Evaluation of Bioactive Compounds, Antioxidant Activity, and Anticancer Potential of Wild Ganoderma lucidum Extracts from High-Altitude Regions of Nepal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
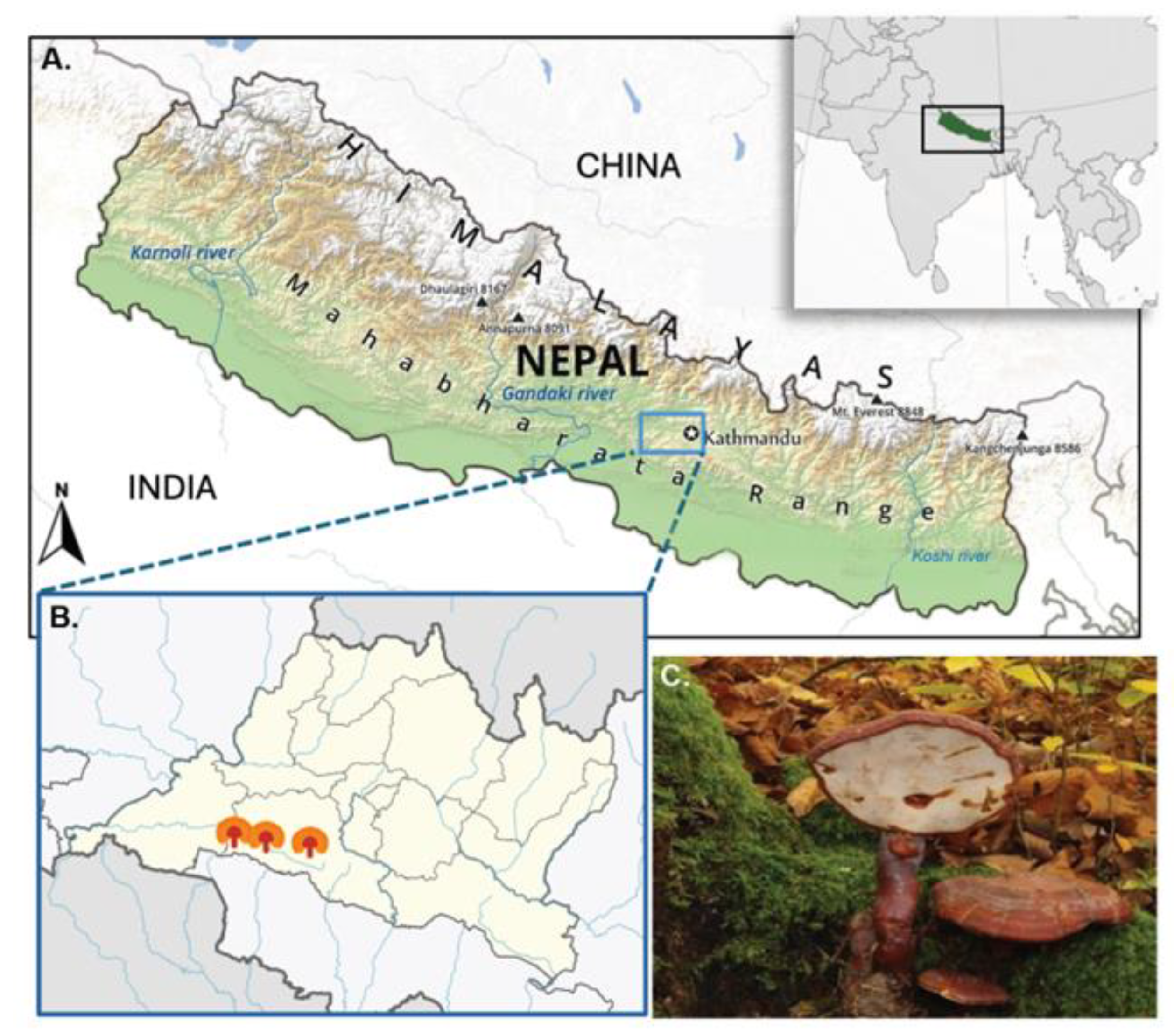
2.2. Collection and Identification
2.3. Sample Preparation and Extraction
2.4. Estimation of Total Phenolic, Flavonoid, β-Carotene, and Lycopene
2.4.1. Total Phenolic Content
2.4.2. Total Flavonoid Content
2.4.3. Estimation of β-Carotene and Lycopene Content
β-carotene = (0.216 × A663) − (0.304 × A505) + (0.452 × A453)
2.5. Determination of In Vitro Antioxidant Activities
2.5.1. DPPH (2,2-Diphenyl-1-Picryl-Hydrazyl) Assay
2.5.2. Superoxide Radical Scavenging Assay
2.5.3. Hydroxyl Radical Scavenging Assay
2.5.4. Nitric Oxide Radical Scavenging Assay
2.5.5. Reducing Power Assay
2.6. MTT Cell Viability Assay
2.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.8. Statistical Analysis
3. Results
3.1. Extraction Yield
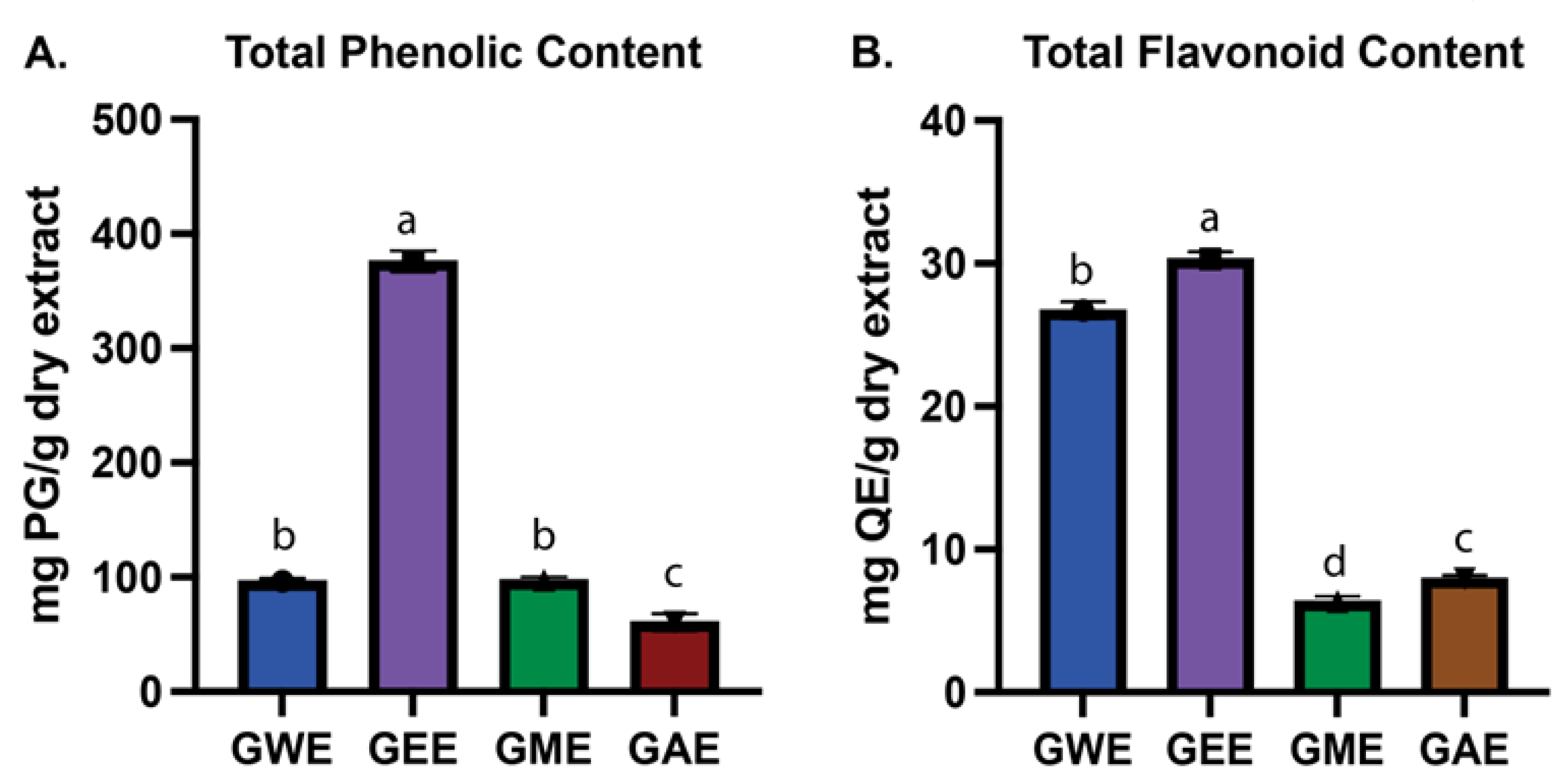
3.2. Estimation of Total Phenolic and Flavonoid Content
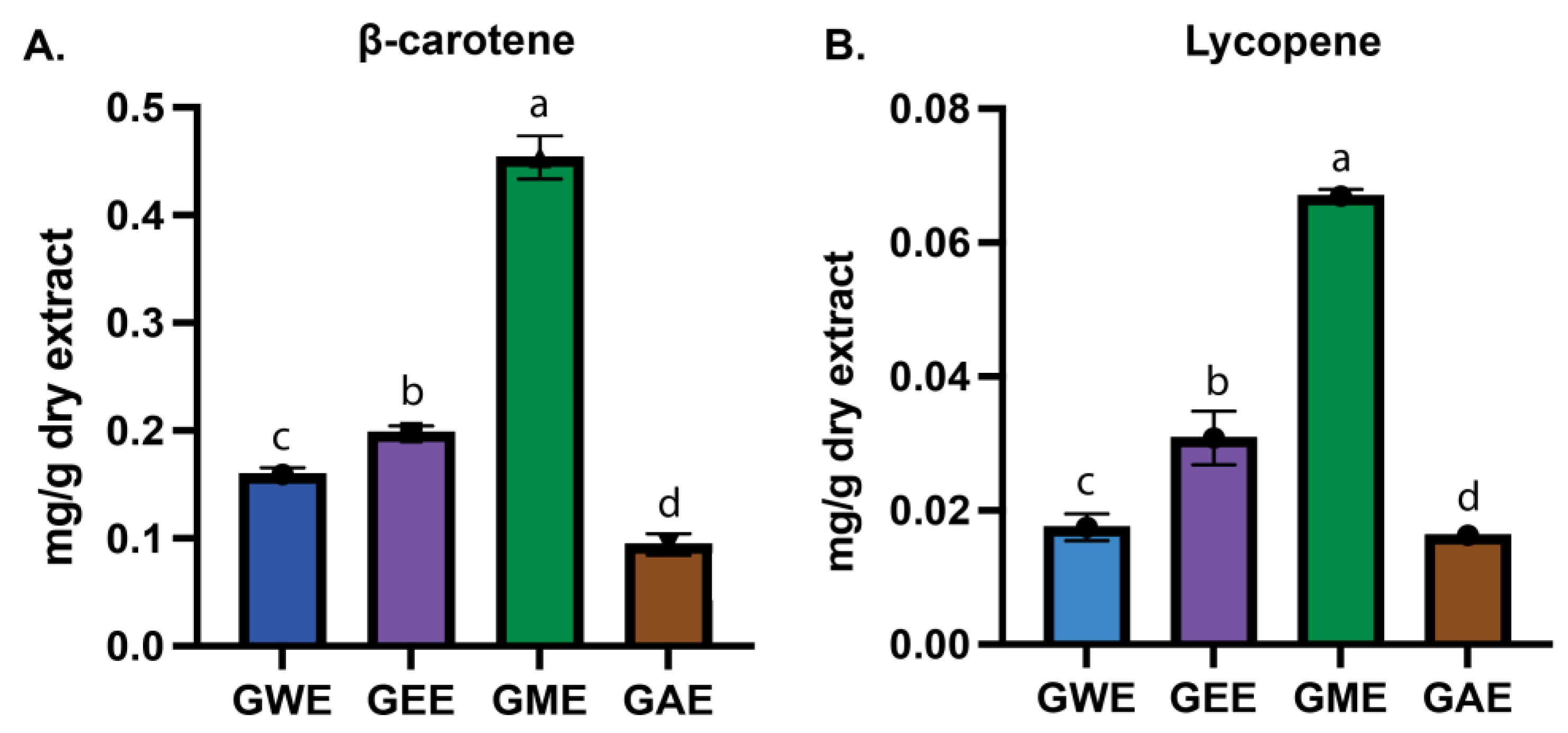
3.3. Estimation of β-Carotene and Lycopene
3.4. Comparative In Vitro Antioxidant Activities
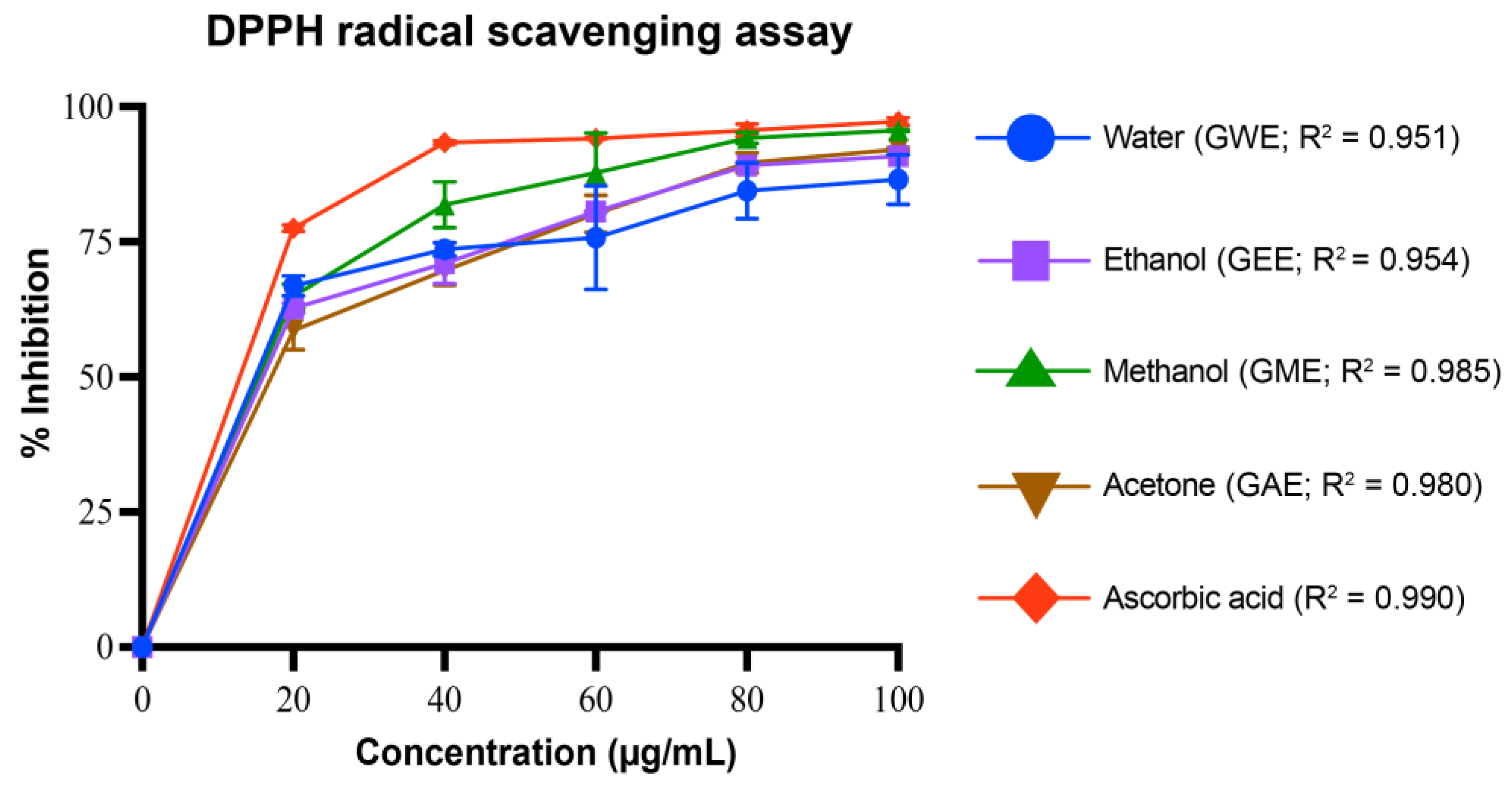
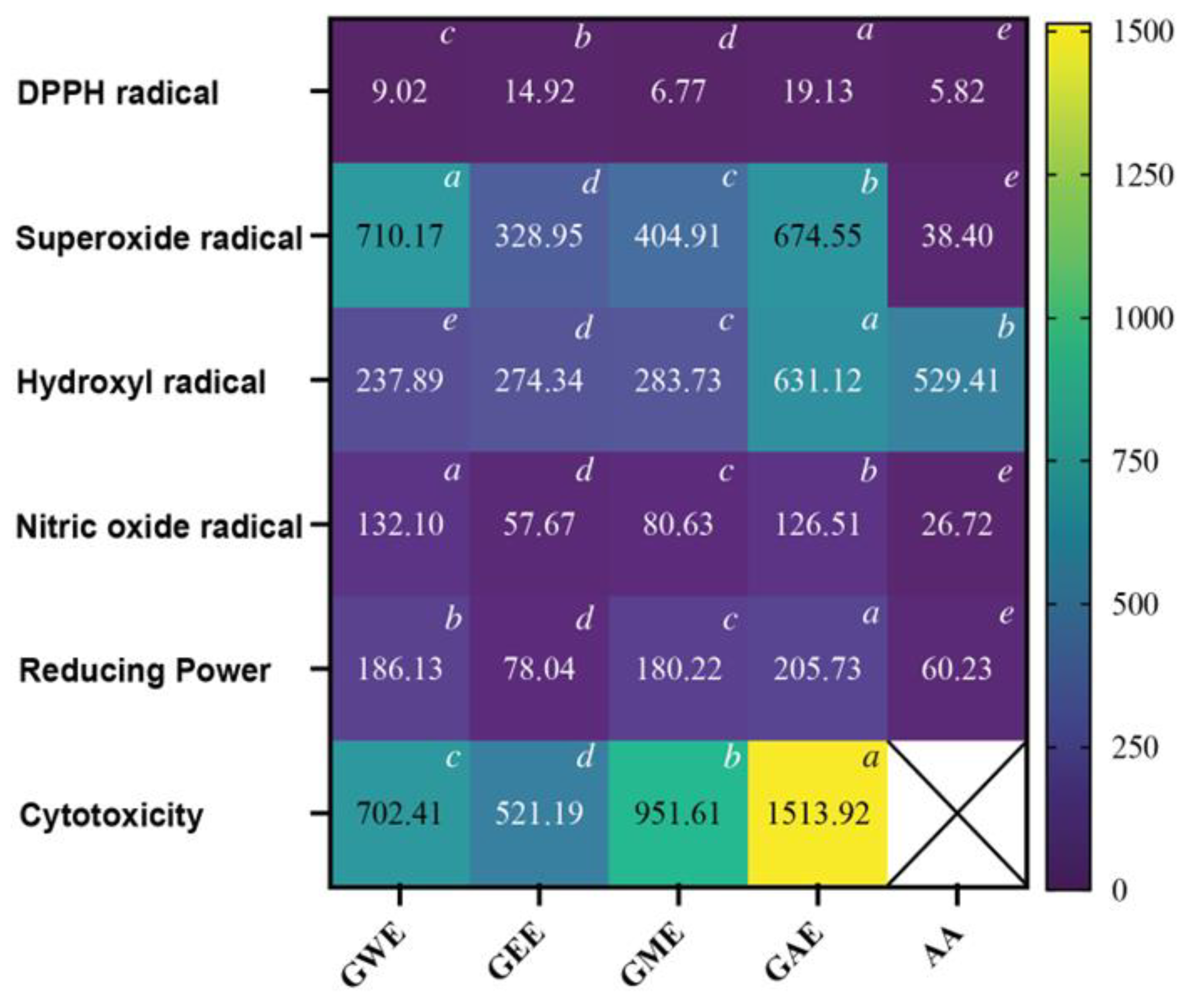
3.4.1. DPPH Radical Scavenging Activity
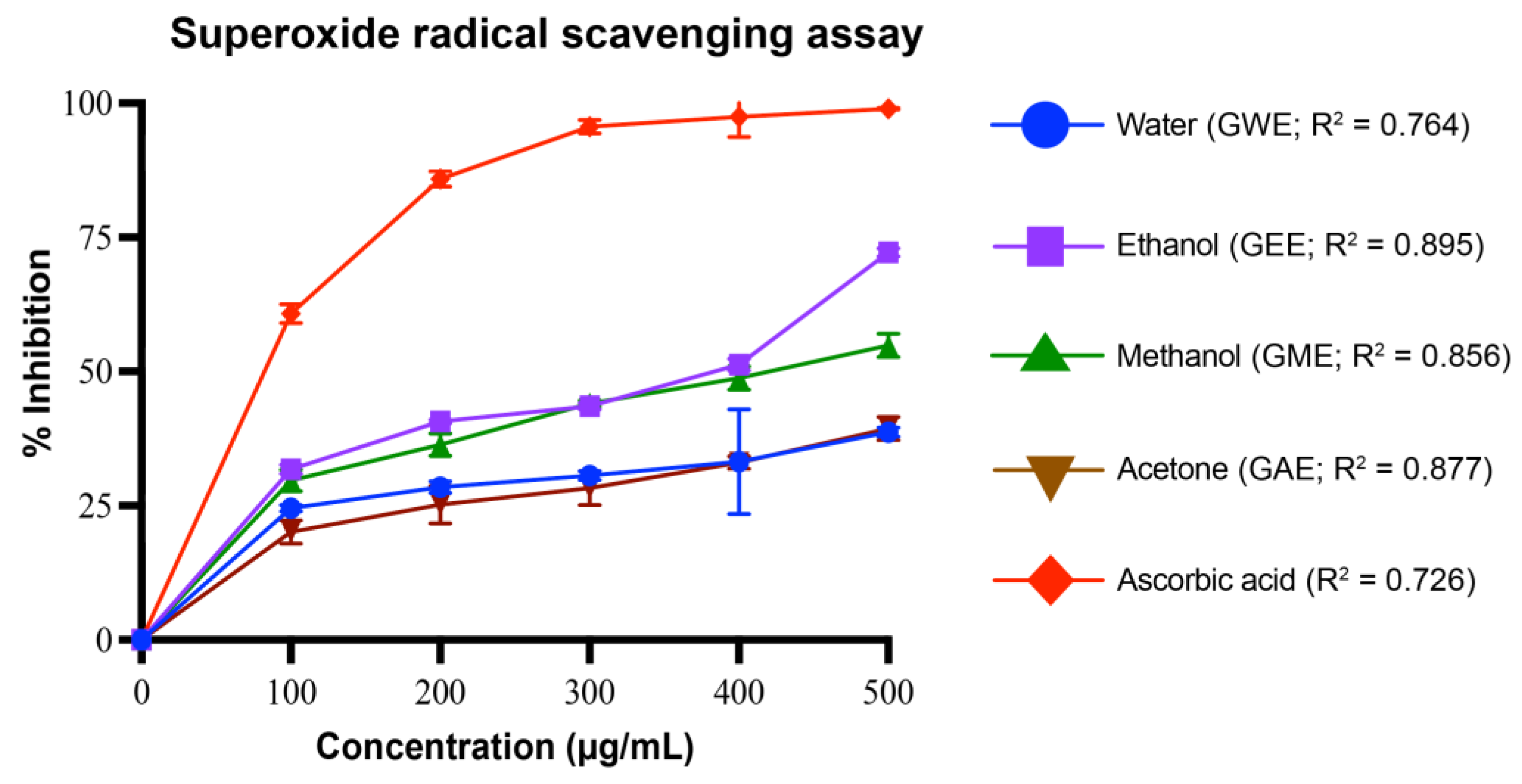
3.4.2. Superoxide Radical Scavenging Activity
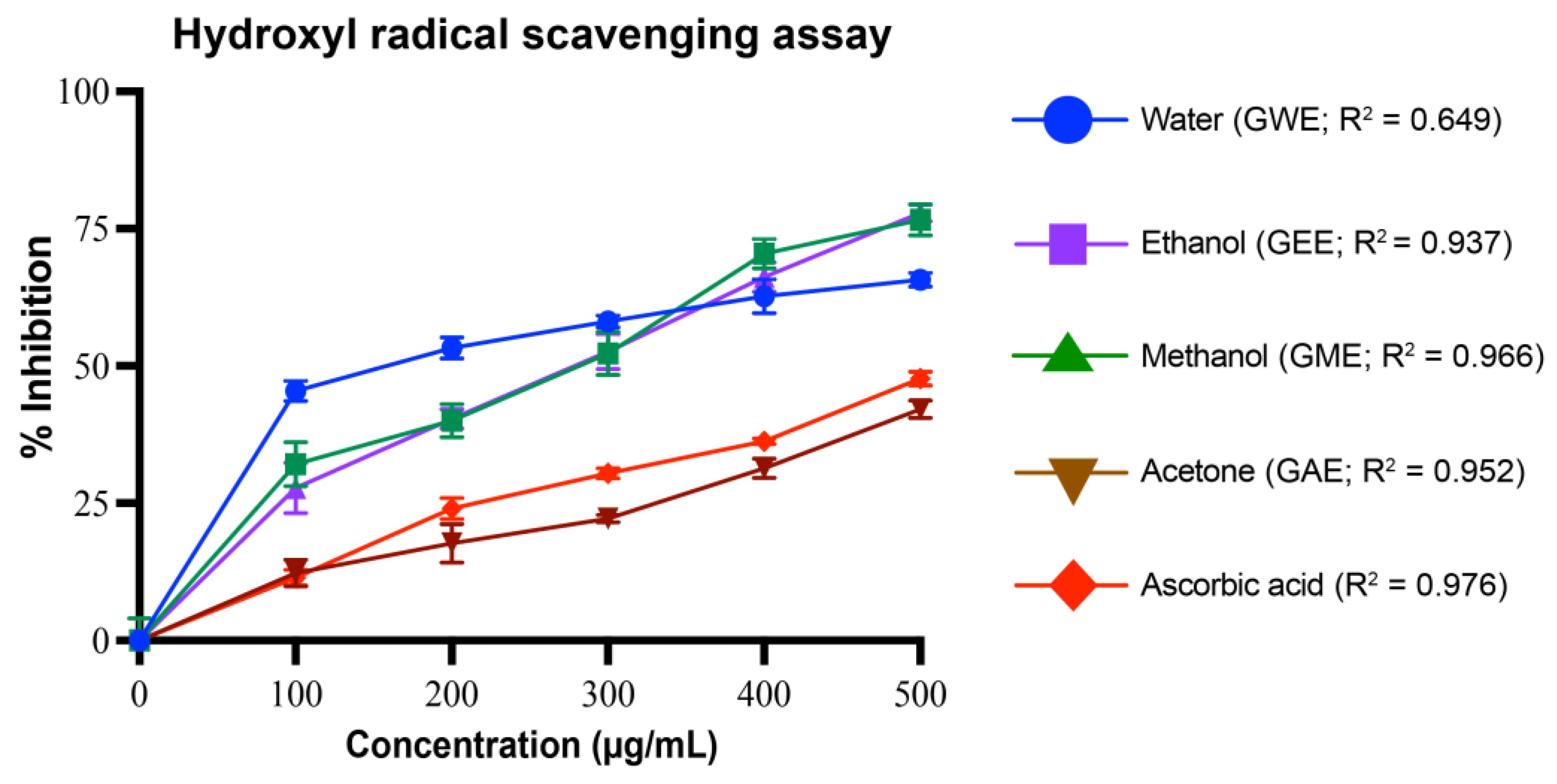
3.4.3. Hydroxyl Radical Scavenging Activity
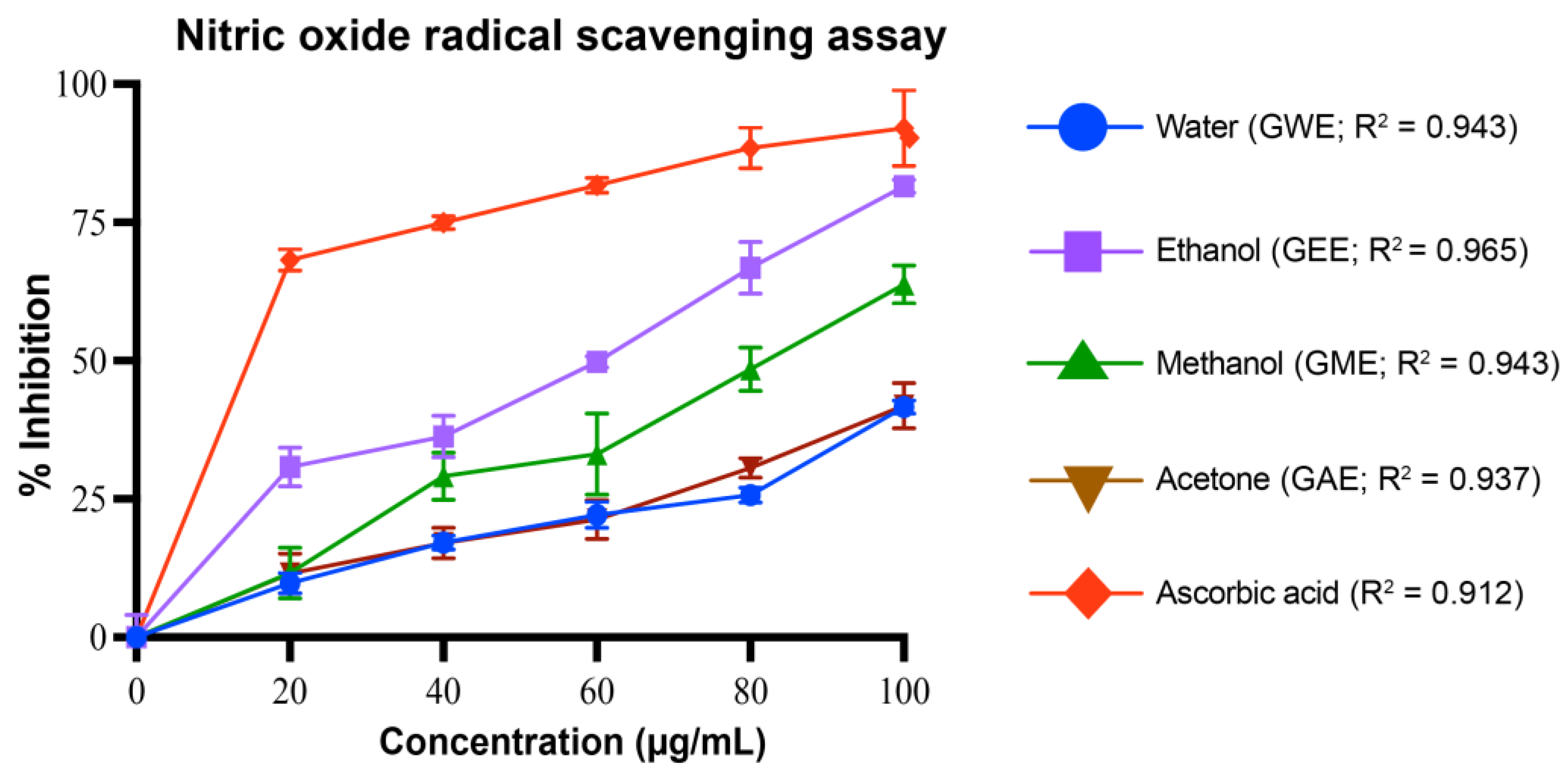
3.4.4. Nitric Oxide Radical Scavenging Activity
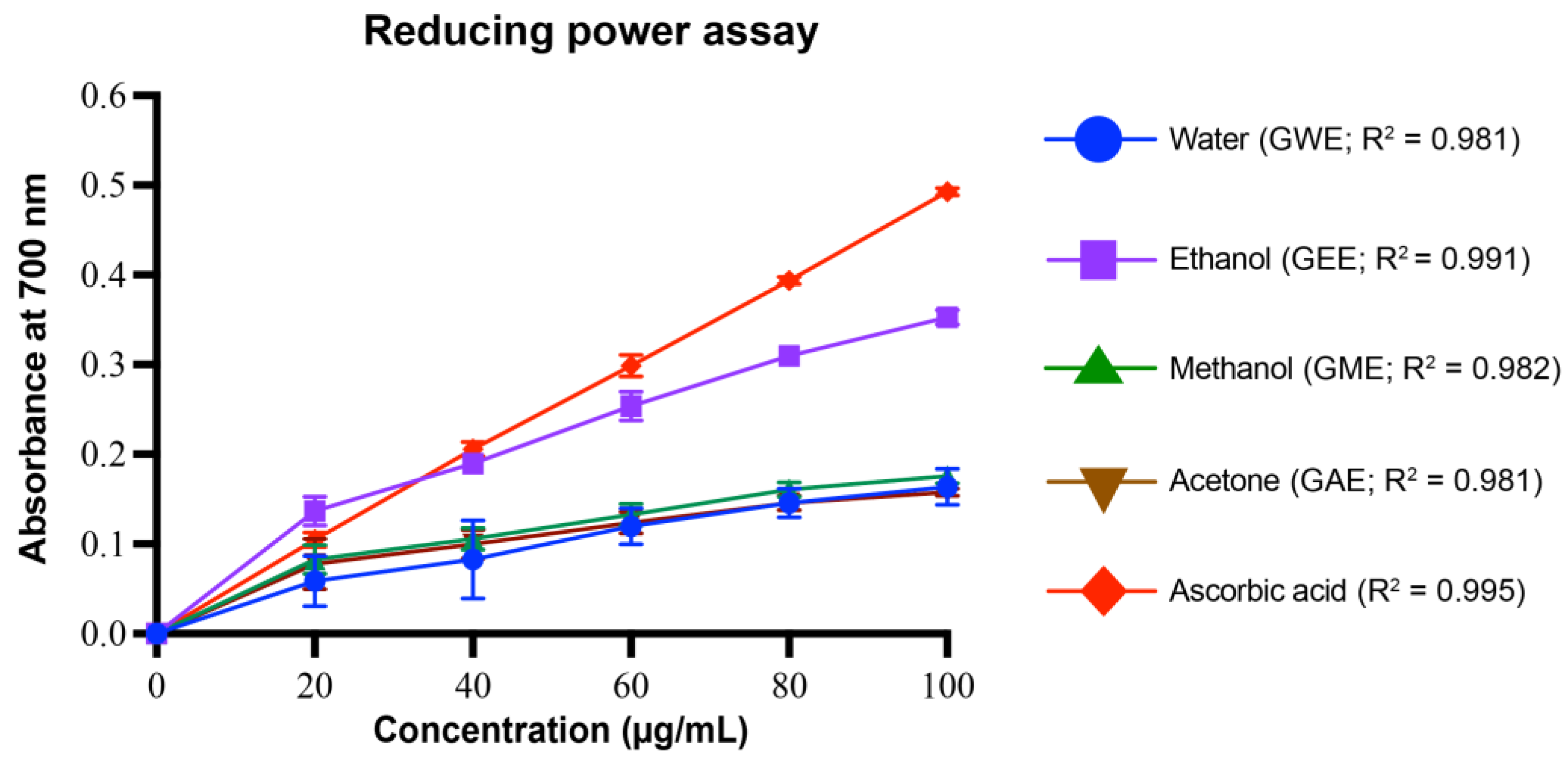
3.4.5. Reducing Power Assay
3.5. MTT-Based Viability Assay in HeLa Cells
3.6. IC50 Comparison of Extraction Solvents for Antioxidant and Cytotoxicity Activities
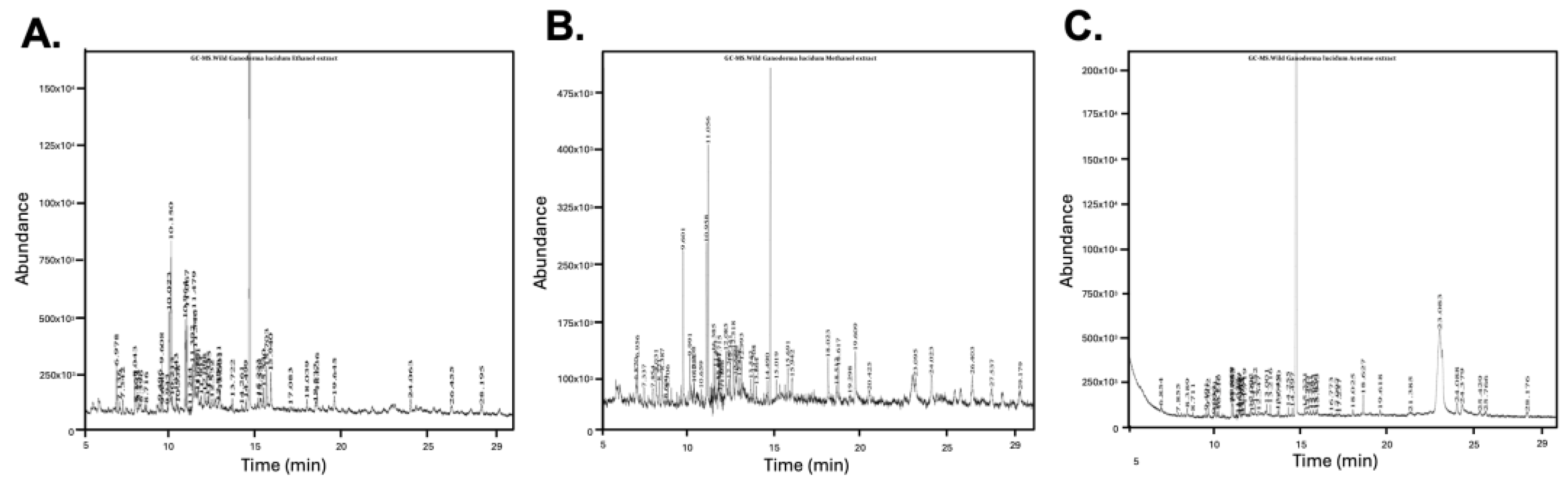
3.7. Solvent-Dependent Variation in Bioactive Compounds via GC-MS Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GWE | Ganoderma lucidum water extract |
| GEE | Ganoderma lucidum ethanol extract |
| GME | Ganoderma lucidum methanol extract |
| GAE | Ganoderma lucidum acetone extract |
| TPC | Total phenolic content |
| TFP | Total flavonoid content |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| GC-MS | Gas chromatography–mass spectrometry |
| LTP | Long-term potentiation |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| HeLa | Human cervical carcinoma cell lines |
References
- Zhou, L.W.; Cao, Y.; Wu, S.H.; Vlasák, J.; Li, D.W.; Li, M.J.; Dai, Y.C. Global Diversity of the Ganoderma lucidum Complex (Ganodermataceae, Polyporales) Inferred from Morphology and Multilocus Phylogeny. Phytochemistry 2015, 114, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.S.; Kao, C.H.J.; Xu, Y.; Glucina, M.P.; Paterson, R.R.M.; Ferguson, L.R. From 2000 Years of Ganoderma lucidum to Recent Developments in Nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.; Tomasevic, I.; Elobeid, T.; Çadırcı, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of Ganoderma lucidum: From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tian, L.; Wang, Y.; Li, Z.; Xu, Z. Chemodiversity, Pharmacological Activity, and Biosynthesis of Specialized Metabolites from Medicinal Model Fungi Ganoderma lucidum. Chin. Med. 2024, 19, 51. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary Metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Zhu, M.; Chang, Q.; Wong, L.K.; Chong, F.S.; Li, R.C. Triterpene Antioxidants from Ganoderma lucidum. Phytother. Res. 1999, 531, 529–531. [Google Scholar] [CrossRef]
- Yuen, J.W.M.; Gohel, M.D.I.; Yuen, J.W.M.; Gohel, M.D.I. Anticancer Effects of Ganoderma lucidum: A Review of Scientific Evidence. Nutr. Cancer 2005, 53, 11–17. [Google Scholar] [CrossRef]
