Inhibitory Effects against Alpha-Amylase of an Enriched Polyphenol Extract from Pericarp of Mangosteen (Garcinia mangostana)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. MPPs Extraction
Optimization of MPP Extraction Conditions
2.3. Identification of Phenolic Compounds in MPPs
2.4. Total Antioxidant Properties of MPPs
2.5. Inhibition of Alpha-Amylase Activity In Vitro
2.5.1. Alpha-Amylase Inhibitory Activity
2.5.2. Alpha-Amylase Inhibition Kinetics
2.5.3. Fluorescence Quenching
2.5.4. Autodock
2.6. Inhibition of Alpha-Amylase Activity In Vivo
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Optimization Conditions of MPPs
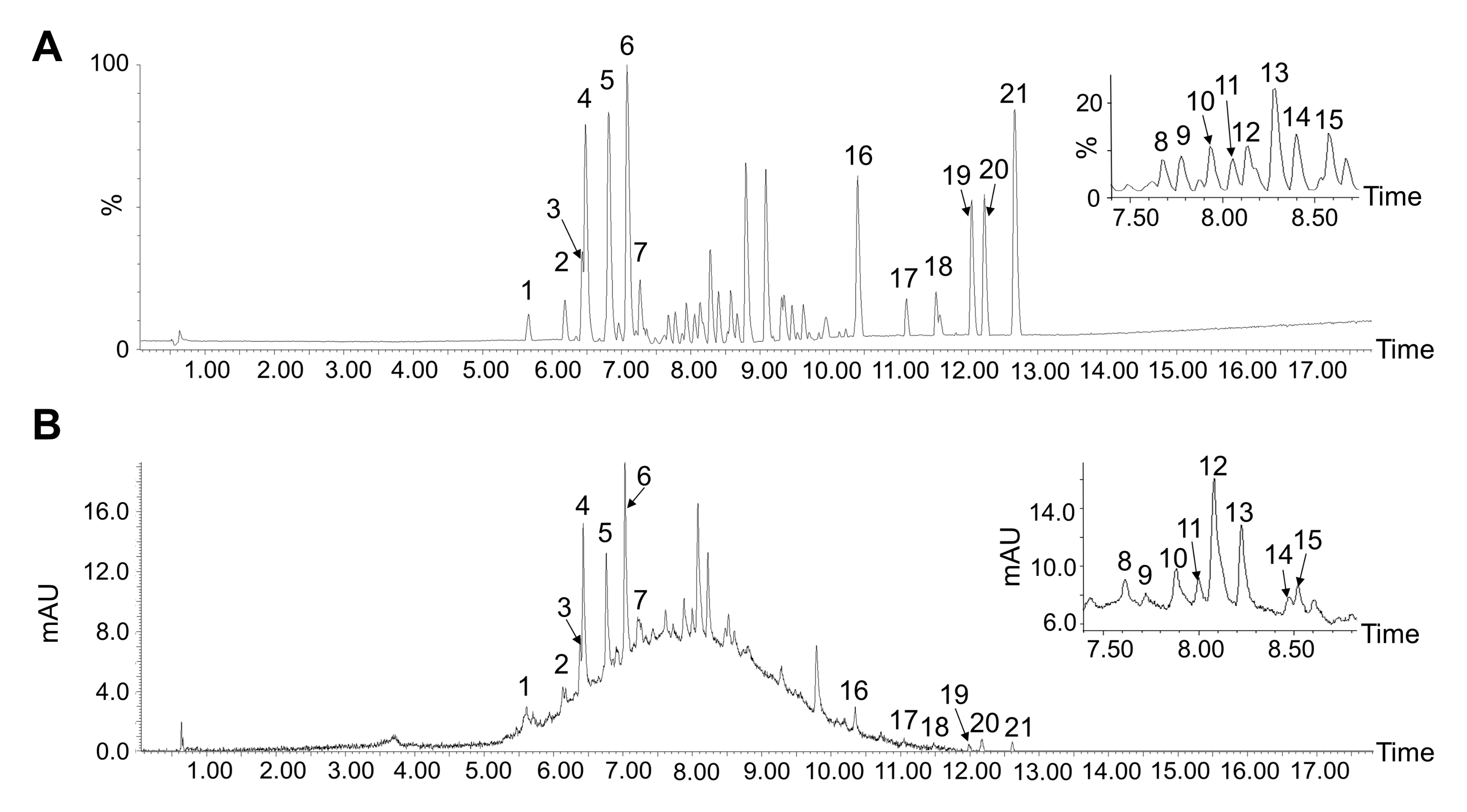
3.2. Tentative Identification of MPPs
3.3. Antioxidant Properties of MPPs
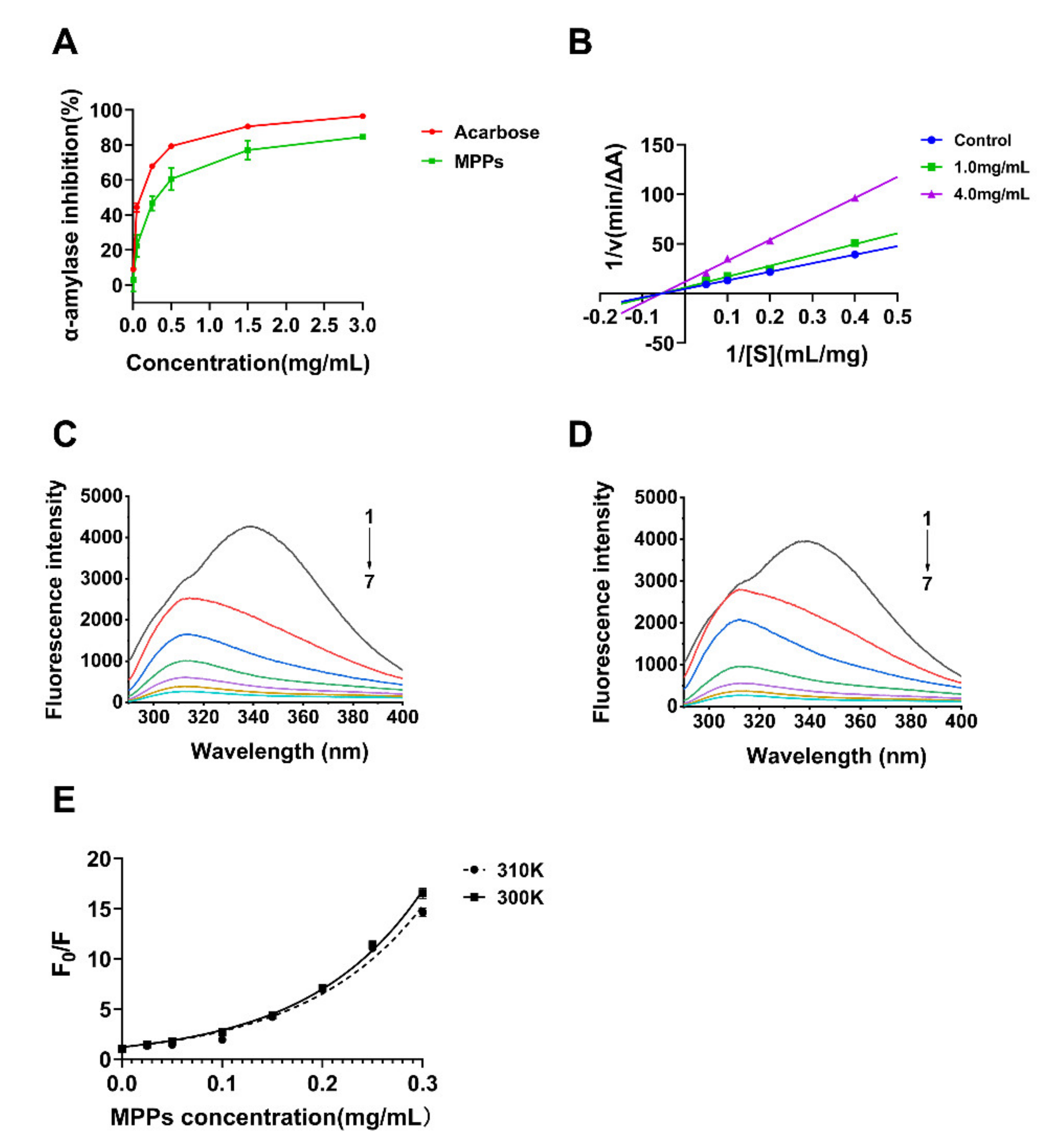
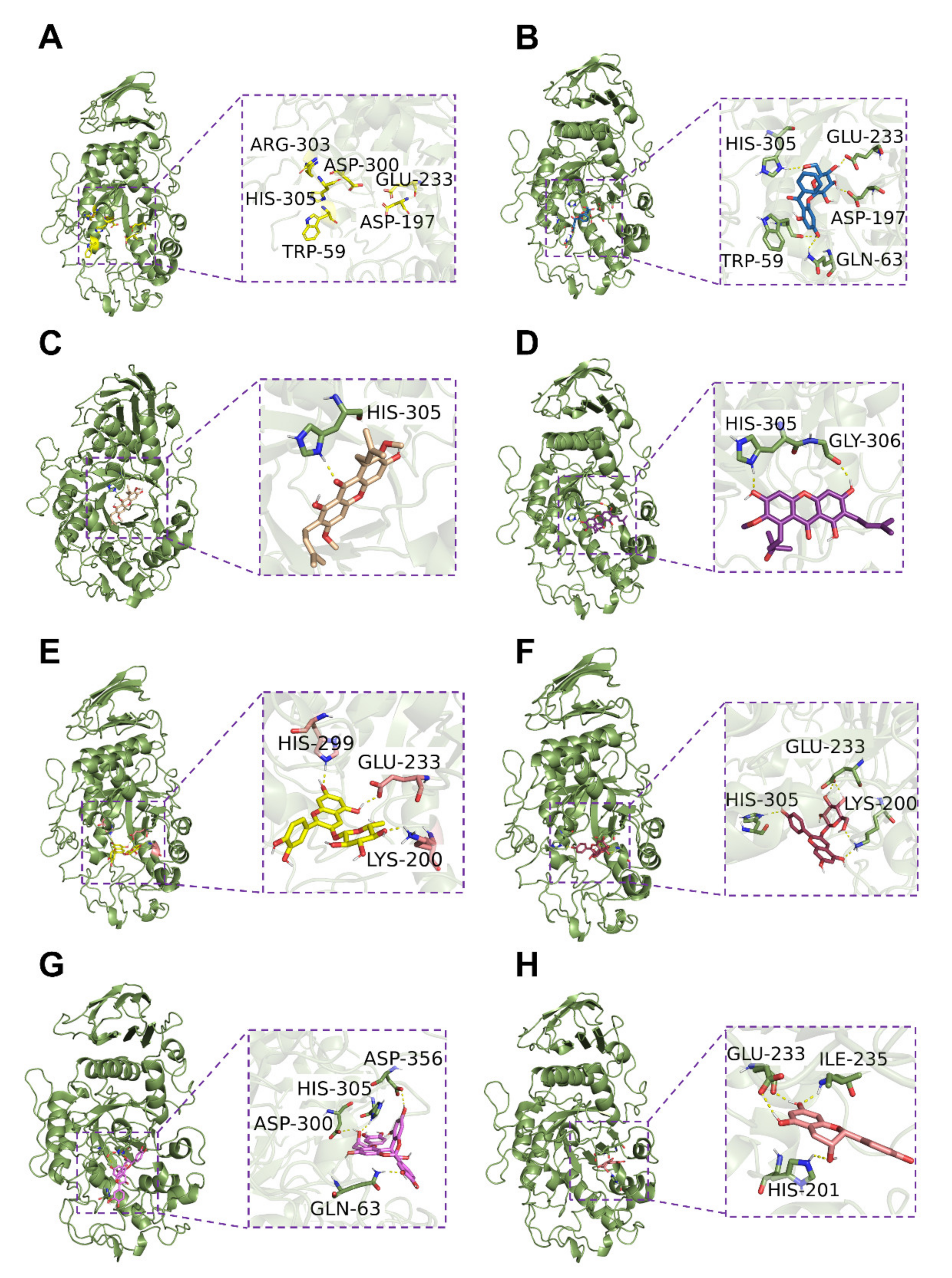
3.4. Inhibition of Alpha-Amylase Activity In Vitro and Possible Mechanism
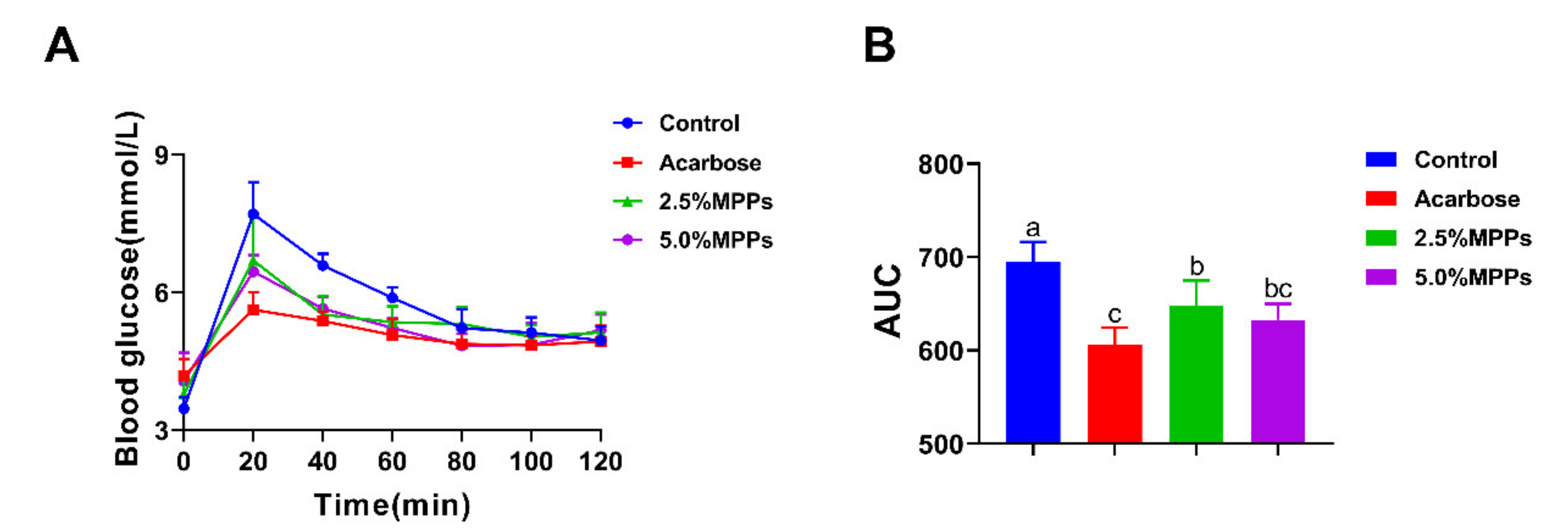
3.5. Postprandial Blood Glucose Response to Starch with MPPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Diabetes Prevention Program Research Group Long-Term Effects of Lifestyle Intervention or Metformin on Diabetes Development and Microvascular Complications over 15-Year Follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [CrossRef] [Green Version]
- Schellenberg, E.S.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients With and at Risk for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 159, 543. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.B.; Hogger, P. Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future Perspectives. CMC 2014, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Bonnet, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. Dietary Antioxidant Capacity and Risk of Type 2 Diabetes in the Large Prospective E3N-EPIC Cohort. Diabetologia 2018, 61, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Schaft, N.; Schoufour, J.D.; Nano, J.; Kiefte-de Jong, J.C.; Muka, T.; Sijbrands, E.J.G.; Ikram, M.A.; Franco, O.H.; Voortman, T. Dietary Antioxidant Capacity and Risk of Type 2 Diabetes Mellitus, Prediabetes and Insulin Resistance: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 853–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young Apple Polyphenols Postpone Starch Digestion In Vitro and In Vivo. J. Funct. Foods 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Muritala, H.F.; Akolade, J.O.; Akande, S.A.; Abdulazeez, A.T.; Aladodo, R.A.; Bello, A.B. Antioxidant and Alpha-Amylase Inhibitory Potentials of Cocos Nucifera Husk. Food Sci. Nutr. 2018, 6, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narita, Y.; Inouye, K. Kinetic Analysis and Mechanism on the Inhibition of Chlorogenic Acid and Its Components against Porcine Pancreas α-Amylase Isozymes I and II. J. Agric. Food Chem. 2009, 57, 9218–9225. