Sensory Circumventricular Organ Insulin Signaling in Cardiovascular and Metabolic Regulation
Abstract
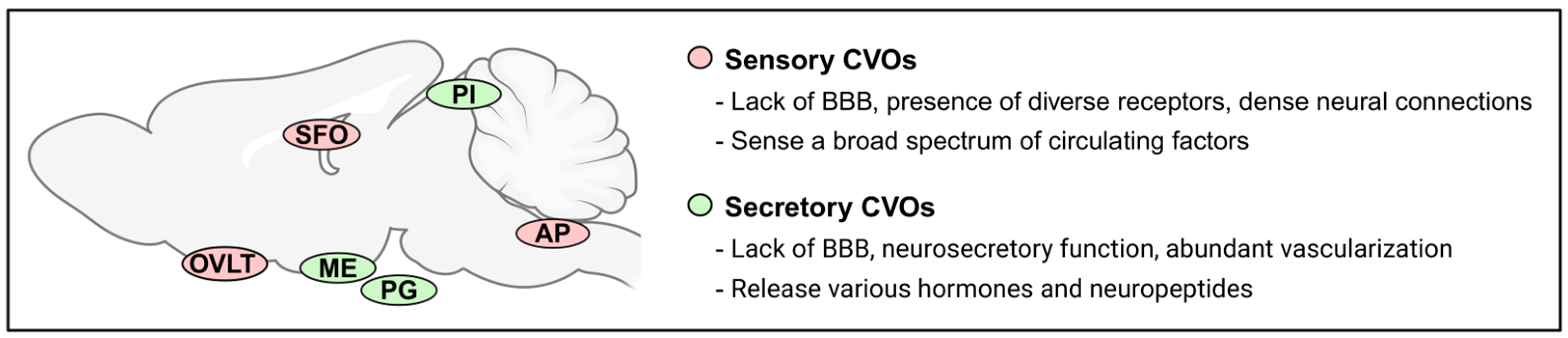
1. Introduction
2. Sensory CVOs as a Potential Key Site for Insulin Action
3. Sensory CVOs Insulin Receptors in Cardiovascular Regulation
3.1. SFO
3.2. OVLT
3.3. AP
4. Sensory CVOs Insulin Receptors in Metabolic Regulation
4.1. SFO
4.2. OVLT
4.3. AP
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef]
- Perez-Tilve, D.; Stern, J.E.; Tschop, M. The brain and the metabolic syndrome: Not a wireless connection. Endocrinology 2006, 147, 1136–1139. [Google Scholar] [CrossRef]
- Jais, A.; Bruning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef]
- Jeong, J.K.; Horwath, J.A.; Simonyan, H.; Blackmore, K.A.; Butler, S.D.; Young, C.N. Subfornical organ insulin receptors tonically modulate cardiovascular and metabolic function. Physiol. Genom. 2019, 51, 333–341. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, S.G.; Knudsen, G.M.; Videbaek, C.; Pinborg, L.H.; Schmidt, J.F.; Holm, S.; Paulson, O.B. No effect of insulin on glucose blood-brain barrier transport and cerebral metabolism in humans. Diabetes 1999, 48, 1915–1921. [Google Scholar] [CrossRef]
- Bingham, E.M.; Hopkins, D.; Smith, D.; Pernet, A.; Hallett, W.; Reed, L.; Marsden, P.K.; Amiel, S.A. The role of insulin in human brain glucose metabolism: An 18fluoro-deoxyglucose positron emission tomography study. Diabetes 2002, 51, 3384–3390. [Google Scholar] [CrossRef]
- Meijer, R.I.; Gray, S.M.; Aylor, K.W.; Barrett, E.J. Pathways for insulin access to the brain: The role of the microvascular endothelial cell. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1132–H1138. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.R.; Bardgett, J.F.; Wolfgang, L.; Stocker, S.D. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 2011, 57, 435–441. [Google Scholar] [CrossRef]
- Luckett, B.S.; Frielle, J.L.; Wolfgang, L.; Stocker, S.D. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1538–H1546. [Google Scholar] [CrossRef]
- Smith, P.M.; Ferguson, A.V. Metabolic signaling to the central nervous system: Routes across the blood brain barrier. Curr. Pharm. Des. 2014, 20, 1392–1399. [Google Scholar] [CrossRef]
- Balland, E.; Dam, J.; Langlet, F.; Caron, E.; Steculorum, S.; Messina, A.; Rasika, S.; Falluel-Morel, A.; Anouar, Y.; Dehouck, B.; et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014, 19, 293–301. [Google Scholar] [CrossRef]
- Jiang, H.; Gallet, S.; Klemm, P.; Scholl, P.; Folz-Donahue, K.; Altmuller, J.; Alber, J.; Heilinger, C.; Kukat, C.; Loyens, A.; et al. MCH Neurons Regulate Permeability of the Median Eminence Barrier. Neuron 2020, 107, 306–319 e309. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E.A. The circumventricular organs. Histol. Histopathol. 2017, 32, 879–892. [Google Scholar] [CrossRef]
- Kiecker, C. The origins of the circumventricular organs. J. Anat. 2018, 232, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.; Nyul-Toth, A.; Suciu, M.; Hermenean, A.; KrizbaiI, A. Heterogeneity of the blood-brain barrier. Tissue Barriers 2016, 4, e1143544. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.K.; Gross, P.M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993, 7, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.C.; Bruning, J.C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism–from embryo to old age. Trends Endocrinol. Metab. 2013, 24, 76–84. [Google Scholar] [CrossRef]
