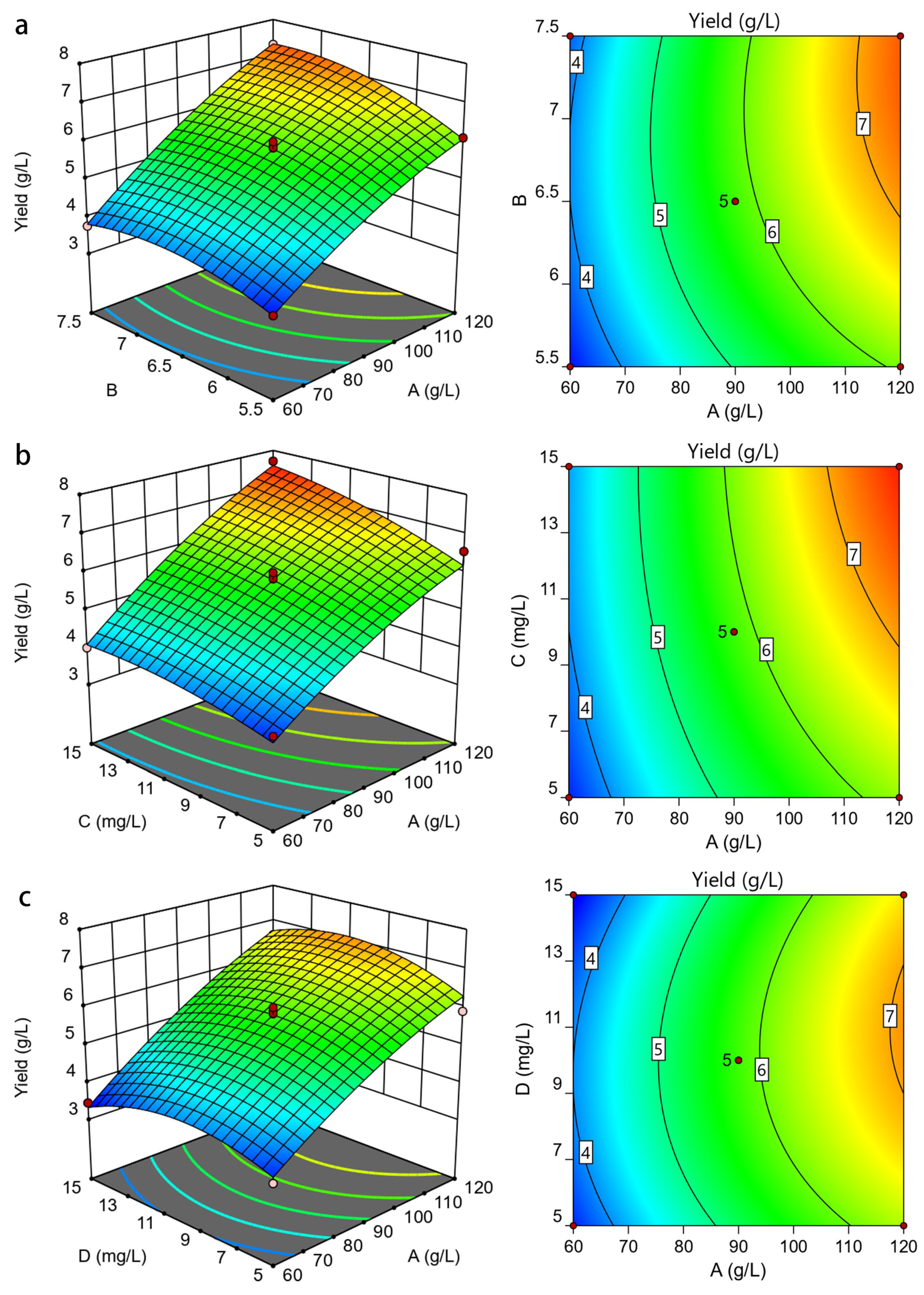
3.2.2. Analysis of Response Surface
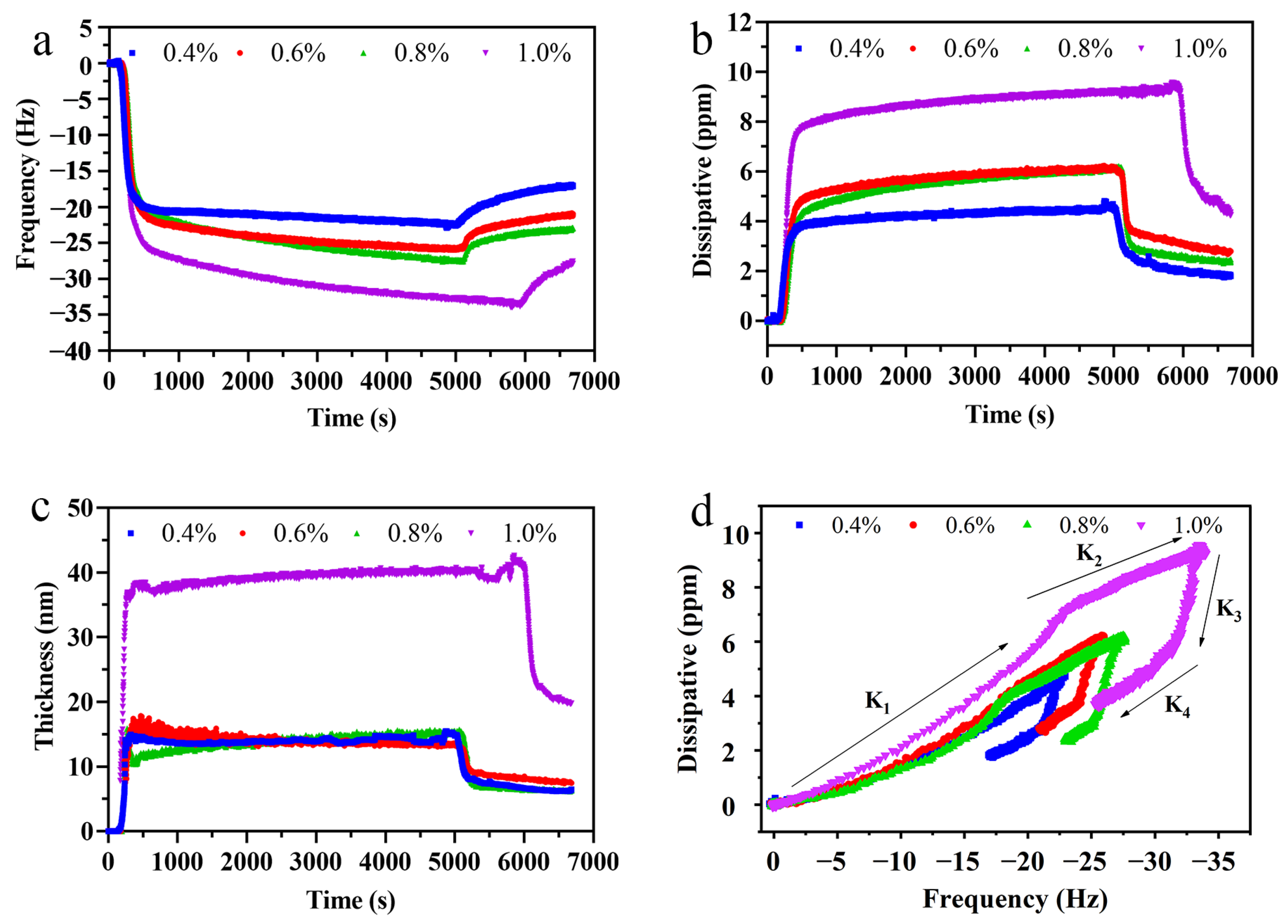
3.3. Rheological Properties of Crude LSEP
3.4. Adsorption of Crude LSEP at the MCT–Water Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.F.; Li, X.L.; Chen, W.X.; Chen, P.S.; Jin, X.F.; Chen, W.J.; Chen, H.M. Organic Acid Content, Antioxidant Capacity, and Fermentation Kinetics of Matured Coconut (Cocos nucifera) Water Fermented by Saccharomyces cerevisiae D254. Int. J. Food Eng. 2018, 14. [Google Scholar] [CrossRef]
- Zhang, G.F.; Chen, W.X.; Chen, W.J.; Chen, H.M. Improving the quality of matured coconut (Cocos nucifera Linn.) water by low alcoholic fermentation with Saccharomyces cerevisiae: Antioxidant and volatile profiles. J. Food Sci. Technol. 2018, 55, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Sankar, T.; Kungumaselvan, T.; Gopalakrishnan, M.; Gunasekaran, Y. Evaluation of mature coconut water on soil nutrient availability and stimulation plant growth and yield of Greengram. Pharma Innov. J. 2020, 9, 136–139. [Google Scholar]
- Anith, K.N. Mature coconut as a bio-fermentor for multiplication of plant growth promoting rhizobacteria. Curr. Sci. A Fortn. J. Res. 2009, 97, 1647–1653. [Google Scholar]
- Halib, N.; Amin, M.; Ahmad, I. Physicochemical Properties and Characterization of Nata de Coco from Local Food Industries as a Source of Cellulose. Sains Malays. 2012, 41, 205–211. [Google Scholar]
- Fan, H.; Wu, Y.; Hu, X.; Wu, J.; Liao, X. Characteristics of thin-layer drying and rehydration of nata de coco. Int. J. Food Sci. Technol. 2011, 46, 1438–1444. [Google Scholar] [CrossRef]
- Li, W.T.; Guo, Y.L.; Chen, H.M.; Chen, W.X.; Zhang, H.L.; Zhang, M.; Zhong, Q.P.; Chen, W.J. Physicochemical Characterization of an Exopolysaccharide Produced by Lipomyces sp. and Investigation of Rheological and Interfacial Behavior. Gels 2021, 7, 156. [Google Scholar] [CrossRef]
- Xu, S.; Ma, Z.; Chen, Y.; Li, J.; Jiang, H.; Qu, T.; Zhang, W.; Li, C.; Liu, S. Characterization of the flavor and nutritional value of coconut water vinegar based on metabolomics. Food Chem. 2022, 369, 130872. [Google Scholar] [CrossRef]
- Thirumal, V.; Chistoserdov, A.; Bajpai, R.; Bader, J.; Popovic, M.K.; Subramaniam, R. Effect of Developed Low Cost Minimal Medium on Lipid and Exopolysaccharide Production by Lipomyces starkeyi Under Repeated Fed-batch and Continuous Cultivation. Chem. Biochem. Eng. Q. 2018, 32, 473–481. [Google Scholar] [CrossRef]