- Acharya, K.; Bera, M.; Tarafder, E.; Dasgupta, A. Pharmacognostic Standardization of Ganoderma lucidum: A Commercially Explored Medicinal Mushroom. Der Pharmacia Lettre 2015, 7, 175–181. [Google Scholar]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical Features of Ganoderma Polysaccharides with Antioxidant, Antitumor and Antimicrobial Activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef]
- Mohan, K.; Padmanaban, M.; Uthayakumar, V. Isolation, Structural Characterization and Antioxidant Activities of Polysaccharide from Ganoderma lucidum (Higher Basidiomycetes). Am. J. Biomed. Life Sci. 2015, 3, 168–175. [Google Scholar]
- Heleno, S.A.; Barros, L.; Martins, A.; João, M.; Queiroz, R.P. Fruiting Body, Spores and in Vitro Produced Mycelium of Ganoderma lucidum from Northeast Portugal: A Comparative Study of the Antioxidant Potential of Phenolic and Polysaccharidic Extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Lindequist, U.; Mentel, R.; Go, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral Terpenoid Constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-Cancer Activities in Vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Cha, K.M.; Kim, S.K.; Lim, B.O. Anti-Inflammatory Activity on Mice of Extract of Ganoderma lucidum Grown on Rice via Modulation of MAPK and NF-ΚB Pathways. Phytochemistry 2015, 114, 125–136. [Google Scholar] [CrossRef]
- Uddin, K.; Shrestha, H.L.; Murthy, M.S.R.; Bajracharya, B.; Shrestha, B.; Gilani, H.; Pradhan, S.; Dangol, B. Development of 2010 National Land Cover Database for the Nepal. J. Environ. Manag. 2015, 148, 82–90. [Google Scholar] [CrossRef]
- Hai Bang, T.; Suhara, H.; Doi, K.; Ishikawa, H.; Fukami, K.; Parajuli, G.P.; Katakura, Y.; Yamashita, S.; Watanabe, K.; Adhikari, M.K.; et al. Wild Mushrooms in Nepal: Some Potential Candidates as Antioxidant and ACE-Inhibition Sources. Evid.-Based Complement. Altern. Med. 2014, 2014, 195305. [Google Scholar] [CrossRef]
- Tamrakar, S.; Tran, H.B.; Nishida, M.; Kaifuchi, S.; Suhara, H.; Doi, K.; Fukami, K.; Parajuli, G.P.; Shimizu, K. Antioxidative Activities of 62 Wild Mushrooms from Nepal and the Phenolic Profile of Some Selected Species. J. Nat. Med. 2016, 70, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Bhattarai, S.; Devkota, S.; Larsen, H.O. Collection and Use of Wild Edible Fungi in Nepal. Econ. Bot. 2008, 62, 12–23. [Google Scholar] [CrossRef]
- Devkota, S.; Fang, W.; Arunachalam, K.; Phyo, K.M.M.; Shakya, B. Systematic Review of Fungi, Their Diversity and Role in Ecosystem Services from the Far Eastern Himalayan Landscape (FHL). Heliyon 2023, 9, e12756. [Google Scholar] [CrossRef]
- Adhikari, M.K. Mushrooms of Nepal; Durrieu, G., Cotter, H.V.T., Eds.; Self-Published: Kathmandu, Nepal, 2014. [Google Scholar]
- Paudel, P.K.; Bhattarai, B.P.; Kindlmann, P. An Overview of the Biodiversity in Nepal. In Himalayan Biodiversity in the Changing World; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–40. ISBN 9789400718029. [Google Scholar]
- Pan, L.; Yang, N.; Sui, Y.; Li, Y.; Zhao, W.; Zhang, L.; Mu, L.; Tang, Z. Altitudinal Variation on Metabolites, Elements, and Antioxidant Activities of Medicinal Plant. Asarum. Metab. 2023, 13, 1193. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, D. Secondary Metabolites as a Survival Strategy in Plants of High Mountain Habitats. Bol. Latinoam. Caribe Plantas Med. Aromat. 2019, 18, 444–458. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Kareparamban, J.A.; Nikam, P.H.; Kadam, V.J. Spectrophotometric Estimation of Ferulic Acid from Ferula Asafoetida by Folin-Ciocalteu’s Reagent. Der Pharm. Sin. 2012, 3, 680–684. [Google Scholar]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Kumari, K.; Prakash, V.; Rana, S.; Sagar, A. In Vitro Antioxidant Activity of Methanolic Extract of Ganoderma lucidum (Curt) P. Karst. Int. J. Adv. Sci. Res. 2016, 1, 51–54. [Google Scholar]