- Muntzel, M.S.; Morgan, D.A.; Mark, A.L.; Johnson, A.K. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am. J. Physiol. 1994, 267, R1350–R1355. [Google Scholar] [CrossRef] [PubMed]
- Pricher, M.P.; Freeman, K.L.; Brooks, V.L. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 2008, 51, 514–520. [Google Scholar] [CrossRef]
- Schulingkamp, R.J.; Pagano, T.C.; Hung, D.; Raffa, R.B. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci. Biobehav. Rev. 2000, 24, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.L.; Ferris, H.A. The brain as an insulin-sensitive metabolic organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef]
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978, 272, 827–829. [Google Scholar] [CrossRef]
- van Houten, M.; Posner, B.I.; Kopriwa, B.M.; Brawer, J.R. Insulin-binding sites in the rat brain: In vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology 1979, 105, 666–673. [Google Scholar] [CrossRef]
- Hill, J.M.; Lesniak, M.A.; Pert, C.B.; Roth, J. Autoradiographic localization of insulin receptors in rat brain: Prominence in olfactory and limbic areas. Neuroscience 1986, 17, 1127–1138. [Google Scholar] [CrossRef]
- Werther, G.A.; Hogg, A.; Oldfield, B.J.; McKinley, M.J.; Figdor, R.; Allen, A.M.; Mendelsohn, F.A. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 1987, 121, 1562–1570. [Google Scholar] [CrossRef]
- Lakhi, S.; Snow, W.; Fry, M. Insulin modulates the electrical activity of subfornical organ neurons. Neuroreport 2013, 24, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Jeong, J.K.; Young, C.N. Cellular Profile of Subfornical Organ Insulin Receptors in Mice. Biomolecules 2024, 14, 1256. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, C.C.; Ferguson, A.V. Physiological roles for the subfornical organ: A dynamic transcriptome shaped by autonomic state. J. Physiol. 2016, 594, 1581–1589. [Google Scholar] [CrossRef]
- Peterson, C.S.; Huang, S.; Lee, S.A.; Ferguson, A.V.; Fry, W.M. The transcriptome of the rat subfornical organ is altered in response to early postnatal overnutrition. IBRO Rep. 2018, 5, 17–23. [Google Scholar] [CrossRef]
- Filippi, B.M.; Yang, C.S.; Tang, C.; Lam, T.K. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012, 16, 500–510. [Google Scholar] [CrossRef]
- Fry, W.M.; Ferguson, A.V. The subfornical organ and organum vasculosum of the lamina terminalis: Critical roles in cardiovascular regulation and the control of fluid balance. Handb. Clin. Neurol. 2021, 180, 203–215. [Google Scholar] [CrossRef]
- Young, C.N.; Li, A.; Dong, F.N.; Horwath, J.A.; Clark, C.G.; Davisson, R.L. Endoplasmic reticulum and oxidant stress mediate nuclear factor-kappaB activation in the subfornical organ during angiotensin II hypertension. Am. J. Physiol. Cell Physiol. 2015, 308, C803–C812. [Google Scholar] [CrossRef]
