Abstract
Type 2 diabetes (T2D) is a major global health issue, influenced by sedentary behavior and obesity. Emerging evidence implicates the gut microbiota in T2D pathophysiology through effects on glucose metabolism, inflammation, and insulin sensitivity. This systematic review included eleven studies, six observational and five interventional, examining the relationship between physical activity, exercise, and gut microbiota in individuals with or at risk of T2D. Observational studies associated low physical activity and high sedentary time with reduced α-diversity and increased abundance of potentially harmful bacteria. Interventional studies showed that structured exercise, including moderate-intensity and sprint interval training, increased beneficial bacteria such as Faecalibacterium, Veillonella, Lachnospira, and Bifidobacterium, linked to anti-inflammatory effects and improved metabolic profiles. However, overall microbial diversity often remained unchanged unless combined with dietary modifications. Exercise also reduced levels of trimethylamine N-oxide, a metabolite linked to cardiovascular risk. Despite increases in butyrate-producing taxa, most studies did not report significant short-term changes in short-chain fatty acid levels, highlighting the complex interaction between microbiota and host metabolism. These findings support physical activity and exercise as modifiable factors that can influence gut microbiota composition, potentially contributing to improved metabolic regulation and better management of T2D.
1. Introduction
The human intestine harbors billions of microorganisms, including bacteria, protozoa, viruses, and archaea, collectively forming a complex ecosystem known as the gut microbiota. This microbial community plays a vital role in maintaining human health by modulating immune and inflammatory responses, regulating neuronal signaling, preserving intestinal barrier integrity, and contributing to the biosynthesis of vitamins, steroid hormones, and neurotransmitters. Moreover, the gut microbiota significantly influences the metabolism of branched-chain and aromatic amino acids, bile acids, and various drugs [1]. The composition of the human microbiome varies widely among individuals and is shaped by ethnicity, genetics, environment, diet, and early microbial exposure. Six dominant bacterial phyla are commonly observed: Actinobacteria, Bacillota, Bacteroidetes, Fusobacteria, Verrucomicrobia, and Proteobacteria [2].
The gut microbiota is mainly analyzed using microbial DNA from fecal samples via 16S rRNA gene amplicon or shotgun metagenomic sequencing, with the latter providing higher resolution data [3,4]. These methods measure α-diversity, reflecting species richness and abundance within samples, and β-diversity, showing differences between samples. Metagenomics can also assess metabolic outputs of the microbiota. Dysbiosis, or an imbalance in the microbiota, reduces short-chain fatty acid (SCFA) synthesis, important for gut health, β-cell proliferation, and insulin biosynthesis [5]. Dysbiosis also affects trimethylamine production, as it is converted to trimethylamine N-oxide (TMAO), a metabolite linked to increased type 2 diabetes (T2D) risk [1,6].
T2D affects over 500 million people worldwide and is characterized by impaired insulin secretion and resistance, causing hyperglycemia [7,8]. This figure is projected to increase, particularly in low-income countries [8]. T2D is a leading cause of death globally, with many cases in younger populations [7]. Although no consensus exists on gut microbiota composition in T2D, common findings include a higher Bacillota/Bacteroidetes ratio and increased Blautia abundance in patients compared to healthy controls [1,9]. Decreased butyrate-producing bacteria, such as Faecalibacterium and Roseburia, also appear central to T2D development [10]. Beneficial taxa like Alistipes, Akkermansia, and Haemophilus, linked to anti-inflammatory effects, gut barrier reinforcement, and glucose tolerance, are generally reduced in T2D [11].
Given the link between gut microbiota and T2D pathophysiology, lifestyle interventions like exercise are crucial to explore. Exercise improves glycemic control and insulin sensitivity; for example, a single session of high-intensity exercise can increase glucose uptake by at least 40%, improve glucose tolerance, and enhance insulin sensitivity within 12 to 48 h post-workout [12,13,14]. Aerobic training boosts short-term insulin sensitivity and enhances mitochondrial function, while resistance training increases muscle mass, bone density, and insulin sensitivity, especially when combined with caloric restriction [15,16,17]. Combined aerobic and resistance training may yield even greater benefits for glucose regulation [17].