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory Effect of Polyphenol-Rich Extracts of Jute Leaf (Corchorus olitorius) on Key Enzyme Linked to Type 2 Diabetes (α-Amylase and α-Glucosidase) and Hypertension (Angiotensin I Converting) in Vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, S.; Xie, J.; Chen, Y.; Dong, R.; Zhang, X.; Liu, S.; Xie, J.; Hu, X.; Yu, Q. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities of Bound Polyphenols Extracted from Mung Bean Skin Dietary Fiber. LWT 2020, 132, 109943. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current Trends of Tropical Fruit Waste Utilization. Crit. Rev. Food Sci. Nutr. 2016, 58, 335–361. [Google Scholar] [CrossRef]
- Sze Lim, Y.; Sze Hui Lee, S.; Chin Tan, B. Antioxidant Capacity and Antibacterial Activity of Different Parts of Mangosteen (Garcinia Mangostana Linn.) Extracts. Fruits 2013, 68, 483–489. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Chong, C.W.; Murugaiyah, V. LC-QTOF-MS Analysis of Xanthone Content in Different Parts of Garcinia Mangostana and Its Influence on Cholinesterase Inhibition. J. Enzym. Inhib. Med. Chem. 2020, 35, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Siemak, J.; Klimowicz, A. Antioxidant Activity and Polyphenol Content in Extracts from Various Parts of Fresh and Frozen Mangosteen. Acta Sci. Pol. Technol. Aliment. 2020, 19, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M. New Benzophenones and a Dihydroflavanonol from Garcinia mangostana Pericarps and Their Antioxidant and Cytotoxic Activities. Phytochem. Lett. 2020, 39, 43–48. [Google Scholar] [CrossRef]
- Wang, A.; Li, D.; Wang, S.; Zhou, F.; Li, P.; Wang, Y.; Lin, L. γ-Mangostin, a Xanthone from Mangosteen, Attenuates Oxidative Injury in Liver via NRF2 and SIRT1 Induction. J. Funct. Foods 2018, 40, 544–553. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Wang, A.; Dong, Z.; Yin, S.; Zhang, Q.; Ye, Y.; Li, L.; Lin, L. Nitric Oxide Inhibitory Xanthones from the Pericarps of Garcinia mangostana. Phytochemistry 2016, 131, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Anti-Inflammatory Effect of Garcinia mangostana Linn. Pericarp Extract in Methicillin-Resistant Staphylococcus aureus-Induced Superficial Skin Infection in Mice. Biomed. Pharmacother. 2019, 111, 705–713. [Google Scholar] [CrossRef] [PubMed]