- Chang, S.S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant Activity of Extracts from Acacia confusa Bark and Heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In Vitro and in Vivo Antioxidant Activity of Ethanolic Extract of White Button Mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Bristi, N.J. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-Scavenging Activity and Ferric Reducing Ability of Juniperus Thurifera (L.), J. Oxycedrus (L.), J. Phoenicea (L.) and Tetraclinis Articulata (L.). Adv. Pharmacol. Sci. 2016, 2016, 6392656. [Google Scholar] [CrossRef]
- Li, X.M.; Luo, X.G.; He, J.F.; Wang, N.; Zhou, H.; Yang, P.L.; Zhang, T.C. Induction of Apoptosis in Human Cervical Carcinoma Hela Cells by Active Compounds from Hypericum ascyron L. Oncol. Lett. 2018, 15, 3944–3950. [Google Scholar] [CrossRef]
- Tiwari, S.; Dhakal, N. Analysis of Variations in Biomolecules during Various Growth Phases of Freshwater Microalgae Chlorella sp. Appl. Food Biotechnol. 2023, 10, 73–84. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Kozhantayeva, A.; Tursynova, N.; Kolpek, A.; Aibuldinov, Y.; Tursynova, A.; Mashan, T.; Mukazhanova, Z.; Ibrayeva, M.; Zeinuldina, A.; Nurlybayeva, A.; et al. Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium. Pharmaceuticals 2024, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective Extraction of Bioactive Compounds from Plants Using Recent Extraction Techniques. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Thang, T.D.; Kuo, P.C.; Hwang, T.L.; Yang, M.L.; Ngoc, N.T.B.; Han, T.T.N.; Lin, C.W.; Wu, T.S. Triterpenoids and Steroids from Ganoderma mastoporum and Their Inhibitory Effects on Superoxide Anion Generation and Elastase Release. Molecules 2013, 18, 14285–14292. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.W.; Zheng, H.C.; Damirin, A.; Ma, C.M. Cytotoxic Constituents of Lasiosphaera fenzlii on Different Cell Lines and the Synergistic Effects with Paclitaxel. Nat. Prod. Res. 2016, 30, 1862–1865. [Google Scholar] [CrossRef]
- Ramos-Ligonio, A.; López-Monteon, A.; de la Soledad Lagunes-Castro, M.; Suárez-Medellín, J.; Espinoza, C.; Mendoza, G.; Trigos, Á. In Vitro Expression of Toll-like Receptors and Proinflammatory Molecules Induced by Ergosta-7,22-Dien-3-One Isolated from a Wild Mexican Strain of Ganoderma oerstedii (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 203–211. [Google Scholar] [CrossRef]
- Krivošija, S.; Nastić, N.; Karadžić Banjac, M.; Kovačević, S.; Podunavac-Kuzmanović, S.; Vidović, S. Supercritical Extraction and Compound Profiling of Diverse Edible Mushroom Species. Foods 2025, 14, 107. [Google Scholar] [CrossRef]
- Parmar, R.; Kumar, D. Study of Chemical Composition in Wild Edible Mushroom Pleurotus cornucopiae (Paulet) from Himachal Pradesh, India by Using Fourier Transforms Infrared Spectrometry (FTIR), Gas Chromatography-Mass Spectrometry (GCMS) and X-Ray Fluorescence (XRF). Biol. Forum–An. Int. J. 2015, 7, 1057–1066. [Google Scholar]
- Akwu, N.A.; Naidoo, Y.; Singh, M.; Lin, J.; Aribisala, J.O.; Sabiu, S.; Lekhooa, M.; Aremu, A.O. Phytochemistry, Antibacterial and Antioxidant Activities of Grewia lasiocarpa E. Mey. Ex Harv. Fungal Endophytes: A Computational and Experimental Validation Study. Chem. Biodivers. 2025, 22, e202402908. [Google Scholar] [CrossRef]
- Das Gupta, S.; Suh, N. Tocopherols in Cancer: An Update. Mol. Nutr. Food Res. 2016, 60, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Y.; Cui, X.X.; Goodin, S.; Wang, H.; Du, Z.Y.; Li, D.; Zhang, K.; Tony Kong, A.N.; Dipaola, R.S.; et al. Potent Inhibitory Effect of β-Tocopherol on Prostate Cancer Cells Cultured in Vitro and Grown as Xenograft Tumors in Vivo. J. Agric. Food Chem. 2014, 62, 10752–10758. [Google Scholar] [CrossRef]