Beyond metabolic effects, exercise and physical activity influence the gut microbiota by reducing gastrointestinal transit time and promoting beneficial bacteria, particularly butyrate producers like Roseburia hominis and Faecalibacterium prausnitzii [12,13,18]. This modulation strengthens the intestinal barrier, reduces inflammation, and improves the Bacteroidota/Bacillota ratio associated with weight control and gut health [14,19,20,21]. Moderate to vigorous intensity exercise appears most effective for these microbial benefits [20].
Despite promising findings, heterogeneity in study designs and exercise protocols limits conclusions. This systematic review therefore synthesizes current evidence on the effects of physical exercise on gut microbiota composition and its potential role in managing T2D. The review includes observational and interventional studies assessing microbiota changes in response to exercise interventions.
2. Materials and Methods
2.1. Protocol Registration and Literature Search Methodology
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD420251052730) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
A scientific literature review was conducted using two electronic databases, PubMed and Web of Science, without applying time restrictions.
The search equation used in PubMed was (Exercise OR “Physical activity” OR “Physical exercise” OR Training AND (Microbiota OR “Gastrointestinal Microbiomes” OR “Gut Microbiome” OR “Gut Microflora” OR “Gut microbiota” OR “Gastrointestinal Flora” OR “Gastrointestinal Microflora” OR “Intestinal microbiome” OR “Intestinal microbiota” OR “Intestinal microflora” OR “Enteric bacteria”) AND (“Diabetes mellitus type 2” OR “Ketosis-Resistant Diabetes Mellitus” OR “Diabetes Mellitus, Non Insulin Dependent” OR “Diabetes Mellitus, Non-Insulin-Dependent” OR “Non-Insulin-Dependent Diabetes Mellitus OR Diabetes Mellitus, Type II” OR “Diabetes Mellitus, Noninsulin Dependent” OR “Type 2 Diabetes Mellitus” OR “Noninsulin-Dependent Diabetes Mellitus” OR “Noninsulin Dependent Diabetes Mellitus” OR “Type 2 Diabetes OR Diabetes, Type 2”).
The search equation used in Web of Science was ((TS = (Exercise OR “Physical activity” OR “Physical exercise” OR “Exercise training”)) AND TS = (Microbiota OR “Gastrointestinal Microbiomes” OR “Gut Microbiome” OR “Gut Microflora” OR “Gut microbiota” OR “Gastrointestinal Flora” OR “Gastrointestinal Microflora” OR “Intestinal microbiome” OR “Intestinal microbiota” OR “Intestinal microflora” OR “Enteric bacteria”)) AND TS = (“Diabetes mellitus type 2” OR “Ketosis-Resistant Diabetes Mellitus” OR “Diabetes Mellitus, Non Insulin Dependent” OR “Diabetes Mellitus, Non-Insulin-Dependent” OR “Non-Insulin-Dependent Diabetes Mellitus” OR “Diabetes Mellitus, Type II” OR “Diabetes Mellitus, Noninsulin Dependent” OR “Type 2 Diabetes Mellitus” OR “Noninsulin-Dependent Diabetes Mellitus” OR “Noninsulin Dependent Diabetes Mellitus” OR “Type 2 Diabetes” OR “Diabetes, Type 2”)”.
2.2. Inclusion/Exclusion Criteria
2.2.1. Inclusion Criteria
The inclusion criteria were defined according to the PICO (Population, Intervention, Comparison, Outcome) methodology:
- Population: We included studies involving humans with prediabetes, T2D, or individuals at risk of developing type 2 diabetes.
- Intervention: Eligible interventions comprised cross-sectional studies analyzing gut microbiota through fecal sample collection and assessing physical activity via accelerometry or questionnaires. Experimental studies implementing a structured exercise program were also included.
- Comparison: Comparison of gut microbiota among individuals with different levels of physical activity or between groups that underwent an exercise intervention and those that did not.
