Analysis of Endoplasmic Reticulum Stress Proteins in Spermatogenic Cells After Paclitaxel Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
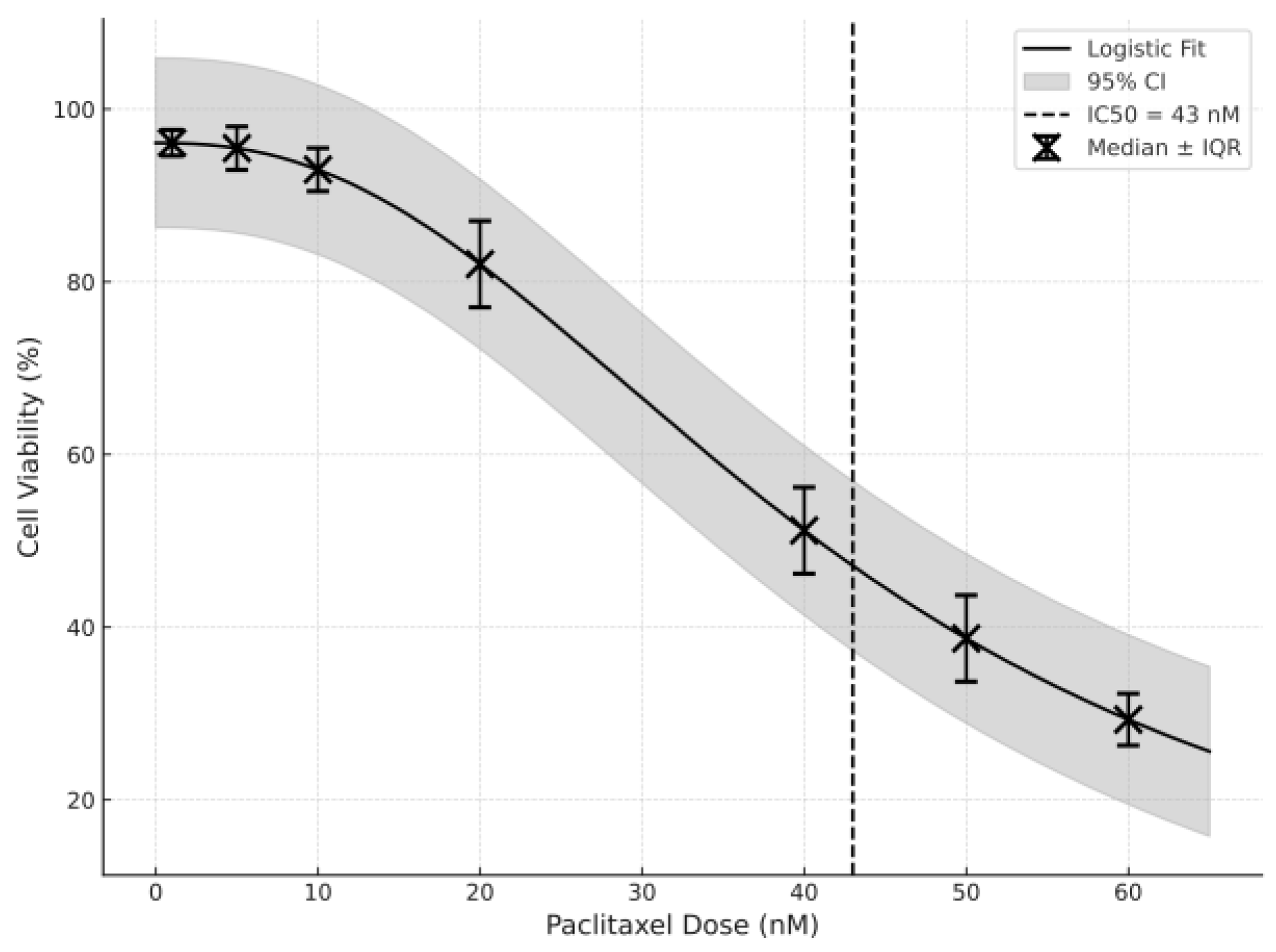
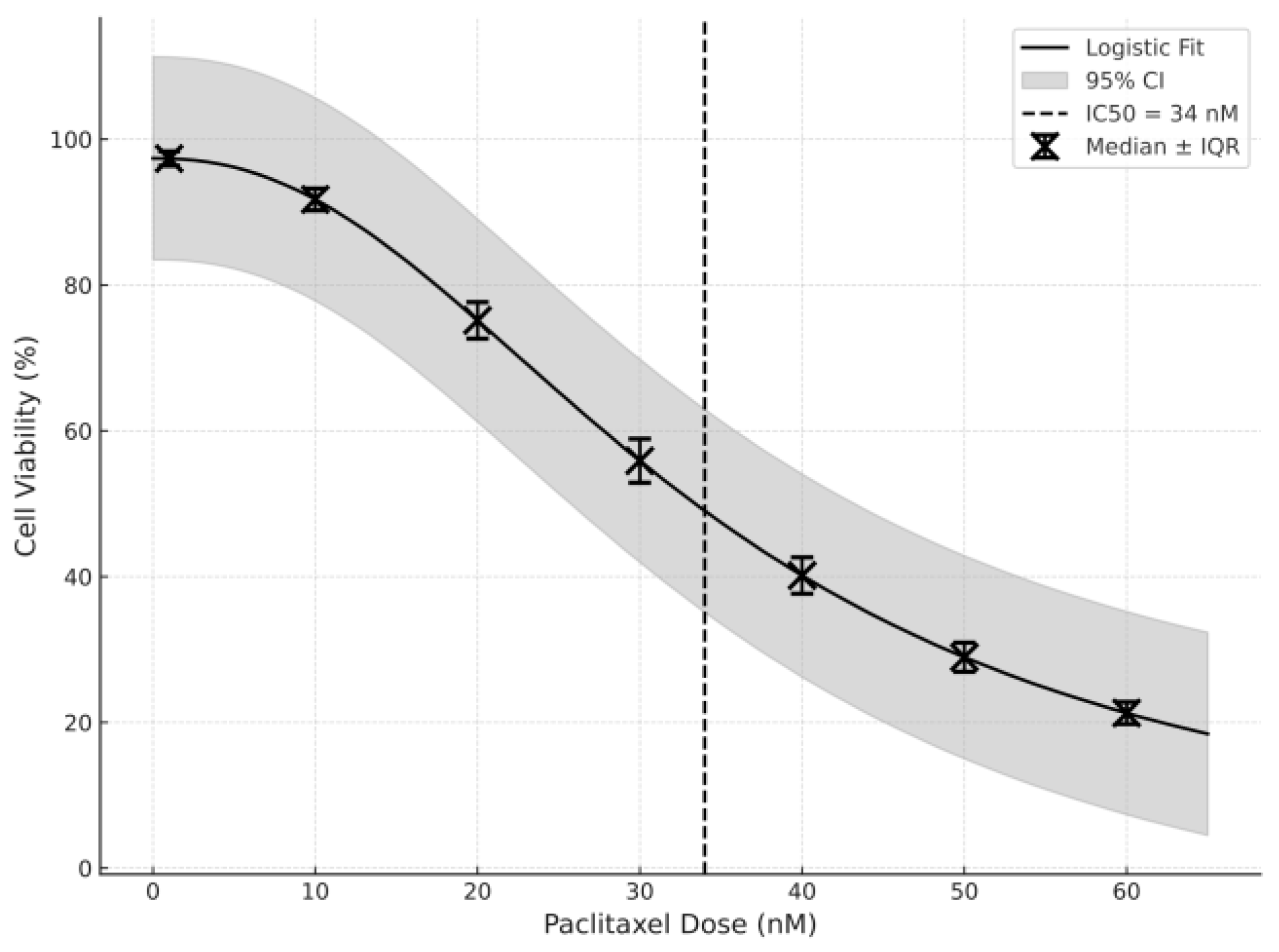
2.2. Paclitaxel IC50 Determination Using an MTT Assay
2.3. Experimental Design and Grouping
2.4. Immunocytochemical Procedure
2.5. Statistical Analysis
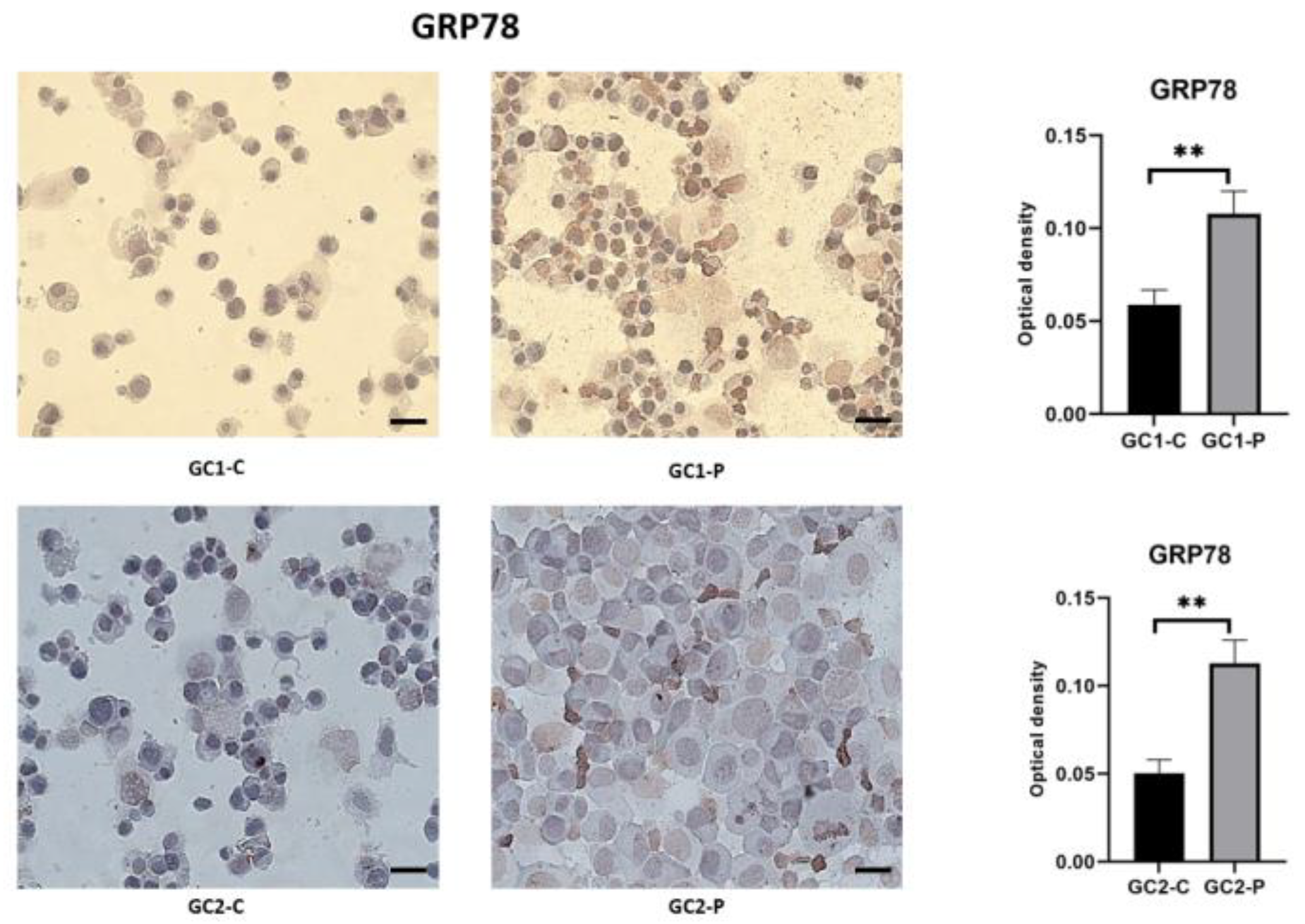
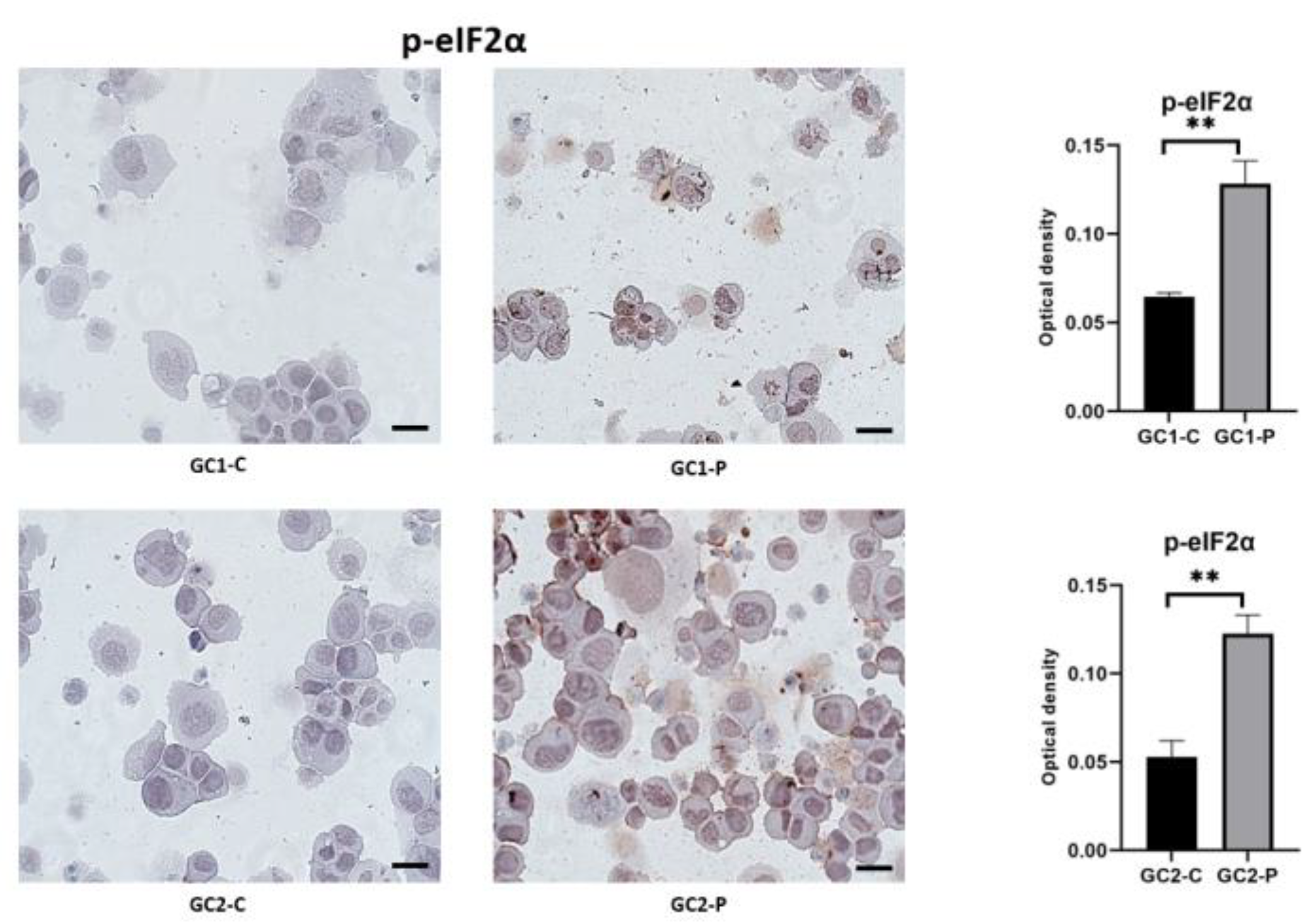
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PERK | Protein Kinase R-like ER Kinase |
| GC1 | Mouse Spermatogonial Cell |
| CHOP | C/EBP Homologous Protein |
| ER | Endoplasmic Reticulum |
| UPR | Unfolded Protein Response |
| IRE1- | Inositol-requiring Enzyme 1 Alpha |
| ATF6 | Activating Transcription Factor 6 |
| GRP78 | Glucose-regulated Protein 78 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| PBS | Phosphate-buffered Saline |
| DMSO | Dimethyl Sulfoxide |
| DAB | 3,3′ Diaminobenzidine |
| SD | Standard Deviation |
References
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in modern oncology and nanomedicine-based cancer therapy. Oxidative Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Y.; Wang, R.; Najafi, M. Mechanisms of cancer cell death induction by paclitaxel: An updated review. Apoptosis 2022, 27, 647–667. [Google Scholar] [CrossRef]
- Wu, C.; Wu, T.; Chen, D.; Wei, S.; Tang, W.; Xue, L.; Xiong, J.; Huang, Y.; Guo, Y.; Chen, Y.; et al. The effects and mechanism of taxanes on chemotherapy-associated ovarian damage: A review of current evidence. Front. Endocrinol. 2022, 13, 1025018. [Google Scholar] [CrossRef]