- Sutanto, S.; Zullaikah, S.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.-H. Lipomyces starkeyi: Its current status as a potential oil producer. Fuel Process. Technol. 2018, 177, 39–55. [Google Scholar] [CrossRef]
- Wild, R.; Patil, S.; Popovic, M.; Zappi, M.; Dufreche, S.; Bajpai, R. Lipids from Lipomyces starkeyi. Food Technol. Biotechnol. 2010, 48, 329–335. [Google Scholar]
- Rahman, S.; Arbter, P.; Popovic, M.; Bajpai, R.; Subramaniam, R. Microbial lipid production from lignocellulosic hydrolyzates: Effect of carbohydrate mixtures and acid-hydrolysis byproducts on cell growth and lipid production by Lipomyces starkeyi. J. Chem. Technol. Biotechnol. 2017, 92, 1980–1989. [Google Scholar] [CrossRef]
- Oguri, E.; Masaki, K.; Naganuma, T.; Iefuji, H. Phylogenetic and biochemical characterization of the oil-producing yeast Lipomyces starkeyi. Antonie van Leeuwenhoek 2012, 101, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Takafumi, N.; Yasuyuki, U.; Kentaro, T. Physiological factors affecting total cell number and lipid content of the yeast, Lipomyces starkeyi. J. Gen. Appl. Microbiol. 1985, 31, 29–37. [Google Scholar]
- Wu, M.; Shi, Z.; Huang, H.; Qu, J.; Dai, X.; Tian, X.; Wei, W.; Li, G.; Ma, T. Network structure and functional properties of transparent hydrogel sanxan produced by Sphingomonas sanxanigenens NX02. Carbohydr. Polym. 2017, 176, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Maalej, H.; Hmidet, N.; Boisset, C.; Bayma, E.; Heyraud, A.; Nasri, M. Rheological and emulsifying properties of a gel-like exopolysaccharide produced by Pseudomonas stutzeri AS22. Food Hydrocoll. 2016, 52, 634–647. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid Bacteria Exopolysaccharides Producers: A Sustainable Tool for Functional Foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Tabernero, A.; Cardea, S. Microbial Exopolysaccharides as Drug Carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Sacco, L.P.; Castellane, T.C.L.; Polachini, T.C.; de Macedo Lemos, E.G.; Alves, L.M.C. Exopolysaccharides produced by Pandoraea shows emulsifying and anti-biofilm activities. J. Polym. Res. 2019, 26, 91. [Google Scholar] [CrossRef]