- Biernacki, K.; Daśko, M.; Ciupak, O.; Kubiński, K.; Rachon, J.; Demkowicz, S. Novel 1,2,4-Oxadiazole Derivatives in Drug Discovery. Pharmaceuticals 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Siwach, A.; Verma, P.K. Therapeutic Potential of Oxadiazole or Furadiazole Containing Compounds. BMC Chem. 2020, 14, 70. [Google Scholar] [CrossRef]
- Ehrsam, D.; Porta, F.; Mori, M.; Meyer Zu Schwabedissen, H.E.; Via, L.D.; Garcia-Argaez, A.N.; Basile, L.; Meneghetti, F.; Villa, S.; Gelain, A. Unravelling the Antiproliferative Activity of 1,2,5-Oxadiazole Derivatives. Anticancer Res. 2019, 39, 3453–3461. [Google Scholar] [CrossRef]
- Luczynski, M.; Kudelko, A. Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture. Appl. Sci. 2022, 12, 3756. [Google Scholar] [CrossRef]
- Su, J.C.; Pan, X.X.; Wei, X.; Lei, X.; Zhang, P. Structurally Diverse Steroids from an Endophyte of Aspergillus tennesseensis 1022LEF Attenuates LPS-Induced Inflammatory Response through the Cholinergic Anti-Inflammatory Pathway. Chem. Biol. Interact. 2022, 362, 109998. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cheng, X.L.; Cui, J.H.; Yan, X.R.; Wei, F.; Bai, X.; Lin, R.C. Effect of Ergosta-4,6,8(14),22-Tetraen-3-One (Ergone) on Adenine-Induced Chronic Renal Failure Rat: A Serum Metabonomic Study Based on Ultra Performance Liquid Chromatography/High-Sensitivity Mass Spectrometry Coupled with MassLynx i-FIT Algorithm. Clin. Chim. Acta 2012, 413, 1438–1445. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Shen, X.; Chao, X.; Ho, C.C.; Cheng, X.L.; Zhang, Y.; Lin, R.C.; Du, K.J.; Luo, W.J.; Chen, J.Y.; et al. Ergosta-4,6,8(14),22-Tetraen-3-One Induces G2/M Cell Cycle Arrest and Apoptosis in Human Hepatocellular Carcinoma HepG2 Cells. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 384–390. [Google Scholar] [CrossRef]
- Nilkhet, S.; Vongthip, W.; Lertpatipanpong, P.; Prasansuklab, A.; Tencomnao, T.; Chuchawankul, S.; Baek, S.J. Ergosterol Inhibits the Proliferation of Breast Cancer Cells by Suppressing AKT/GSK-3beta/Beta-Catenin Pathway. Sci. Rep. 2024, 14, 19664. [Google Scholar] [CrossRef]
- Dupont, S.; Fleurat-Lessard, P.; Cruz, R.G.; Lafarge, C.; Grangeteau, C.; Yahou, F.; Gerbeau-Pissot, P.; Abrahão Júnior, O.; Gervais, P.; Simon-Plas, F.; et al. Antioxidant Properties of Ergosterol and Its Role in Yeast Resistance to Oxidation. Antioxidants 2021, 10, 1024. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Wang, X.; Zhang, Y.; Sun, Q.; Jiang, Y.; Yao, J.; Li, C.; Wang, Y.; Wang, W. Ferruginol Restores SIRT1-PGC-1α-Mediated Mitochondrial Biogenesis and Fatty Acid Oxidation for the Treatment of DOX-Induced Cardiotoxicity. Front. Pharmacol. 2021, 12, 773834. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, M.; Philippot, S.; González-Cardenete, M.A. Anticoronavirus Evaluation of Antimicrobial Diterpenoids: Application of New Ferruginol Analogues. Viruses 2023, 15, 1342. [Google Scholar] [CrossRef] [PubMed]
- Bispo de Jesus, M.; Zambuzzi, W.F.; Ruela de Sousa, R.R.; Areche, C.; Santos de Souza, A.C.; Aoyama, H.; Schmeda-Hirschmann, G.; Rodríguez, J.A.; Monteiro de Souza Brito, A.R.; Peppelenbosch, M.P.; et al. Ferruginol Suppresses Survival Signaling Pathways in Androgen-Independent Human Prostate Cancer Cells. Biochimie 2008, 90, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.M.; Al-Qurainy, F.; Tarroum, M.; Khan, S.; Nadeem, M.; Shaikhaldein, H.O.; Alansi, S. Phytochemical Compound Profile and the Estimation of the Ferruginol Compound in Different Parts (Roots, Leaves, and Seeds) of Juniperus Procera. Separations 2022, 9, 352. [Google Scholar] [CrossRef]