- John, O.D.; Mouatt, P.; Panchal, S.K.; Brown, L. Rind from Purple Mangosteen (Garcinia mangostana) Attenuates Diet-Induced Physiological and Metabolic Changes in Obese Rats. Nutrients 2021, 13, 319. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Wang, Y.-T.; Lin, L.-G. New Insights into the Anti-Obesity Activity of Xanthones from Garcinia mangostana. Food Funct. 2015, 6, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Do, H.T.T.; Cho, J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. Int. J. Mol. Sci. 2020, 21, 6211. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Chang, A.K.; Li, Y.; Tao, X.; Liu, W.; Wang, Z.; Su, W.; Li, Z.; Liang, X. Screening and Tissue Distribution of Protein Tyrosine Phosphatase 1B Inhibitors in Mice Following Oral Administration of Garcinia mangostana L. Ethanolic Extract. Food Chem. 2021, 357, 129759. [Google Scholar] [CrossRef] [PubMed]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal Properties of Mangosteen (Garcinia mangostana L.): A Comprehensive Update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Tsai, M.-J.; Fu, Y.-S.; Weng, C.-F. The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia Linii with Comprehensive Review of the Garcinia Family. Biomolecules 2019, 9, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthy, M.; Sundralingam, U.; Palanisamy, U.D. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299. [Google Scholar] [CrossRef]
- Peng, J.; Jia, Y.; Du, X.; Wang, Y.; Yang, Z.; Li, K. Study of Physicochemical Stability of Anthocyanin Extracts from Black Peanut Skin and Their Digestion Enzyme and Adipogenesis Inhibitory Activities. LWT 2019, 107, 107–116. [Google Scholar] [CrossRef]
- Sun, H.-N.; Mu, T.-H.; Xi, L.-S. Effect of PH, Heat, and Light Treatments on the Antioxidant Activity of Sweet Potato Leaf Polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-Amylase, α-Glucosidase and Lipase Inhibitory Activity of the Phenolic Substances in Two Black Legumes of Different Genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, S.; Mateus, N.; de Freitas, V. Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching. J. Agric. Food Chem. 2007, 55, 6726–6735. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, W.; Gong, S.; Gu, X.; Lan, X.; Wu, J.; Wang, Z. Investigating Lignin from Canna Edulis Ker Residues Induced Activation of α-Amylase: Kinetics, Interaction, and Molecular Docking. Food Chem. 2019, 271, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.L.; Chong, G.H. Effect of Drum Drying on Physico-Chemical Characteristics of Dragon Fruit Peel (Hylocereus polyrhizus). Int. J. Food Eng. 2015, 11, 285–293. [Google Scholar] [CrossRef]
- Da Silva, J.K.; Cazarin, C.B.B.; Batista, Â.G.; Maróstica, M. Effects of Passion Fruit (Passiflora edulis) Byproduct Intake in Antioxidant Status of Wistar Rats Tissues. LWT Food Sci. Technol. 2014, 59, 1213–1219. [Google Scholar] [CrossRef]
- Da Silva, J.K.; Cazarin, C.B.B.; Bogusz Junior, S.; Augusto, F.; Maróstica Junior, M.R. Passion Fruit (Passiflora edulis) Peel Increases Colonic Production of Short-Chain Fatty Acids in Wistar Rats. LWT Food Sci. Technol. 2014, 59, 1252–1257. [Google Scholar] [CrossRef]