- Seviour, T.; Pijuan, M.; Nicholson, T.; Keller, J.; Yuan, Z. Gel-forming exopolysaccharides explain basic differences between structures of aerobic sludge granules and floccular sludges. Water Res. 2009, 43, 4469–4478. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Tilwani, Y.M.; Arul, V. Physicochemical and functional characterization of mannan exopolysaccharide from Weissella confusa MD1 with bioactivities. Int. J. Biol. Macromol. 2020, 143, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Furuta, H.; Maeda, H. Rheological properties of water-soluble soybean polysaccharides extracted under weak acidic condition. Food Hydrocoll. 1999, 13, 267–274. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Y.P.; Cai, Z.X.; Guo, Y.L.; Wu, M.; Wang, P.G.; Li, R.Q.; Zhang, H.B. Interfacial and emulsion characterisation of chemically modified polysaccharides through a multiscale approach. J. Colloid Interface Sci. 2020, 580, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Alkharfy, K.M.; Wani, T.A.; Raish, M. Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015, 72, 990–997. [Google Scholar] [CrossRef]
- Luo, D.H. Optimization of total polysaccharide extraction from Dioscorea nipponica Makino using response surface methodology and uniform design. Carbohydr. Polym. 2012, 90, 284–288. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide production of Pantoea sp. BCCS 001 GH: Physical characterizations, emulsification, and antioxidant activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef]
- Farhoosh, R.; Riazi, A. A compositional study on two current types of salep in Iran and their rheological properties as a function of concentration and temperature. Food Hydrocoll. 2007, 21, 660–666. [Google Scholar] [CrossRef]
- Kohnke, T.; Ostlund, A.; Brelid, H. Adsorption of arabinoxylan on cellulosic surfaces: Influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromolecules 2011, 12, 2633–2641. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.Y.; Xiao, G.N.; Yang, H.L. Efficient Production of Exopolysaccharide by Submerged Fermentation of Hypsizygus marmoreus Using a Two-stage pH Control Strategy. Chem. Biochem. Eng. Q. 2017, 31, 519–526. [Google Scholar] [CrossRef]
- McHargue, J.S.; Calfee, R.K. Effect of Manganese, Copper and Zinc on the Growth of Yeast. Plant Physiol. 1931, 6, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hao, Y.; Chu, T.W.; Li, C.Q.; Zhang, Z.F.; Zhou, Y. Ultrasonic-assisted extraction, chemical characterization of polysaccharides from Yunzhi mushroom and its effect on osteoblast cells. Carbohydr. Polym. 2010, 80, 922–926. [Google Scholar] [CrossRef]