- Hilzendeger, A.M.; Cassell, M.D.; Davis, D.R.; Stauss, H.M.; Mark, A.L.; Grobe, J.L.; Sigmund, C.D. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension 2013, 61, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wei, S.G.; Weiss, R.M.; Felder, R.B. Angiotensin II Type 1a Receptors in the Subfornical Organ Modulate Neuroinflammation in the Hypothalamic Paraventricular Nucleus in Heart Failure Rats. Neuroscience 2018, 381, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Prager-Khoutorsky, M.; Bourque, C.W. Anatomical organization of the rat organum vasculosum laminae terminalis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R324–R337. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, B.J.; Simmonds, S.S.; Browning, K.N.; Stocker, S.D. Organum Vasculosum of the Lamina Terminalis Detects NaCl to Elevate Sympathetic Nerve Activity and Blood Pressure. Hypertension 2017, 69, 163–170. [Google Scholar] [CrossRef]
- Nomura, K.; Hiyama, T.Y.; Sakuta, H.; Matsuda, T.; Lin, C.H.; Kobayashi, K.; Kobayashi, K.; Kuwaki, T.; Takahashi, K.; Matsui, S.; et al. [Na(+)] Increases in Body Fluids Sensed by Central Na(x) Induce Sympathetically Mediated Blood Pressure Elevations via H(+)-Dependent Activation of ASIC1a. Neuron 2019, 101, 60–75 e66. [Google Scholar] [CrossRef]
- Collister, J.P.; Nahey, D.B.; Hartson, R.; Wiedmeyer, C.E.; Banek, C.T.; Osborn, J.W. Lesion of the OVLT markedly attenuates chronic DOCA-salt hypertension in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R568–R575. [Google Scholar] [CrossRef] [PubMed]
- Collister, J.P.; Taylor-Smith, H.; Drebes, D.; Nahey, D.; Tian, J.; Zimmerman, M.C. Angiotensin II-Induced Hypertension Is Attenuated by Overexpressing Copper/Zinc Superoxide Dismutase in the Brain Organum Vasculosum of the Lamina Terminalis. Oxid. Med. Cell. Longev. 2016, 2016, 3959087. [Google Scholar] [CrossRef] [PubMed]
- Muntzel, M.; Beltz, T.; Mark, A.L.; Johnson, A.K. Anteroventral third ventricle lesions abolish lumbar sympathetic responses to insulin. Hypertension 1994, 23, 1059–1062. [Google Scholar] [CrossRef]
- Muntzel, M.S.; Thunhorst, R.L.; Johnson, A.K. Effects of subfornical organ lesions on sympathetic nerve responses to insulin. Hypertension 1997, 29, 1020–1024. [Google Scholar] [CrossRef]
- Cai, Y.; Hay, M.; Bishop, V.S. Synaptic connections and interactions between area postrema and nucleus tractus solitarius. Brain Res. 1996, 724, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, C.C.; Fry, M.; Smith, P.M.; Yao, S.T.; Hazell, G.G.; Lolait, S.J.; Paton, J.F.; Ferguson, A.V.; Murphy, D. The transcriptome of the medullary area postrema: The thirsty rat, the hungry rat and the hypertensive rat. Exp. Physiol. 2011, 96, 495–504. [Google Scholar] [CrossRef]
- Abukar, Y.; Ramchandra, R.; Hood, S.G.; McKinley, M.J.; Booth, L.C.; Yao, S.T.; May, C.N. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res. Cardiol. 2018, 113, 35. [Google Scholar] [CrossRef]
- Korim, W.S.; Elsaafien, K.; Basser, J.R.; Setiadi, A.; May, C.N.; Yao, S.T. In renovascular hypertension, TNF-alpha type-1 receptors in the area postrema mediate increases in cardiac and renal sympathetic nerve activity and blood pressure. Cardiovasc. Res. 2019, 115, 1092–1101. [Google Scholar] [CrossRef]
- Ford, R.; Lu, H.; Duanmu, Z.; Scislo, T.; Dunbar, J.C. The effect of the removal of the area postrema on insulin and IGF-1-induced cardiovascular and sympathetic nervous responses. Int. J. Exp. Diabetes Res. 2000, 1, 59–67. [Google Scholar] [CrossRef]
- Huang, H.N.; Lu, P.J.; Lo, W.C.; Lin, C.H.; Hsiao, M.; Tseng, C.J. In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation 2004, 110, 2476–2483. [Google Scholar] [CrossRef]