- Outcome: Physical activity level, blood biomarkers, fecal microbiome composition, insulin resistance, and cardiorespiratory fitness (VO2max).
2.2.2. Exclusion Criteria
Regarding the exclusion criteria, studies that did not measure physical activity or exercise, as well as individuals who were taking prebiotic or probiotic supplementation, were excluded from the review.
2.3. Screening Process
The search results were imported into the Mendeley Reference Manager platform to review and remove duplicate documents. The initial screening of titles and abstracts was conducted independently and blindly by both researchers. In cases where no clear consensus was reached, the principal investigator made the final decision on inclusion or exclusion.
2.4. Data Extraction
The selected studies underwent a full-text review for data extraction. A dedicated data extraction form was developed to collect key trial information, including author, year of publication, population characteristics, age, clinical condition, study type, type of intervention, sample size, training modality and intensity, volume, duration of the intervention, and weekly training frequency. Outcome values were considered at the end of the intervention period, regardless of the total study duration. Data was independently extracted by two researchers, who also conducted a cross-validation. Subsequently, a third researcher reviewed all the collected information to ensure the consistency and accuracy of the values.
3. Results
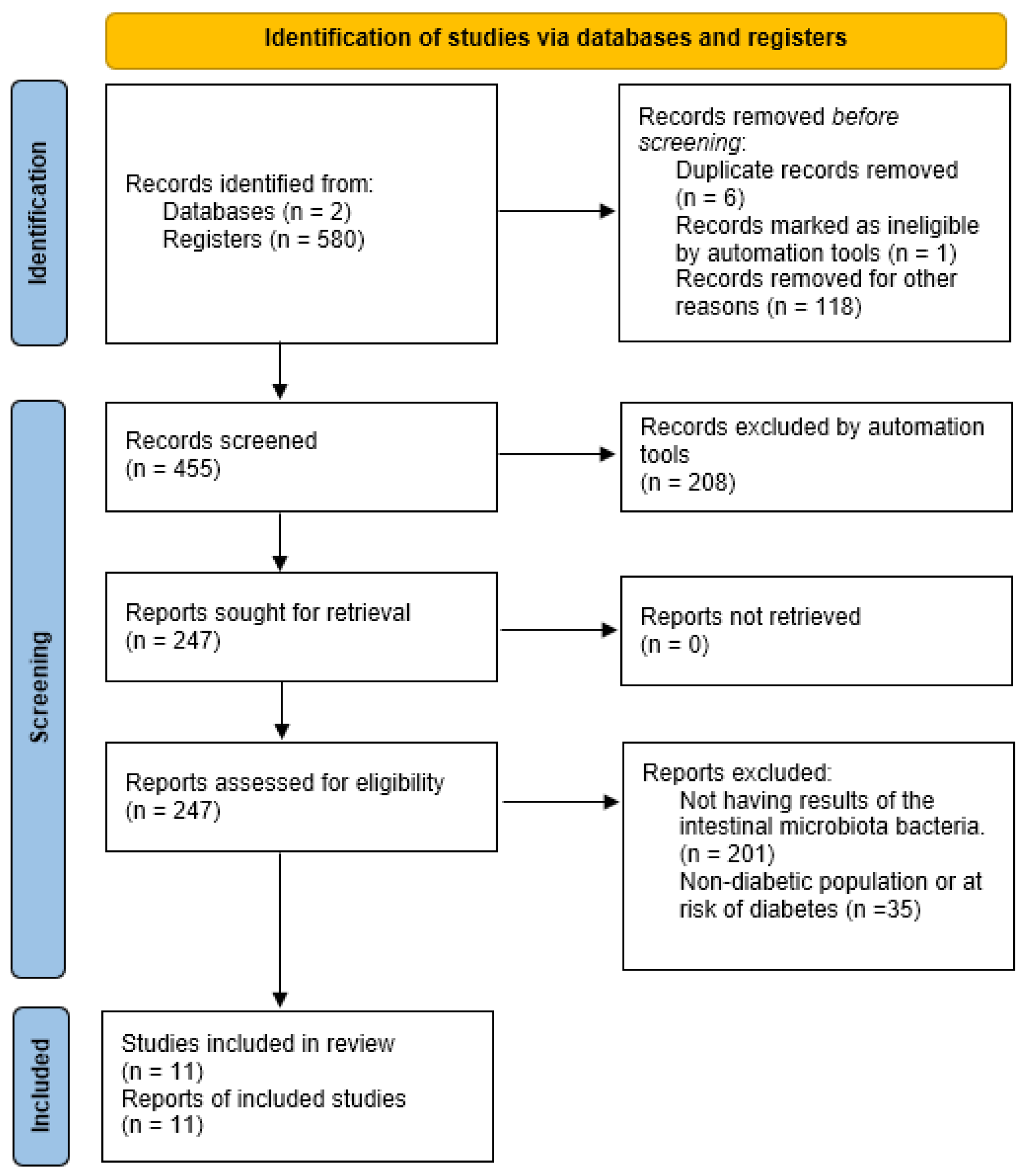
A total of 580 studies were collected: 171 from PubMed and 409 from Web of Science. Applying the “Humans” filter in both databases automatically excluded 118 studies (42 from PubMed and 76 from Web of Science) that had been conducted on animals. Additionally, six studies were removed as duplicates, and one was excluded for being marked as ineligible by automation tools.