- Shao, L.; González-Cardenete, M.A.; Prieto-Garcia, J.M. In Vitro Cytotoxic Effects of Ferruginol Analogues in Sk-MEL28 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 16322. [Google Scholar] [CrossRef]
- Ho, S.T.; Tung, Y.T.; Kuo, Y.H.; Lin, C.C.; Wu, J.H. Ferruginol Inhibits Non-Small Cell Lung Cancer Growth by Inducing Caspase-Associated Apoptosis. Integr. Cancer Ther. 2015, 14, 86–97. [Google Scholar] [CrossRef]
- Wang, X.; Cao, G.; Ding, D.; Li, F.; Zhao, X.; Wang, J.; Yang, Y. Ferruginol Prevents Degeneration of Dopaminergic Neurons by Enhancing Clearance of α-Synuclein in Neuronal Cells. Fitoterapia 2022, 156, 105066. [Google Scholar] [CrossRef]
- Sediva, A.; Orlicky, M.; Vrabcova, P.; Klocperk, A.; Kalina, T.; Fujiwara, H.; Hsu, F.-F.; Bambouskova, M. Geranylgeraniol Supplementation Leads to an Improvement in Inflammatory Parameters and Reversal of the Disease Specific Protein Signature in Patients with Hyper-IgD Syndr. medRxiv 2024. [Google Scholar] [CrossRef]
- Gheith, R.; Sharp, M.; Stefan, M.; Ottinger, C.; Lowery, R.; Wilson, J. The Effects of Geranylgeraniol on Blood Safety and Sex Hormone Profiles in Healthy Adults: A Dose-Escalation, Randomized, Placebo-Controlled Trial. Nutraceuticals 2023, 3, 605–618. [Google Scholar] [CrossRef]
- Chung, E.; Elmassry, M.M.; Cao, J.J.; Kaur, G.; Dufour, J.M.; Hamood, A.N.; Shen, C.L. Beneficial Effect of Dietary Geranylgeraniol on Glucose Homeostasis and Bone Microstructure in Obese Mice Is Associated with Suppression of Proinflammation and Modification of Gut Microbiome. Nutr. Res. 2021, 93, 27–37. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ekeuku, S.O.; Trias, A. The Role of Geranylgeraniol in Managing Bisphosphonate-Related Osteonecrosis of the Jaw. Front. Pharmacol. 2022, 13, 878556. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.W.; Zhang, Y.; Wang, W.H.; Wu, X.Y.; Liu, S.Z.; Bin, Y.L.; Cai, B.P.; Huang, S.Y.; Fang, M.J.; et al. Hinokione: An Abietene Diterpene with Pancreatic β Cells Regeneration and Hypoglycemic Activity, and Other Derivatives with Novel Structures from the Woods of Agathis dammara. J. Nat. Med. 2024, 78, 849–862. [Google Scholar] [CrossRef]
- Gáborová, M.; Šmejkal, K.; Kubínová, R. Abietane Diterpenes of the Genus Plectranthus sensu lato. Molecules 2022, 27, 166. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A.; Topcu, G.; Johansson, C.B. Norditerpenoids and Diterpenoids from Salvia Multicaulis with Antituberculous Activity. J. Nat. Prod. 1997, 60, 1275–1280. [Google Scholar] [CrossRef]
- Dong, J.R.; Chang, W.W.; Chen, S.M. Nerolidol Inhibits Proliferation of Leiomyoma Cells via Reactive Oxygen Species-Induced DNA Damage and Downregulation of the ATM/Akt Pathway. Phytochemistry 2021, 191, 112901. [Google Scholar] [CrossRef]
- Judzentiene, A.; Butkiene, R.; Budiene, J.; Tomi, F.; Casanova, J. Composition of Seed Essential Oils of Rhododendron tomentosu. Nat. Prod. Commun. 2012, 7, 227–230. [Google Scholar] [PubMed]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Pouso, M.R.; Cairrao, E. Effect of Retinoic Acid on the Neurovascular Unit: A Review. Brain Res. Bull. 2022, 184, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Casadesus, G.; Zhu, X.; Lee, H.G.; Perry, G.; Smith, M.A.; Gustaw-Rothenberg, K.; Lerner, A. All-Trans Retinoic Acid as a Novel Therapeutic Strategy for Alzheimer’s Disease. Expert Rev. Neurother. 2009, 9, 1615–1621. [Google Scholar] [CrossRef]