- Da Silva, L.M.R.; de Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of Bioactive Compounds in Pulps and By-Products of Tropical Fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, N.A.; Abang Zaidel, D.N.; Muhamad, I.I.; Abdul Hamid, M.; Yaakob, H.; Mohd Jusoh, Y.M. Optimization of the Antioxidant-Rich Xanthone Extract from Mangosteen (Garcinia mangostana L.) Pericarp via Microwave-Assisted Extraction. Heliyon 2019, 5, e02571. [Google Scholar] [CrossRef] [Green Version]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Ultrasonic-Assisted Virgin Coconut Oil Based Extraction for Maximizing Polyphenol Recovery and Bioactivities of Mangosteen Peels. J. Food Sci. Technol. 2020, 57, 4032–4043. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Chen, C.-C.; Chen, Y.-J.; Huang, R.-L.; Shieh, B.-J. Three Xanthones and a Benzophenone from Garcinia mangostana. J. Nat. Prod. 2001, 64, 903–906. [Google Scholar] [CrossRef]
- Jung, H.-A.; Su, B.-N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant Xanthones from the Pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef]
- Lin, J.; Gao, Y.; Li, H.; Zhang, L.; Li, X. DNA Protective Effect of Mangosteen Xanthones: An in Vitro Study on Possible Mechanisms. Adv. Pharm. Bull. 2014, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of Apoptosis by Xanthones from Mangosteen in Human Leukemia Cell Lines. J. Nat. Prod. 2003, 66, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.B. HPLC Analysis of Selected Xanthones in Mangosteen Fruit. J. Sep. Sci. 2007, 30, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Le Huyen, T.; Tran, T.M.; Nguyen, T.A.; Pham, T.B.; Nguyen Tien, D. A New Megastigmane Sulphoglycoside and Polyphenolic Constituents from Pericarps of Garcinia mangostana. Nat. Prod. Res. 2016, 30, 1598–1604. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ninomiya, K.; Tagashira, Y.; Maejima, K.; Yoshida, T.; Amakura, Y. Polyphenolic Constituents of the Pericarp of Mangosteen (Garcinia mangostana L.). J. Agric. Food Chem. 2015, 63, 7670–7674. [Google Scholar] [CrossRef]
- Maliński, M.P.; Kikowska, M.A.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Thiem, B. Phytochemical Screening, Phenolic Compounds and Antioxidant Activity of Biomass from Lychnis Flos-cuculi L. In Vitro Cultures and Intact Plants. Plants 2021, 10, 206. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Bosso, A.; Cassino, C.; Motta, S.; Panero, L.; Tsolakis, C.; Guaita, M. Polyphenolic Composition and In Vitro Antioxidant Activity of Red Grape Seeds as Byproducts of Short and Medium-Long Fermentative Macerations. Foods 2020, 9, 1451. [Google Scholar] [CrossRef]