- Bal-Price, A.; Meek, M.B. Adverse outcome pathways: Application to enhance mechanistic understanding of neurotoxicity. Pharmacol. Ther. 2017, 179, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Yarana, C.; St Clair, D.K. Chemotherapy-induced tissue injury: An insight into the role of extracellular vesicles-mediated oxidative stress responses. Antioxidants 2017, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Fromenty, B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol. Res. Perspect. 2016, 4, e00211. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Spencer, B.G.; Finnie, J.W. The role of endoplasmic reticulum stress in cell survival and death. J. Comp. Pathol. 2020, 181, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, M.M.; Torabizadeh, S.A.; Karimabad, M.N. The Glucose-Regulated Protein78 (GRP78) in the unfolded protein response (UPR) pathway: A potential therapeutic target for breast cancer. Anti-Cancer Agents Med. Chem. 2023, 23, 505–524. [Google Scholar] [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef]
- Shi, J.; Li, Z.; Xu, R.; Zhang, J.; Zhou, Q.; Gao, R.; Lu, H.; Lan, Y.; Zhao, K.; He, H.; et al. The PERK/PKR-eIF2α pathway negatively regulates porcine hemagglutinating encephalomyelitis virus replication by attenuating global protein translation and facilitating stress granule formation. J. Virol. 2022, 96, e01695-21. [Google Scholar] [CrossRef]
- Mäkelä, J.A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef]