- Yeh, T.C.; Liu, C.P.; Cheng, W.H.; Chen, B.R.; Lu, P.J.; Cheng, P.W.; Ho, W.Y.; Sun, G.C.; Liou, J.C.; Tseng, C.J. Caffeine intake improves fructose-induced hypertension and insulin resistance by enhancing central insulin signaling. Hypertension 2014, 63, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.; Lu, P.J.; Huang, H.N.; Lo, W.C.; Ho, W.Y.; Lai, T.C.; Chiang, H.T.; Tseng, C.J. Defective phosphatidylinositol 3-kinase signaling in central control of cardiovascular effects in the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens. Res. 2008, 31, 1209–1218. [Google Scholar] [CrossRef]
- Young, C.N.; Morgan, D.A.; Butler, S.D.; Rahmouni, K.; Gurley, S.B.; Coffman, T.M.; Mark, A.L.; Davisson, R.L. Angiotensin type 1a receptors in the forebrain subfornical organ facilitate leptin-induced weight loss through brown adipose tissue thermogenesis. Mol. Metab. 2015, 4, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Horwath, J.A.; Hurr, C.; Butler, S.D.; Guruju, M.; Cassell, M.D.; Mark, A.L.; Davisson, R.L.; Young, C.N. Obesity-induced hepatic steatosis is mediated by endoplasmic reticulum stress in the subfornical organ of the brain. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Kim, H.R.; Young, C.N. Circumventricular organ-hypothalamic circuit endoplasmic reticulum stress drives hepatic steatosis during obesity. obesity (Silver Spring) 2024, 32, 59–69. [Google Scholar] [CrossRef]
- Blackmore, K.; Houchen, C.J.; Simonyan, H.; Arestakesyan, H.; Stark, A.K.; Dow, S.A.; Kim, H.R.; Jeong, J.K.; Popratiloff, A.; Young, C.N. A forebrain-hypothalamic ER stress driven circuit mediates hepatic steatosis during obesity. Mol. Metab. 2024, 79, 101858. [Google Scholar] [CrossRef]
- Scherer, T.; Lindtner, C.; O’Hare, J.; Hackl, M.; Zielinski, E.; Freudenthaler, A.; Baumgartner-Parzer, S.; Todter, K.; Heeren, J.; Krssak, M.; et al. Insulin Regulates Hepatic Triglyceride Secretion and Lipid Content via Signaling in the Brain. Diabetes 2016, 65, 1511–1520. [Google Scholar] [CrossRef]
- Abbott, S.B.; Machado, N.L.; Geerling, J.C.; Saper, C.B. Reciprocal Control of Drinking Behavior by Median Preoptic Neurons in Mice. J. Neurosci. 2016, 36, 8228–8237. [Google Scholar] [CrossRef]
- Bealer, S.L.; Carithers, J.; Johnson, A.K. Fluid regulation, body weight and drinking responses following hypothalamic knife cuts. Brain Res. 1984, 305, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.N.; Sirinathsinghji, D.J.; Delaney, C.E. The effects of a reversible colchicine-induced lesion of the anterior ventral region of the third cerebral ventricle in rats. Brain Res. 1987, 437, 339–344. [Google Scholar] [CrossRef]
- Bathgate, R.A.; Halls, M.L.; van der Westhuizen, E.T.; Callander, G.E.; Kocan, M.; Summers, R.J. Relaxin family peptides and their receptors. Physiol. Rev. 2013, 93, 405–480. [Google Scholar] [CrossRef]
- de Avila, C.; Chometton, S.; Lenglos, C.; Calvez, J.; Gundlach, A.L.; Timofeeva, E. Differential effects of relaxin-3 and a selective relaxin-3 receptor agonist on food and water intake and hypothalamic neuronal activity in rats. Behav. Brain Res. 2018, 336, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.A.; Leib, D.E.; Knight, Z.A. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 2017, 18, 459–469. [Google Scholar] [CrossRef]