- Dembinska-Kiec, A.; Mykkänen, O.; Kiec-Wilk, B.; Mykkänen, H. Antioxidant Phytochemicals against Type 2 Diabetes. Br. J. Nutr. 2008, 99, ES109–ES117. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous Optimization of the Ultrasound-Assisted Extraction for Phenolic Compounds Content and Antioxidant Activity of Lycium Ruthenicum Murr. Fruit Using Response Surface Methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-L.; Si, X.; Wang, Y.-H.; Gong, E.-S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive Flavonoids from Rubus corchorifolius Inhibit α-Glucosidase and α-Amylase to Improve Postprandial Hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. α-Glucosidase Inhibition and Antihyperglycemic Activity of Prenylated Xanthones from Garcinia mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef]
- Fatmawati, S.; Ersam, T.; Shimizu, K. The Inhibitory Activity of Aldose Reductase in Vitro by Constituents of Garcinia mangostana Linn. Phytomedicine 2015, 22, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, F.; Gao, F.; Shan, F.; Bian, J.; Zhao, C. Study on the Interaction between 3 Flavonoid Compounds and α-Amylase by Fluorescence Spectroscopy and Enzymatic Kinetics. J. Food Sci. 2009, 74, C199–C203. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, X.; Qi, W.; Su, R.; He, Z. Affinity of Rosmarinic Acid to Human Serum Albumin and Its Effect on Protein Conformation Stability. Food Chem. 2016, 192, 178–187. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. Investigation of the Interaction between Gallic Acid and α-Amylase by Spectroscopy. Int. J. Food Prop. 2016, 19, 2481–2494. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between Polyphenols in Thinned Young Apples and Porcine Pancreatic α-Amylase: Inhibition, Detailed Kinetics and Fluorescence Quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef]
- Naczk, M.; Towsend, M.; Zadernowski, R.; Shahidi, F. Protein-Binding and Antioxidant Potential of Phenolics of Mangosteen Fruit (Garcinia mangostana). Food Chem. 2011, 128, 292–298. [Google Scholar] [CrossRef]
- Sun, L.; Gidley, M.J.; Warren, F.J. The Mechanism of Interactions between Tea Polyphenols and Porcine Pancreatic Alpha-Amylase: Analysis by Inhibition Kinetics, Fluorescence Quenching, Differential Scanning Calorimetry and Isothermal Titration Calorimetry. Mol. Nutr. Food Res. 2017, 61, 1700324. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zuo, H.; Shu, L. Comparison of the Interaction between Three Anthocyanins and Human Serum Albumins by Spectroscopy. J. Lumin. 2014, 153, 54–63. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Brayer, G.D.; Sidhu, G.; Maurus, R.; Rydberg, E.H.; Braun, C.; Wang, Y.; Nguyen, N.T.; Overall, C.M.; Withers, S.G. Subsite Mapping of the Human Pancreatic α-Amylase Active Site through Structural, Kinetic, and Mutagenesis Technique. Biochemistry 2000, 39, 4778–4791. [Google Scholar] [CrossRef] [PubMed]