- Delessard, M.; Saulnier, J.; Rives, A.; Dumont, L.; Rondanino, C.; Rives, N. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis and male fertility. Int. J. Mol. Sci. 2020, 21, 1454. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.M. In vitro spermatogenesis; past, present, and future. Spermatozoa Facts Perspect. 2018, 13, 25–51. [Google Scholar]
- Walker, T. Progress in the study of toxic effects of drugs on the male reproductive system. Asia-Pac. J. Pharmacother. Toxicol. 2024, 4, 76–84. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Tyagi, S.; Mani, S. Media and buffer preparation for cell culture. In Animal Cell Culture: Principles and Practice; Springer International Publishing: Cham, Switzerland, 2023; pp. 77–88. [Google Scholar]
- Hingorani, M.T. A Neuroengineering Platform for ex vivo Analysis of Single-Axon Dynamics of Serotonergic Neurons. Ph.D. Thesis, University of California, Santa Barbara, CA, USA, 2022. [Google Scholar]
- Riquelme, M.V.; Zhao, H.; Srinivasaraghavan, V.; Pruden, A.; Vikesland, P.; Agah, M. Optimizing blocking of nonspecific bacterial attachment to impedimetric biosensors. Sens. Bio-Sens. Res. 2016, 8, 47–54. [Google Scholar] [CrossRef]
- Oztatlici, M.; Oztatlici, H.; Saygili, S.K.; Kaya, K.; Cingoz, I.D. Cyclophosphamide stimulates endoplasmic reticulum stress and induces apoptotic cell death in human glioblastoma cell lines. Rom. J. Morphol. Embryol. 2024, 65, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ileriturk, M.; Kandemir, O.; Akaras, N.; Simsek, H.; Genc, A.; Kandemir, F.M. Hesperidin has a protective effect on paclitaxel-induced testicular toxicity through regulating oxidative stress, apoptosis, inflammation and endoplasmic reticulum stress. Reprod. Toxicol. 2023, 118, 108369. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. OncoTargets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The impact of drugs on male fertility: A review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef]