- Pino-Lagos, K.; Benson, M.J.; Noelle, R.J. Retinoic Acid in the Immune System. Ann. N. Y. Acad. Sci. 2008, 1143, 170–187. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Amina, B.; Kaoutar, A.; Saidi, R.; Lamrani, Z.; Pinto, E.; Esteves da Silva, J.C.G.; Maouni, A.; Pinto da Silva, L. Chemical Characterization and Evaluation of Antimicrobial Properties of the Wild Medicinal Mushroom Ganoderma lucidum Growing in Northern Moroccan Forests. Life 2023, 13, 1217. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Effects of Extraction Solvent on Wheat Bran Antioxidant Activity Estimation. LWT–Food Sci. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Yousuf, B.; Panesar, P.S.; Chopra, H.K.; Gul, K. Characterization of Secondary Metabolites from Various Solvent Extracts of Saffron Floral Waste. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 87, 89–100. [Google Scholar] [CrossRef]
- Packialakshmi, N.; Naziya, S. Fourier Transform Infrared Spectroscopy Analysis of Various Solvent Extracts of Caralluma Fimbriyata. Asian J. Biomed. Pharm. Sci. 2014, 4, 20–25. [Google Scholar]
- Tomsone, L.; Kruma, Z.; Galoburda, R. Comparison of Different Solvents and Extraction Methods for Isolation of Phenolic Compounds from Horseradish Roots. Int. Sch. Sci. Res. Innov. 2012, 6, 1164–1169. [Google Scholar]
- Pham, H.; Nguyen, V.; Vuong, Q.; Bowyer, M.; Scarlett, C. Effect of Extraction Solvents and Drying Methods on the Physicochemical and Antioxidant Properties of Helicteres hirsuta lour Leaves. Technologies 2015, 3, 285–301. [Google Scholar] [CrossRef]
- Avcı, E.; Avcı, G.A.; Kose, D.A. Determination of Antioxidant and Antimicrobial Activities of Medically Important Mushrooms Using Different Solvents and Chemical Composition via GC / MS Analyses. J. Food Nutr. Res. 2014, 2, 429–434. [Google Scholar] [CrossRef][Green Version]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma Species: Profiling of Phenolic Compounds by HPLC-DAD, Antioxidant, Antimicrobial and Inhibitory Activities on Key Enzymes Linked to Diabetes Mellitus, Alzheimer’s Disease and Skin Disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Nagaraj, K.; Mallikarjun, N.; Naika, R.; Venugopal, T.M. Antioxdative Activities of Wild Macro Fungi Ganoderma applanatum (PERS.) PAT. Asian J. Pharm. Clin. Res. 2014, 7, 166–171. [Google Scholar]
- Tel, G.; Ozturk, M.; Duru, M.E.; Turkoglu, A. Antioxidant and Anticholinesterase Activities of Five Wild Mushroom Species with Total Bioactive Contents. Pharm. Biol. 2015, 53, 824–830. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total Phenols, Ascorbic Acid, B-Carotene and Lycopene in Portuguese Wild Edible Mushrooms and Their Antioxidant Activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Bravo, L.; Sources, D.; Significance, N. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Sonboli, A. Antioxidant, Free Radical Scavenging Activities of Salvia brachyantha and Its Protective Effect against Oxidative Cardiac Cell Injury. Food Chem. Toxicol. 2010, 48, 846–853. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, I.G. Ganoderma lucidum Extract Protects DNA from Strand Breakage Caused by Hydroxyl Radical and UV Irradiation. Int. J. Mol. Med. 1999, 4, 273–277. [Google Scholar] [CrossRef]
- Lakshmi, B.; Ajith, T.A.; Sheena, N.; Gunapalan, N.; Janardhanan, K.K. Antiperoxidative, Anti-Inflammatory, and Antimutagenic Activities of Ethanol Extract of the Mycelium of Ganoderma lucidum Occurring in South India. Teratog. Carcinog. Mutagen. 2003, 23 (Suppl. 1), 85–97. [Google Scholar] [CrossRef]
- Lee, J.M.; Kwon, H.; Jeong, H.; Lee, J.W.; Lee, S.Y.; Baek, S.J.; Surh, Y.J. Inhibition of Lipid Peroxidation and Oxidative DNA Damage by Ganoderma lucidum. Phytother. Res. 2001, 15, 245–249. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S.S. Antioxidant Capacity and the Correlation with Major Phenolic Compounds, Anthocyanin, and Tocopherol Content in Various Extracts from the Wild Edible Boletus edulis Mushroom. BioMed Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef]
- Jayakumar, T.; Thomas, P.A.; Geraldine, P. In-Vitro Antioxidant Activities of an Ethanolic Extract of the Oyster Mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol. 2009, 10, 228–234. [Google Scholar] [CrossRef]