- Jokura, H.; Watanabe, I.; Umeda, M.; Hase, T.; Shimotoyodome, A. Coffee Polyphenol Consumption Improves Postprandial Hyperglycemia Associated with Impaired Vascular Endothelial Function in Healthy Male Adults. Nutr. Res. 2015, 35, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.-I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and Blackcurrant Polyphenol-Rich Drinks Decrease Postprandial Glucose, Insulin and Incretin Response to a High-Carbohydrate Meal in Healthy Men and Women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serina, J.J.C.; Castilho, P.C.M.F. Using Polyphenols as a Relevant Therapy to Diabetes and Its Complications, a Review. Crit. Rev. Food Sci. Nutr. 2021, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary Polyphenols and Type 2 Diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Sun, L.; Gidley, M.J.; Warren, F.J. Tea Polyphenols Enhance Binding of Porcine Pancreatic α-Amylase with Starch Granules but Reduce Catalytic Activity. Food Chem. 2018, 258, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of Porcine Pancreatic α-Amylase Activity by Chlorogenic Acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Polyphenols from Acorn Leaves (Quercus liaotungensis) Protect Pancreatic Beta Cells and Their Inhibitory Activity against α-Glucosidase and Protein Tyrosine Phosphatase 1B. Molecules 2018, 23, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory Mechanism of Gallic Acid against Advanced Glycation End Products Induced Cardiac Remodeling in Experimental Rats. Chem.-Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Mangostanaxanthones III and IV: Advanced Glycation End-Product Inhibitors from the Pericarp of Garcinia mangostana. J. Nat. Med. 2017, 71, 216–226. [Google Scholar] [CrossRef]
- Karunakaran, T.; Ee, G.C.L.; Ismail, I.S.; Mohd Nor, S.M.; Zamakshshari, N.H. Acetyl- and O-Alkyl-Derivatives of β-Mangostin from Garcinia mangostana and Their Anti-Inflammatory Activities. Nat. Prod. Res. 2018, 32, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.; El-Bassossy, H.; Mohamed, G.; El-Halawany, A.; Alshali, K.; Banjar, Z. Phenolics from Garcinia mangostana Inhibit Advanced Glycation Endproducts Formation: Effect on Amadori Products, Cross-Linked Structures and Protein Thiols. Molecules 2016, 21, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonini, E.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin Improves Insulin Sensitivity in High Fat Diet-Fed Mice. Arch. Biochem. Biophys. 