- Meistrich, M.L. Risks of genetic damage in offspring conceived using spermatozoa produced during chemotherapy or radiotherapy. Andrology 2020, 8, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Ili, P.; Sari, F.; Bucak, M.N.; Öztürk, C.; Güngör, Ş.; Ataman, M.B. DNA damaging effect of paclitaxel in the epididymal sperms as a chemotherapeutic agent and possible remedies to prevent this effect: A study on reproductive potential of male cancer patients of reproductive age. Theriogenology 2019, 132, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Odle, A.K.; Aykin-Burns, N.; Allen, A.R. Chemotherapy induced oxidative stress in the ovary: Drug-dependent mechanisms and potential interventions. Biol. Reprod. 2023, 108, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Fernández-Pascual, E.; Ochoa Arvizo, M.A.; Vishwakarma, R.; Martínez-Salamanca, J.I. Male Infertility and the Risk of Developing Testicular Cancer: A Critical Contemporary Literature Review. Medicina 2023, 59, 1305. [Google Scholar] [CrossRef]
- Kankılıç, N.A.; Küçükler, S.; Gür, C.; Akarsu, S.A.; Akaras, N.; Şimşek, H.; İleritürk, M.; Kandemir, F.M. Naringin protects against paclitaxel-induced toxicity in rat testicular tissues by regulating genes in pro-inflammatory cytokines, oxidative stress, apoptosis, and JNK/MAPK signaling pathways. J. Biochem. Mol. Toxicol. 2024, 38, e23751. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef]