- Shen, S.A.; Chen, D.J.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.H.; Ding, C.B. Optimization of extraction process and antioxidant activity of polysaccharides from leaves of Paris polyphylla. Carbohydr. Polym. 2014, 104, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Zhang, R.T.; Liu, C. Extraction and antioxidant activity of phenolic compounds from wheat bran treated by steam explosion. Trop. J. Pharm. Res. 2015, 14, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Luft, L.; Confortin, T.C.; Todero, I.; Brun, T.; Mazutti, M.A. Production of bioemulsifying compounds from Phoma dimorpha using agroindustrial residues as additional carbon sources. Biocatal. Agric. Biotechnol. 2021, 35, 102079. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Gouveia, A.R.; Pinheiro, C.; Torres, C.A.V.; Grandfils, C.; Reis, M.A.M. Controlled Production of Exopolysaccharides from Enterobacter A47 as a Function of Carbon Source with Demonstration of Their Film and Emulsifying Abilities. Appl. Biochem. Biotechnol. 2014, 172, 641–657. [Google Scholar] [CrossRef]
- Li, L.; Liao, B.Y.; Thakur, K.; Zhang, J.G.; Wei, Z.J. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 109, 761–771. [Google Scholar] [CrossRef]
- Khondkar, P.; Aidoo, K.E.; Tester, R.F. The Effects of Temperature, pH, and Cations on the Rheological Properties of the Extracellular Polysaccharides of Medicinal Species of Genus Tremella Pers. (Heterobasidiomycetes). Int. J. Med. Mushrooms 2010, 12, 73–85. [Google Scholar] [CrossRef]
- Bao, H.H.; You, S.G.; Cao, L.K.; Zhou, R.; Wang, Q.; Cui, S.W. Chemical and rheological properties of polysaccharides from fruit body of Auricularia auricular-judae. Food Hydrocoll. 2016, 57, 30–37. [Google Scholar] [CrossRef]
- de Oliveira, J.M.; Amaral, S.A.; Burkert, C.A.V. Rheological, textural and emulsifying properties of an exopolysaccharide produced by Mesorhizobium loti grown on a crude glycerol-based medium. Int. J. Biol. Macromol. 2018, 120, 2180–2187. [Google Scholar] [CrossRef]
- Xu, L.; Dong, M.Z.; Gong, H.J.; Sun, M.J.; Li, Y.J. Effects of inorganic cations on the rheology of aqueous welan, xanthan, gellan solutions and their mixtures. Carbohydr. Polym. 2015, 121, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Foudazi, R.; Qavi, S.; Masalova, I.; Malkin, A.Y. Physical chemistry of highly concentrated emulsions. Adv. Colloid Interface Sci. 2015, 220, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. Relationships between the behavior of three different sources of pectin at the oil-water interface and the stability of the emulsion. Food Hydrocoll. 2022, 128, 107566. [Google Scholar] [CrossRef]