- Rajasekaran, M.; Rajasekaran, M.; Kalaimagal, C. In Vitro Antioxidant Activity of Ethanolic Extract of a Medicinal Mushroom, Ganoderma Lucidum. J. Pharm. Sci. Res. 2011, 3, 1427–1433. [Google Scholar]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evid.-Based Complement. Altern. Med. 2012, 2012, 464238. [Google Scholar] [CrossRef]
- Lim, C.S.H.; Lim, S.L. Ferric Reducing Capacity Versus Ferric Reducing Antioxidant Power for Measuring Total Antioxidant Capacity. Lab. Med. 2013, 44, 51–55. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Omidifar, N.; Chiang, W.H.; Neralla, V.R.; Yousefi, K.; Shokripour, M. Ganoderma lucidum Methanolic Extract as a Potent Phytoconstituent: Characterization, In-Vitro Antimicrobial and Cytotoxic Activity. Sci. Rep. 2023, 13, 17326. [Google Scholar] [CrossRef]
- Veljović, S.; Veljović, M.; Nikićević, N.; Despotović, S.; Radulović, S.; Nikšić, M.; Filipović, L. Chemical Composition, Antiproliferative and Antioxidant Activity of Differently Processed Ganoderma lucidum Ethanol Extracts. J. Food Sci. Technol. 2017, 54, 1312–1320. [Google Scholar] [CrossRef]
- Fronza, M.; Murillo, R.; Ślusarczyk, S.; Adams, M.; Hamburger, M.; Heinzmann, B.; Laufer, S.; Merfort, I. In Vitro Cytotoxic Activity of Abietane Diterpenes from Peltodon Longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011, 19, 4876–4881. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Deng, G.; Yuan, W.; Su, Z. Cytotoxic Compounds from Invasive Giant Salvinia (Salvinia molesta) against Human Tumor Cells. Bioorg. Med. Chem. Lett. 2013, 23, 6682–6687. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin, D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
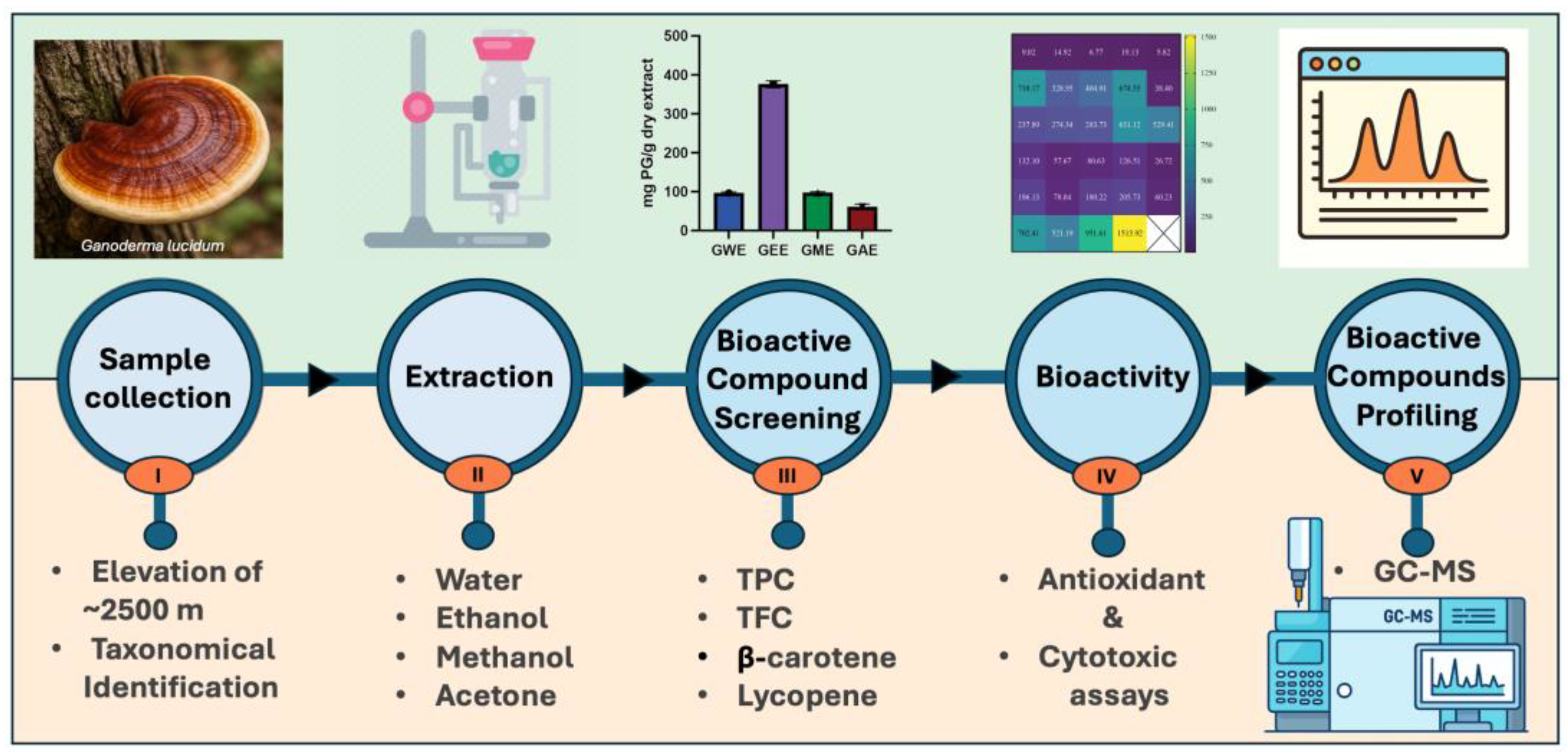


| Parameter | Description |
|---|---|
| Collection month | September–October |
| Location | Chandragiri Hill, Kathmandu, Nepal |
| Elevation | 7482 ft (2280 m) above sea level |
| Coordinates | Latitude: 27.67402° N; Longitude: 85.19874° E |
| Ecosystem type | Solitary |
| Substrate | Wood, stump, log, stick, base of tree, bark |
| Host tree | Quercus lanata |
| Rot type | White-rot |
| Surrounding trees | Predominantly hardwoods |
| Basidiocarp size Texture | 6–10 cm × 4–16 cm × 1.3 cm Woody to corky |
| Stipe | Sub-sessile to laterally stipitate, 2–3 cm |
| Pileus shape | Reniform |
| Upper surface | Laccate, dark reddish to purplish, yellowish at margins; brittle, soft |
| Margin | Blunt, rounded, brown-white |
| Pore surface | Creamy to milky coffee; ~5 pores/mm |
| Tube layer | 2–9 mm long, white turning brown when brushed or aged |
| Context | 9 mm thick, brown, without horny deposition |
| Cutis type | Thick-walled claviform with diverticula; 35–42 × 6–8.5 µm |
| Hyphal system | Trimitic: Generative (3.3 µm, hyaline, thin-walled, with clamp); Skeletal (5.8–7.5 µm, brown, thick); Binding (5–7.5 µm, brown) |
| Basidiospores | 8.3–10 × 6.6 µm; yellowish-brown |
| Identification authority | Prof. Mahesh Kumar Adhikari, Dept. of Plant Resources, Kathmandu |
| Extract | Weight of Sample Before Extraction (gm) | Weight Obtained After Extraction (gm) | % Yield Value |
|---|---|---|---|
| Water | 10 | 0.229 | 2.29 d |
| Ethanol | 10 | 0.343 | 3.43 b |
| Methanol | 10 | 0.298 | 2.98 c |
| Acetone | 10 | 0.501 | 5.01 a |
| Compound Name | Solvent Extracts (% Area) | Compound Class | Key Pharmacological Relevance | Reference | ||
|---|---|---|---|---|---|---|
| GEE | GME | GAE | ||||
| 7,22-Ergostadienone | 3.54 | 2.90 | 2.56 | Sterol | Antithrombotic activity with cardiovascular benefit; antidiabetic, anticancer, and neuroprotective effects; pro-inflammatory properties (activating Toll-like receptors, cytokines, and chemokines) | [37,40,41,42,43] |