- Zhu, G.; Lee, A.S. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J. Cell. Physiol. 2015, 230, 1413–1420. [Google Scholar] [CrossRef]
- Wek, R.C. Role of eIF2α kinases in translational control and adaptation to cellular stress. Cold Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef]
- Sisinni, L.; Pietrafesa, M.; Lepore, S.; Maddalena, F.; Condelli, V.; Esposito, F.; Landriscina, M. Endoplasmic reticulum stress and unfolded protein response in breast cancer: The balance between apoptosis and autophagy and its role in drug resistance. Int. J. Mol. Sci. 2019, 20, 857. [Google Scholar] [CrossRef]
- Boye, E.; Grallert, B. eIF2α phosphorylation and the regulation of translation. Curr. Genet. 2020, 66, 293–297. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Finelli, R.; Baskaran, S.; Agarwal, A. Dysregulation of key proteins associated with sperm motility and fertility potential in cancer patients. Int. J. Mol. Sci. 2020, 21, 6754. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lun, J.; Zhang, H.; Wang, L.; Zhang, G.; Zhang, H.; Fang, J. Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy. Acta Biochim. Biophys. Sin. 2021, 53, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, C.; Zhao, H.; Zhou, Y.; Chen, Z.; Wang, L. Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage. Reprod. Biol. Endocrinol. 2018, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Samaddar, S.; Redhwan, M.A.M.; Eraiah, M.M. Endoplasmic Reticulum Stress and Protein Misfolding in Neurodegenerative Diseases. In Proteostasis: Investigating Molecular Dynamics in Neurodegenerative Disorders; Springer Nature: Singapore, 2025; pp. 329–351. [Google Scholar]