| Level | −1 | 0 | 1 |
|---|---|---|---|
| A | 60 | 90 | 120 |
| B | 5.5 | 6.5 | 7.5 |
| C | 5 | 10 | 15 |
| D | 5 | 10 | 15 |
| Run | A | B | C | D | Yield (g/L) | |
|---|---|---|---|---|---|---|
| Glucose (g/L) | pH | Mn2+ (mg/L) | Zn2+ (mg/L) | Experimental | Predicted | |
| 1 | 60 | 5.5 | 10 | 10 | 3.45 ± 0.03 p | 3.45 |
| 2 | 120 | 5.5 | 10 | 10 | 6.10 ± 0.05 d | 6.07 |
| 3 | 60 | 7.5 | 10 | 10 | 3.75 ± 0.04 o | 3.80 |
| 4 | 120 | 7.5 | 10 | 10 | 7.30 ± 0.06 b | 7.32 |
| 5 | 90 | 6.5 | 5 | 5 | 4.45 ± 0.05 l | 4.62 |
| 6 | 90 | 6.5 | 15 | 5 | 5.90 ± 0.07 f | 5.39 |
| 7 | 90 | 6.5 | 5 | 15 | 4.00 ± 0.02 n | 4.53 |
| 8 | 90 | 6.5 | 15 | 15 | 5.85 ± 0.03 f | 5.69 |
| 9 | 60 | 6.5 | 10 | 5 | 3.40 ± 0.01 p | 3.55 |
| 10 | 120 | 6.5 | 10 | 5 | 5.90 ± 0.06 f | 6.29 |
| 11 | 60 | 6.5 | 10 | 15 | 3.45 ± 0.03 p | 3.33 |
| 12 | 120 | 6.5 | 10 | 15 | 6.60 ± 0.05 c | 6.72 |
| 13 | 90 | 5.5 | 5 | 10 | 4.35 ± 0.05 m | 4.38 |
| 14 | 90 | 7.5 | 5 | 10 | 5.35 ± 0.03 h | 5.15 |
| 15 | 90 | 5.5 | 15 | 10 | 4.85 ± 0.02 k | 5.32 |
| 16 | 90 | 7.5 | 15 | 10 | 5.90 ± 0.06 f | 6.14 |
| 17 | 60 | 6.5 | 5 | 10 | 3.70 ± 0.01 o | 3.54 |
| 18 | 120 | 6.5 | 5 | 10 | 6.55 ± 0.05 c | 6.18 |
| 19 | 60 | 6.5 | 15 | 10 | 4.00 ± 0.03 n | 4.08 |
| 20 | 120 | 6.5 | 15 | 10 | 7.70 ± 0.08 a | 7.57 |
| 21 | 90 | 5.5 | 10 | 5 | 4.45 ± 0.03 l | 4.26 |
| 22 | 90 | 7.5 | 10 | 5 | 5.40 ± 0.05 h | 5.38 |
| 23 | 90 | 5.5 | 10 | 15 | 4.95 ± 0.03 j | 4.68 |
| 24 | 90 | 7.5 | 10 | 15 | 5.25 ± 0.05 i | 5.16 |
| 25 | 90 | 6.5 | 10 | 10 | 5.45 ± 0.02 g | 5.81 |
| 26 | 90 | 6.5 | 10 | 10 | 5.83 ± 0.04 f | 5.81 |
| 27 | 90 | 6.5 | 10 | 10 | 5.90 ± 0.03 f | 5.81 |
| 28 | 90 | 6.5 | 10 | 10 | 5.85 ± 0.06 f | 5.81 |
| Source | Sum of Squares | df a | Mean Square | F-Value | p-Value | Significance b |
|---|---|---|---|---|---|---|
| Model | 36.24 | 14 | 2.59 | 22.79 | <0.0001 | ** |
| A-Glucose | 28.21 | 1 | 28.21 | 248.44 | <0.0001 | ** |
| B-pH | 1.92 | 1 | 1.92 | 16.91 | 0.0011 | ** |
| C-Mn | 2.80 | 1 | 2.80 | 24.69 | 0.0002 | ** |
| D-Zn | 0.0300 | 1 | 0.0300 | 0.2642 | 0.6153 | |
| AB | 0.2025 | 1 | 0.2025 | 1.78 | 0.2031 | |