Of the remaining 455 studies, 208 were excluded for not meeting the required design criteria (cross-sectional or clinical trials studies), leaving 247 studies from both databases. None were excluded due to retrieval issues.
Among those 247 studies, 201 were excluded for not presenting results on fecal microbiota composition, and 35 were excluded for not including a diabetic population or individuals with characteristics associated with developing diabetes.
Ultimately, 11 articles were included in the systematic review. A synthesis of this process can be seen in Figure 1.
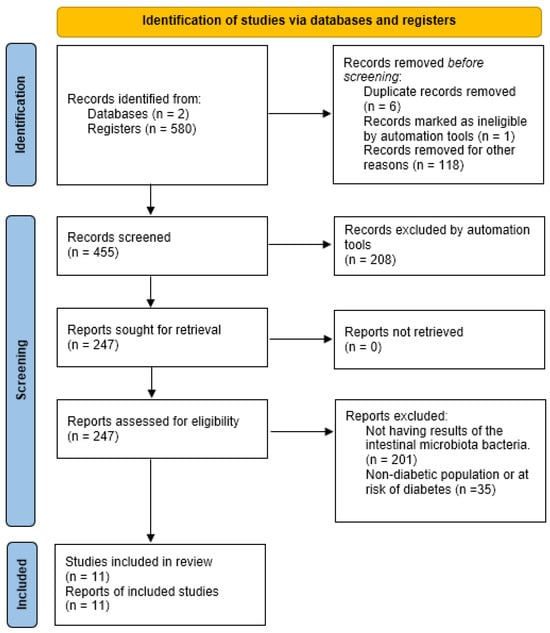
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram, including searches of databases, registers, and other resources.
Of the 11 articles included in this systematic review, 6 were cross-sectional studies and 5 were studies that included experimental protocols. Both study designs are represented in Table 1 and Table 2, respectively.

Table 1.
Observational evidence on physical activity and gut microbiota in T2D.
3.1. Observational Evidence on Physical Activity and Gut Microbiota in T2D
3.1.1. Physical Activity
Two observational studies assessed the relationship between physical activity levels and gut microbiota composition in adults with or at risk of T2D. Houttu et al. [25] reported that physically active individuals exhibited a higher abundance of Lachnospiraceae and Veillonella (Bacillota), whereas sedentary individuals had elevated levels of Roseburia hominis, Erysipelatoclostridium, Lachnoclostridium, and members of the Bacteroidetes phylum, particularly Prevotella_2. Predictive microbial signatures of physical activity were distributed across the dominant phyla Bacillota, Bacteroidetes, Proteobacteria, and Actinobacteria, and other types of phyla such as Lentisphaerae.