- Song, Y.; Zhao, Q.L.; Ogawa, R.; Mizukami, T.; Li, Y.M.; Cui, Z.G.; Saitoh, J.I.; Noguchi, K. Exploring the therapeutic potential of 4, 4′-dimethoxychalcone: Inducing apoptosis in cancer cells via ER stress and autophagy disruption. Cell. Signal. 2025, 132, 111854. [Google Scholar] [CrossRef]
- Li, M.; Yin, L.; Wu, L.; Zhu, Y.; Wang, X. Paclitaxel inhibits proliferation and promotes apoptosis through regulation ROS and endoplasmic reticulum stress in osteosarcoma cell. Mol. Cell. Toxicol. 2020, 16, 377–384. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadeniz Saygılı, S.; Sahin, M.C.; Yukcu, F.; Sanli, S. Analysis of Endoplasmic Reticulum Stress Proteins in Spermatogenic Cells After Paclitaxel Administration. Curr. Issues Mol. Biol. 2025, 47, 620. https://doi.org/10.3390/cimb47080620
Karadeniz Saygılı S, Sahin MC, Yukcu F, Sanli S. Analysis of Endoplasmic Reticulum Stress Proteins in Spermatogenic Cells After Paclitaxel Administration. Current Issues in Molecular Biology. 2025; 47(8):620. https://doi.org/10.3390/cimb47080620
Chicago/Turabian StyleKaradeniz Saygılı, Suna, Meryem Cansu Sahin, Fulya Yukcu, and Senem Sanli. 2025. "Analysis of Endoplasmic Reticulum Stress Proteins in Spermatogenic Cells After Paclitaxel Administration" Current Issues in Molecular Biology 47, no. 8: 620. https://doi.org/10.3390/cimb47080620
APA StyleKaradeniz Saygılı, S., Sahin, M. C., Yukcu, F., & Sanli, S. (2025). Analysis of Endoplasmic Reticulum Stress Proteins in Spermatogenic Cells After Paclitaxel Administration. Current Issues in Molecular Biology, 47(8), 620. https://doi.org/10.3390/cimb47080620