2016, 599, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (–)-Epicatechin Mitigates Oxidative Stress, NO Metabolism Alterations, and Inflammation in Renal Cortex from Fructose-Fed Rats. Free. Radic. Biol. Med. 2016, 90, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josic, J.; Olsson, A.T.; Wickeberg, J.; Lindstedt, S.; Hlebowicz, J. Does Green Tea Affect Postprandial Glucose, Insulin and Satiety in Healthy Subjects: A Randomized Controlled Trial. Nutr. J. 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, M.; van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin Attenuates Atherosclerosis and Exerts Anti-Inflammatory Effects on Diet-Induced Human-CRP and NFκB in Vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Cimino, F.; Molonia, M.S.; Ferrari, D.; Saija, A.; Virgili, F.; Speciale, A. Cyanidin-3-O-Glucoside Ameliorates Palmitate-Induced Insulin Resistance by Modulating IRS-1 Phosphorylation and Release of Endothelial Derived Vasoactive Factors. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 351–357. [Google Scholar] [CrossRef] [PubMed]
| Number | Mangosteen Pericarp Powder/g | Solid to Solvent Ratio (A) | pH (B) | Temperature (C) | Time (D) | Extraction Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 3.00 | 1 | 1 | 1 | 1 | 2.65 ± 0.07 |
| 2 | 3.00 | 1 | 2 | 2 | 2 | 2.77 ± 0.09 |
| 3 | 3.00 | 1 | 3 | 3 | 3 | 2.98 ± 0.05 |
| 4 | 3.00 | 2 | 1 | 2 | 3 | 2.91 ± 0.04 |
| 5 | 3.00 | 2 | 2 | 3 | 1 | 3.07 ± 0.08 |
| 6 | 3.00 | 2 | 3 | 1 | 2 | 2.85 ± 0.05 |
| 7 | 3.00 | 3 | 1 | 3 | 2 | 3.08 ± 0.07 |
| 8 | 3.00 | 3 | 2 | 1 | 3 | 2.99 ± 0.06 |
| 9 | 3.00 | 3 | 3 | 2 | 1 | 3.09 ± 0.08 |
| K1 | 8.40 | 8.64 | 8.49 | 8.81 | ||
| K2 | 8.83 | 8.83 | 8.77 | 8.70 | ||
| K3 | 9.16 | 8.92 | 9.13 | 8.88 | ||
| k1 | 2.80 | 2.88 | 2.83 | 2.94 | ||
| k2 | 2.94 | 2.94 | 2.92 | 2.90 | ||
| k3 | 3.05 | 2.97 | 3.04 | 2.96 | ||
| R | 0.25 | 0.09 | 0.21 | 0.06 | ||
| Order | A > C > B > D | |||||
| Optimum Levels | A3 | B3 | C3 | D3 | ||
| Optimum Factors | A3B3C3D3 | |||||
| No. | Retention Time | Absorption Peak Wavelength (nm) | Precursor Ion [M-H]−(m/z) | Main Fragment Ions (m/z) | Proposed Molecular Formula | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 5.66 | 225 | 423.0916 | 423.0912 | C25H28O6 | β-Mangostin |
| 2 | 6.18 | 278 | 577.1353 | 577.1341 451.1021 425.0861 407.0760 289.0695 | C30H26O12 | B-type (E)C dimer |
| 3 | 6.44 | 280 | 523.1481 | 523.1480 331.0799 289.0659 | Unknown | |
| 4 | 6.48 | 288 | 449.1087 | 449.1073 359.0753 329.0648 301.0659 | C21H21O11+ | Cyanidin-3-O-glucoside |
| 5 | 6.82 | 279 | 577.1353 | 577.1341 451.1021 425.0861 407.0760 289.0695 | C30H26O12 | B-type (E)C dimer |
| 6 | 7.10 | 280 | 289.0705 | 289.0694 245.0792 | C15H14O6 | (E)C |
| 7 | 7.27 | 280 | 865.2007 | 865.1994 577.1340 407.0758 289.0695 | C45H38O18 | B-type (E)C trimer |
| 8 | 7.68 | 279 | 577.1353 | 577.1352 449.0882 407.0765 289.0701 | C30H26O12 | B-type (E)C dimer |