| AC | 0.1806 | 1 | 0.1806 | 1.59 | 0.2279 | |
| AD | 0.1056 | 1 | 0.1056 | 0.9301 | 0.3512 | |
| BC | 0.0006 | 1 | 0.0006 | 0.0055 | 0.9419 | |
| BD | 0.1056 | 1 | 0.1056 | 0.9301 | 0.3512 | |
| CD | 0.0400 | 1 | 0.0400 | 0.3522 | 0.5623 | |
| A2 | 0.4864 | 1 | 0.4864 | 4.28 | 0.0575 | |
| B2 | 0.9065 | 1 | 0.9065 | 7.98 | 0.0135 | * |
| C2 | 0.2252 | 1 | 0.2252 | 1.98 | 0.1809 | |
| D2 | 2.04 | 1 | 2.04 | 18.00 | 0.0008 | ** |
| Residual | 1.59 | 14 | 0.1136 | |||
| Lack of Fit | 1.41 | 10 | 0.1414 | 3.22 | 0.1356 | Not significant |
| Pure Error | 0.1757 | 4 | 0.0439 | |||
| Cor Total | 37.83 | 28 | ||||
| Source | Sum of Squares | df a | Mean Square | F-value | p-value | Significance b |
| K1 (LR) | K2 (LR) | K3 (LR) | K4 (LR) | |
|---|---|---|---|---|
| 0.4% | −0.1242 ± 0.00199 (0.9650) a | −0.2780 ± 0.00088 (0.9711) c | −1.2710 ± 0.02397 (0.9386) c | −0.2389 ± 0.00149 (0.9622) a |
| 0.6% | −0.1959 ± 0.00187 (0.9797) c | −0.2759 ± 0.00068 (0.9837) c | −1.1649 ± 0.01166 (0.9574) b | −0.3420 ± 0.00213 (0.9640) c |
| 0.8% | −0.1851 ± 0.00225 (0.9630) b | −0.2401 ± 0.00045 (0.9901) b | −0.9431 ± 0.02375 (0.8561) a | −0.3819 ± 0.00523 (0.8424) d |
| 1.0% | −0.2852 ± 0.00205 (0.9880) d | −0.1859 ± 0.00044 (0.9803) a | −1.8835 ± 0.05091 (0.9435) d | −0.3298 ± 0.00202 (0.9632) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, W.; Chen, H.; Chen, W.; Zhang, M.; Zhong, Q.; Chen, W. Optimization and Rheological Study of an Exopolysaccharide Obtained from Fermented Mature Coconut Water with Lipomyces starkeyi. Foods 2022, 11, 999. https://doi.org/10.3390/foods11070999
Guo Y, Li W, Chen H, Chen W, Zhang M, Zhong Q, Chen W. Optimization and Rheological Study of an Exopolysaccharide Obtained from Fermented Mature Coconut Water with Lipomyces starkeyi. Foods. 2022; 11(7):999. https://doi.org/10.3390/foods11070999
Chicago/Turabian StyleGuo, Yilin, Wentian Li, Haiming Chen, Weijun Chen, Ming Zhang, Qiuping Zhong, and Wenxue Chen. 2022. "Optimization and Rheological Study of an Exopolysaccharide Obtained from Fermented Mature Coconut Water with Lipomyces starkeyi" Foods 11, no. 7: 999. https://doi.org/10.3390/foods11070999
APA StyleGuo, Y., Li, W., Chen, H., Chen, W., Zhang, M., Zhong, Q., & Chen, W. (2022). Optimization and Rheological Study of an Exopolysaccharide Obtained from Fermented Mature Coconut Water with Lipomyces starkeyi. Foods, 11(7), 999. https://doi.org/10.3390/foods11070999






