Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise
Abstract
1. Introduction
2. Materials and Methods
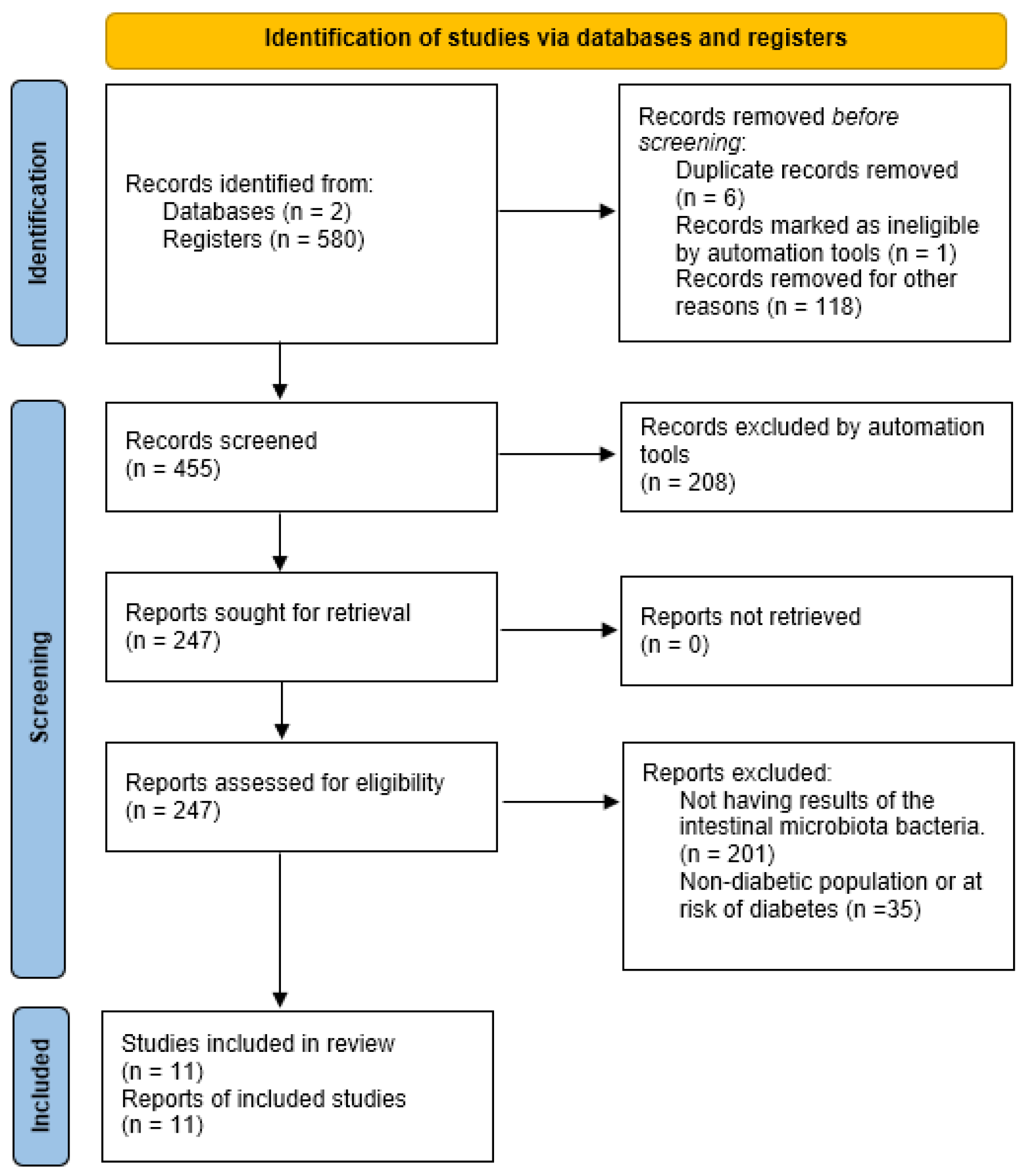
2.1. Protocol Registration and Literature Search Methodology
2.2. Inclusion/Exclusion Criteria
2.2.1. Inclusion Criteria
- Population: We included studies involving humans with prediabetes, T2D, or individuals at risk of developing type 2 diabetes.
- Intervention: Eligible interventions comprised cross-sectional studies analyzing gut microbiota through fecal sample collection and assessing physical activity via accelerometry or questionnaires. Experimental studies implementing a structured exercise program were also included.
- Comparison: Comparison of gut microbiota among individuals with different levels of physical activity or between groups that underwent an exercise intervention and those that did not.
- Outcome: Physical activity level, blood biomarkers, fecal microbiome composition, insulin resistance, and cardiorespiratory fitness (VO2max).