| 9 | 7.77 | 238 | 391.1021 | 391.1013 289.0699 229.0484 | C19H20O9 | Garcimangosone D |
| 10 | 7.93 | 280 | 863.1870 | 863.1870 575.119 289.0697 | C45H36O18 | A-type (E)C trimer |
| 11 | 8.03 | 278 | 275.0540 | 275.0542 243.0275 | C14H12O6 | 4,6,3′,4′-Tetrahydroxy-2-methoxybenzophenone |
| 12 | 8.14 | 285 | 575.1205 | 575.1189 449.0891 407.0762 285.0383 | C30H24O12 | Proanthocyanidin A2 |
| 13 | 8.28 | 523 | 449.1080 | 449.1064 303.00487 285.0383 151.0014 | C21H21O11+ | Cyanidin-3-O-glucoside |
| 14 | 8.40 | 540 | 610.4193 | 610.4177 564.4135 289.0701 | C27H31O16+ | Cyanidin-3-O-sophoroside |
| 15 | 8.57 | 239 | 505.1376 | 505.1376 449.0899 381.1173 241.0000 | C21H30O12S | 4-O-sulpho-β-D-glucopyranosyl abscisate |
| 16 | 10.42 | 443.1698 | 443.1703 428.1467 369.0966 297.0387 | Unknown | ||
| 17 | 11.10 | 249 | 345.0958 | 345.0958 272.0303 243.0274 | C19H22O6 | Garcimangosxanthone C |
| 18 | 11.54 | 250 | 413.1585 | 413.1584 357.0958 329.1005 | C23H26O7 | Garcinone C |
| 19 | 12.05 | 320 | 431.1708 | 431.1689 358.1031 | C21H21O10+ | Pelargonidin-3-O-glucoside |
| 20 | 12.23 | 306 | 427.1754 | 427.1754 353.1013 297.0386 | C24H28O7 | Garcinone D |
| 21 | 12.67 | 317 | 445.1861 | 445.1857 371.1123 357.0964 | Unknown |
| Antioxidant | Hydroxyl Radical | Superoxide Radical | DPPH |
|---|---|---|---|
| MPPs (mg/mL) | 2.24 ± 0.10 a | 1.47 ± 0.11 b | 0.15 ± 0.004 b |
| VC * (mg/mL) | 1.93 ± 0.01 b | 0.17 ± 0.003 c | 0.11 ± 0.01 b |
| BHT * (mg/mL) | 0.83 ± 0.02 c | 3.60 ± 0.47 a | 1.15 ± 0.18 a |
| T/K | Regression Equation | R2 | KFQ (L·g−1) | Kq (108L·g−1·s−1) | |
|---|---|---|---|---|---|
| MPPs + α-amylase | 300 | y = 1.207 exp (8.781x) | 0.9960 | 8.781 | 2.927 |
| MPPs + α-amylase | 310 | y = 1.193 exp (8.503x) | 0.9839 | 8.503 | 2.834 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, H.; Jia, Y.; Peng, J.; Li, C. Inhibitory Effects against Alpha-Amylase of an Enriched Polyphenol Extract from Pericarp of Mangosteen (Garcinia mangostana). Foods 2022, 11, 1001. https://doi.org/10.3390/foods11071001
Li X, Chen H, Jia Y, Peng J, Li C. Inhibitory Effects against Alpha-Amylase of an Enriched Polyphenol Extract from Pericarp of Mangosteen (Garcinia mangostana). Foods. 2022; 11(7):1001. https://doi.org/10.3390/foods11071001
Chicago/Turabian StyleLi, Xiaofang, Haoze Chen, Yan Jia, Jinming Peng, and Chunmei Li. 2022. "Inhibitory Effects against Alpha-Amylase of an Enriched Polyphenol Extract from Pericarp of Mangosteen (Garcinia mangostana)" Foods 11, no. 7: 1001. https://doi.org/10.3390/foods11071001
APA StyleLi, X., Chen, H., Jia, Y., Peng, J., & Li, C. (2022). Inhibitory Effects against Alpha-Amylase of an Enriched Polyphenol Extract from Pericarp of Mangosteen (Garcinia mangostana). Foods, 11(7), 1001. https://doi.org/10.3390/foods11071001