- Moreno, M.L.; Meza, E.; Morgado, E.; Juarez, C.; Ramos-Ligonio, A.; Ortega, A.; Caba, M. Activation of organum vasculosum of lamina terminalis, median preoptic nucleus, and medial preoptic area in anticipation of nursing in rabbit pups. Chronobiol. Int. 2013, 30, 1272–1282. [Google Scholar] [CrossRef]
- Tsai, J.P. The association of serum leptin levels with metabolic diseases. Tzu Chi Med. J. 2017, 29, 192–196. [Google Scholar] [CrossRef]
- Filippi, B.M.; Bassiri, A.; Abraham, M.A.; Duca, F.A.; Yue, J.T.; Lam, T.K. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes 2014, 63, 892–899. [Google Scholar] [CrossRef]
- Blake, C.B.; Smith, B.N. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R711–R720. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.O.; Briggs, D.B. Insulin excites neurons of the area postrema and causes emesis. Neurosci. Lett. 1986, 68, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Perez-Torres, S.; De Caro, R.; Porzionato, A.; Macchi, V.; Beleta, J.; Gavalda, A.; Palacios, J.M.; Mengod, G. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J. Chem. Neuroanat. 2010, 40, 36–42. [Google Scholar] [CrossRef]
- Panicker, A.K.; Wade, G.N. Insulin-induced repartitioning of metabolic fuels inhibits hamster estrous behavior: Role of area postrema. Am. J. Physiol. 1998, 274, R1094–R1098. [Google Scholar] [CrossRef]
- Panicker, A.K.; Mangels, R.A.; Powers, J.B.; Wade, G.N.; Schneider, J.E. AP lesions block suppression of estrous behavior, but not estrous cyclicity, in food-deprived Syrian hamsters. Am. J. Physiol. 1998, 275, R158–R164. [Google Scholar] [CrossRef]
- Alberry, B.; Silveira, P.P. Brain insulin signaling as a potential mediator of early life adversity effects on physical and mental health. Neurosci. Biobehav. Rev. 2023, 153, 105350. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Fang, T.C.; Huang, W.C. Angiotensin receptor blockade blunts hyperinsulinemia-induced hypertension in rats. Hypertension 1998, 32, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Holscher, C. Insulin Signaling Impairment in the Brain as a Risk Factor in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Ricart, W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr. Rev. 2003, 24, 278–301. [Google Scholar] [CrossRef]
- Purkayastha, S.; Cai, D. Neuroinflammatory basis of metabolic syndrome. Mol. Metab. 2013, 2, 356–363. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.R.; Jeong, J.K.; Young, C.N. Sensory Circumventricular Organ Insulin Signaling in Cardiovascular and Metabolic Regulation. Curr. Issues Mol. Biol. 2025, 47, 595. https://doi.org/10.3390/cimb47080595
Kim HR, Jeong JK, Young CN. Sensory Circumventricular Organ Insulin Signaling in Cardiovascular and Metabolic Regulation. Current Issues in Molecular Biology. 2025; 47(8):595. https://doi.org/10.3390/cimb47080595
Chicago/Turabian StyleKim, Han Rae, Jin Kwon Jeong, and Colin N. Young. 2025. "Sensory Circumventricular Organ Insulin Signaling in Cardiovascular and Metabolic Regulation" Current Issues in Molecular Biology 47, no. 8: 595. https://doi.org/10.3390/cimb47080595
APA StyleKim, H. R., Jeong, J. K., & Young, C. N. (2025). Sensory Circumventricular Organ Insulin Signaling in Cardiovascular and Metabolic Regulation. Current Issues in Molecular Biology, 47(8), 595. https://doi.org/10.3390/cimb47080595