2.2.2. Exclusion Criteria
2.3. Screening Process
2.4. Data Extraction
3. Results
3.1. Observational Evidence on Physical Activity and Gut Microbiota in T2D
3.1.1. Physical Activity
3.1.2. Circulating TMAO and Physical Activity
3.1.3. Microbial Diversity and Insulin Resistance
3.1.4. Body Composition and Gut Microbiota
| Reference | Population | Intervention | Measured Variables | Main Findings |
|---|---|---|---|---|
| Beals et al. (2023) [28] |
|
|
|
|
| Motiani et al. (2020) [29] |
|
|
|
|
| Torquiati et al. (2023) [30] |
|
|
|
|
| Verheggen et al. (2021) [31] |
|
|
|
|
| Wei et al. (2022) [32] |
|
|
|
|
3.2. Interventional Studies: Effects of Exercise Type and Intensity on Gut Microbiota
3.2.1. Type and Intensity of Exercise
3.2.2. Short-Chain Fatty Acids (SCFAs)
3.2.3. Microbial Diversity
3.2.4. Gut Microbiota Composition
4. Discussion
4.1. Observational Evidence on Physical Activity and Gut Microbiota in T2D
4.2. Interventional Studies: Effects of Exercise Type and Intensity on Gut Microbiota
4.3. Limitations of This Systematic Review
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DNA | Deoxyribonucleic Acid |
| HbA1c | Hemoglobin A1c |
| HIIT | High-Intensity Interval Training |
| MICT | Moderate-Intensity Continuous Training |
| N | Total participants |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| rRNA | Ribosomal Ribonucleic Acid |
| SCFAs | Short-Chain Fatty Acids |
| SIT | Sprint Interval Training |
| TMAO | Trimethylamine N-oxide |
| TS | Topic Search |
| VO2max | Maximal oxygen uptake |
References
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diabetes Rep. 2018, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, I.; Fulci, V.; Palone, F.; Stronati, L.; Cucchiara, S.; Carissimi, C. Quantitative Assessment of Shotgun Metagenomics and 16S rDNA Amplicon Sequencing in the Study of Human Gut Microbiome. OMICS A J. Integr. Biol. 2018, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V. Human Microbiome Acquisition and Bioinformatic Challenges in Metagenomic Studies. Int. J. Mol. Sci. 2018, 19, 383. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 121, pp. 91–119. ISBN 978-0-12-800100-4. [Google Scholar]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between Microbiota-Dependent Metabolite Trimethylamine-N-Oxide and Type 2 Diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Zhang, N.; Der Pluijm, W.V. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018–2028). Diabetes 2018, 67, 202-LB. [Google Scholar] [CrossRef]
- Egshatyan, L.; Kashtanova, D.; Popenko, A.; Tkacheva, O.; Tyakht, A.; Alexeev, D.; Karamnova, N.; Kostryukova, E.; Babenko, V.; Vakhitova, M.; et al. Gut Microbiota and Diet in Patients with Different Glucose Tolerance. Endocr. Connect. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediat. Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise Influence on the Microbiome–Gut–Brain Axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Pourabdi, R.; Shahidi, F.; Tabandeh, M.R.; Salehpour, M. Aerobic Exercise Timing Affects Mitochondrial Dynamics and Insulin Resistance by Regulating the Circadian Clock Protein Expression and NAD+-SIRT1-PPARα-MFN2 Pathway in the Skeletal Muscle of High-Fat-Diet-Induced Diabetes Mice. J. Physiol. Biochem. 2025, 81, 199–214. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. Impact of Different Training Modalities on Glycaemic Control and Blood Lipids in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Cutuli, D.; Decandia, D.; Giacovazzo, G.; Coccurello, R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership. Int. J. Mol. Sci. 2023, 24, 14686. [Google Scholar] [CrossRef]
- Silva, J.S.C.; Seguro, C.S.; Naves, M.M.V. Gut Microbiota and Physical Exercise in Obesity and Diabetes–A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 863–877. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Díaz, J.; Jorquera-Aguilera, C.; Rodríguez-Rodríguez, A.; Rodríguez-Rodríguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9518. [Google Scholar] [CrossRef]
- Antush, M.T.; Balemba, O.B.; Hendricks, S.A.; Flynn, M.; Geidl, R.; Vella, C.A. Associations of Sedentary Behavior and Screen Time with Human Gut Microbiome Composition and Diversity. Life 2024, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Argyridou, S.; Bernieh, D.; Henson, J.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Suzuki, T.; Yates, T. Associations between Physical Activity and Trimethylamine N -Oxide in Those at Risk of Type 2 Diabetes. BMJ Open Diab. Res. Care 2020, 8, e001359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity: A Microbiome-Wide Analysis from Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef] [PubMed]