| 9(11)-Dehydroergosteryl 3,5-dinitrobenzoate | 2.90 | 3.13 | 2.70 | Sterol conjugate | Anti-inflammatory; antibacterial (MRSA and S. aureus); and cytotoxic properties | [44,45] |
| δ-Tocopherol | 2.13 | 3.91 | 0.75 | Tocopherol | Antioxidant; anti-inflammatory (primarily Via inhibiting protein kinase C and reducing eicosanoid production); anticancer (both in vitro and in vivo prostate xenograft models); cardioprotective and neuroprotective | [46,47] |
| 4-[5-(2-bromophenyl)-1,2,4-oxadiazol-3-yl]-1,2,5-oxadiazol-3-amine | - | - | 0.35 | Synthetic heterocycle | Anticancer (potentially via targeting topoisomerase II relaxation activity); antibacterial; anti-inflammatory; analgesic properties; antioxidant | [48,49,50,51,52] |
| Ergosta-tetraenone | 3.86 | - | 1.67 | Sterol derivative | Anticancer (Via G2/M arrest and apoptosis induction); nephroprotection (mitigation of renal damage in mouse model); anti-inflammatory | [53,54,55] |
| Ergosterol | - | - | 73.99 | Sterol | Vitamin D2 precursor; lipid soluble antioxidant; anticancer effects (cell cycle arrest and modulates Wnt/β-catenin signaling pathway); antimicrobial; antidiabetic; immunomodulatory effects | [56,57,58] |
| Ferruginol | 3.18 | - | - | Abietane diterpene | Anticancer (apoptosis induction in melanoma, prostate, lung, and ovarian cancer cells); neuroprotective (reduces α-synuclein toxicity and restores LTP in Alzheimer’s models); cardioprotective (both in vitro and in vivo models); antimicrobial and antiviral | [59,60,61,62,63,64,65] |
| Geranylgeraniol | 5.26 | - | 0.89 | Diterpenoid alcohol | Anti-inflammatory (NF-κB inhibition; decreased IL-1β, TNF-α, IL-6, COX-2); pain relief; bone and muscle support (muscle regeneration and prevents bisphosphonate-related bone damage); antimicrobial activity; hormonal balance; glucose homeostasis | [66,67,68,69] |
| Hinokione | 2.9 | 5.5 | 0.9 | Abietane diterpene | Anticancer; anti-inflammatory; hypoglycemic and β-Cell regenerative properties (promotes β-cell differentiation and improved glycemia in zebrafish); antibacterial; antioxidant | [70,71,72] |
| Nerolidol acetate | - | 1.70 | - | Sesquiterpene ester | Anticancer; anti-inflammatory; neuroprotective; antimicrobial; antifungal; antioxidant | [73,74,75] |
| Retinoic acid | - | - | 0.50 | Retinoid | Acne and photoaging (promotes cell differentiation and skin repair); anticancer (induces differentiation of malignant promyelocytes in acute promyeloid leukemia); neuroprotective | [76,77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, I.; Pandey, A.; Tiwari, S.; Awal, S.C. Evaluation of Bioactive Compounds, Antioxidant Activity, and Anticancer Potential of Wild Ganoderma lucidum Extracts from High-Altitude Regions of Nepal. Curr. Issues Mol. Biol. 2025, 47, 624. https://doi.org/10.3390/cimb47080624
Thapa I, Pandey A, Tiwari S, Awal SC. Evaluation of Bioactive Compounds, Antioxidant Activity, and Anticancer Potential of Wild Ganoderma lucidum Extracts from High-Altitude Regions of Nepal. Current Issues in Molecular Biology. 2025; 47(8):624. https://doi.org/10.3390/cimb47080624
Chicago/Turabian StyleThapa, Ishor, Ashmita Pandey, Sunil Tiwari, and Suvash Chandra Awal. 2025. "Evaluation of Bioactive Compounds, Antioxidant Activity, and Anticancer Potential of Wild Ganoderma lucidum Extracts from High-Altitude Regions of Nepal" Current Issues in Molecular Biology 47, no. 8: 624. https://doi.org/10.3390/cimb47080624
APA StyleThapa, I., Pandey, A., Tiwari, S., & Awal, S. C. (2025). Evaluation of Bioactive Compounds, Antioxidant Activity, and Anticancer Potential of Wild Ganoderma lucidum Extracts from High-Altitude Regions of Nepal. Current Issues in Molecular Biology, 47(8), 624. https://doi.org/10.3390/cimb47080624