- Houttu, V.; Boulund, U.; Nicolaou, M.; Holleboom, A.G.; Grefhorst, A.; Galenkamp, H.; Van Den Born, B.-J.; Zwinderman, K.; Nieuwdorp, M. Physical Activity and Dietary Composition Relate to Differences in Gut Microbial Patterns in a Multi-Ethnic Cohort—The HELIUS Study. Metabolites 2021, 11, 858. [Google Scholar] [CrossRef]
- Kalagi, N.A.; Thota, R.N.; Stojanovski, E.; Alburikan, K.A.; Garg, M.L. Association between Plasma Trimethylamine N-Oxide Levels and Type 2 Diabetes: A Case Control Study. Nutrients 2022, 14, 2093. [Google Scholar] [CrossRef]
- Somnuk, S.; Komindr, S.; Monkhai, S.; Poolsawat, T.; Nakphaichit, M.; Wanikorn, B. Metabolic and Inflammatory Profiles, Gut Microbiota and Lifestyle Factors in Overweight and Normal Weight Young Thai Adults. PLoS ONE 2023, 18, e0288286. [Google Scholar] [CrossRef]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary Weight Loss-Induced Improvements in Metabolic Function Are Enhanced by Exercise in People with Obesity and Prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.-J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.R.G.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of Exercise Intensity on Gut Microbiome Composition and Function in People with Type 2 Diabetes. Eur. J. Sport Sci. 2023, 23, 530–541. [Google Scholar] [CrossRef]
- Verheggen, R.J.H.M.; Konstanti, P.; Smidt, H.; Hermus, A.R.M.M.; Thijssen, D.H.J.; Hopman, M.T.E. Eight-week Exercise Training in Humans with Obesity: Marked Improvements in Insulin Sensitivity and Modest Changes in Gut Microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef]
- Wei, S.; Brejnrod, A.D.; Trivedi, U.; Mortensen, M.S.; Johansen, M.Y.; Karstoft, K.; Vaag, A.A.; Ried-Larsen, M.; Sørensen, S.J. Impact of Intensive Lifestyle Intervention on Gut Microbiota Composition in Type 2 Diabetes: A Post-Hoc Analysis of a Randomized Clinical Trial. Gut Microbes 2022, 14, 2005407. [Google Scholar] [CrossRef] [PubMed]
- Da, C.; Pinaffi-Langley, A.C.; Melia, E.; Hays, F.A. Exploring the Gut–Mitochondrial Axis: p66Shc Adapter Protein and Its Implications for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 3656. [Google Scholar] [CrossRef]
- Thome, T.; Salyers, Z.R.; Kumar, R.A.; Hahn, D.; Berru, F.N.; Ferreira, L.F.; Scali, S.T.; Ryan, T.E. Uremic Metabolites Impair Skeletal Muscle Mitochondrial Energetics through Disruption of the Electron Transport System and Matrix Dehydrogenase Activity. Am. J. Physiol. Cell Physiol. 2019, 317, C701–C713. [Google Scholar] [CrossRef]
- Jiang, J.-Y.; Liu, W.-M.; Zhang, Q.-P.; Ren, H.; Yao, Q.-Y.; Liu, G.-Q.; Lu, P.-R. Trimethylamine N-Oxide Aggravates Vascular Permeability and Endothelial Cell Dysfunction under Diabetic Condition: In Vitro and in Vivo Study. Int. J. Ophthalmol. 2024, 17, 25–33. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; Von Scholten, B.J. Trimethylamine N-Oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Łagowska, K.; Drzymała-Czyż, S. A Low Glycemic Index, Energy-Restricted Diet but Not Lactobacillus Rhamnosus Supplementation Changes Fecal Short-Chain Fatty Acid and Serum Lipid Concentrations in Women with Overweight or Obesity and Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 917–926. [Google Scholar] [CrossRef]
- Hecker, M.; Sommer, N.; Mayer, K. Assessment of Short- and Medium-Chain Fatty Acids on Mitochondrial Function in Severe Inflammation. In Mitochondrial Medicine; Weissig, V., Edeas, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2277, pp. 125–132. ISBN 978-1-0716-1269-9. [Google Scholar]
| Reference | Population | Measured Variables | Main Findings |
|---|---|---|---|
| Antush et al. (2024) [22] |
|
|
|
| Argyridou et al. (2020) [23] |
|
|
|
| Chen et al. (2021) [24] |
|
|
|
| Houttu et al. (2021) [25] |
|
|
|
| Kalagi et al. (2022) [26] |
|
|
|
| Somnuk et al. (2023) [27] |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muguerza-Rodríguez, L.; Mier, A.; Ponce-González, J.G.; Casals, C.; Corral-Pérez, J. Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise. Curr. Issues Mol. Biol. 2025, 47, 505. https://doi.org/10.3390/cimb47070505
Muguerza-Rodríguez L, Mier A, Ponce-González JG, Casals C, Corral-Pérez J. Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise. Current Issues in Molecular Biology. 2025; 47(7):505. https://doi.org/10.3390/cimb47070505
Chicago/Turabian StyleMuguerza-Rodríguez, Luis, Alba Mier, Jesus G. Ponce-González, Cristina Casals, and Juan Corral-Pérez. 2025. "Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise" Current Issues in Molecular Biology 47, no. 7: 505. https://doi.org/10.3390/cimb47070505
APA StyleMuguerza-Rodríguez, L., Mier, A., Ponce-González, J. G., Casals, C., & Corral-Pérez, J. (2025). Systematic Review on the Importance of Gut Microbiota in the Regulation of Type 2 Diabetes Through Physical Activity and Exercise. Current Issues in Molecular Biology, 47(7), 505. https://doi.org/10.3390/cimb47070505