Similarly, Antush et al. [22] found that higher sedentary behavior correlated with lower α-diversity and a higher Bacillota/Bacteroidetes ratio, both markers previously linked with metabolic dysfunction.
3.1.2. Circulating TMAO and Physical Activity
Two studies explored the impact of physical activity on levels of TMAO, a gut-derived metabolite associated with T2D risk. Argyridou et al. [23] demonstrated that moderate to vigorous physical activity was associated with reduced TMAO levels. Kalagi et al. [26] confirmed that elevated TMAO was linked to T2D and identified lower levels of Clostridiaceae, Peptostreptococcaceae, Clostridium, Itestinibacter, and Romboutsia in individuals with T2D, suggesting potential microbial targets influenced by physical activity.
3.1.3. Microbial Diversity and Insulin Resistance
Only one observational study [24] reported that greater α- and β-microbial diversity were inversely associated with insulin resistance. Specifically, the Ruminococcaceae NK4A214 group was positively correlated with improved insulin sensitivity, reinforcing the metabolic relevance of microbial diversity in physically active populations.
3.1.4. Body Composition and Gut Microbiota
Somnuk et al. [27] examined gut microbiota in relation to body weight and found that overweight individuals had a higher abundance of Clostridiaceae-1, Bacillaceae-1, and Wohlfahrtiimonas. In contrast, Prevotella was more prevalent in individuals with normal weight, while taxa such as Eggerthellaceae, Rikenellaceae, Nocardioidaceae, and Chitinophagaceae were depleted in those with obesity.

Table 2.
Interventional studies: effects of exercise type and intensity on gut microbiota.
Table 2.
Interventional studies: effects of exercise type and intensity on gut microbiota.
| Reference | Population | Intervention | Measured Variables | Main Findings |
|---|---|---|---|---|
| Beals et al. (2023) [28] |
|
|
|
|
| Motiani et al. (2020) [29] |
|
|
|
|
| Torquiati et al. (2023) [30] |
|
|
|
|
| Verheggen et al. (2021) [31] |
|
|
|
|
| Wei et al. (2022) [32] |
|
|
|
|
Abbreviations: DEXA: dual-energy X-ray absorptiometry; ELISA: enzyme-linked immunoassay kit; FFQ: Food Frequency Questionnaire; HbA1c: glycated hemoglobin; HIIT: high-intensity interval training; HR: heart rate; MICT: moderate-intensity continuous training; N: number of persons; PET: positron emission tomography; SCFAs: short-chain fatty acids; SIT: sprint interval training; VO2max: maximum oxygen uptake.
3.2. Interventional Studies: Effects of Exercise Type and Intensity on Gut Microbiota
3.2.1. Type and Intensity of Exercise
Two trials compared different exercise modalities. Motiani et al. [29] found that both Sprint Interval Training (SIT) and Moderate-Intensity Continuous Training (MICT) reduced the Bacillota/Bacteroidetes ratio—largely due to an increase in Bacteroidetes—and decreased levels of Blautia and Clostridium. SIT promoted the growth of Lachnospira, whereas MICT led to increases in Veillonella, Veillonella dispar, and Faecalibacterium. Both protocols also increased levels of beneficial genera like Bifidobacterium and Escherichia A. municiphila.
Torquati et al. [30] reported that MICT increased butyrate-producing taxa such as Lachnospirales and Clostridium cluster IV, while High-Intensity Interval Training (HIIT) enhanced the presence of Rysipelotrichales and Oscillospirales—microbial groups potentially linked to improved metabolic health.
3.2.2. Short-Chain Fatty Acids (SCFAs)
Torquati et al. [30] was the only study to assess SCFAs, reporting no significant post-intervention changes in SCFA concentrations. Nevertheless, both HIIT and MICT increased the abundance of butyrate-producing taxa, suggesting a potential for longer-term metabolic benefits.
3.2.3. Microbial Diversity
Four intervention studies [28,29,31,32] investigated microbial diversity following exercise and generally reported no significant changes in α- or β-diversity. However, Beals et al. [28] noted a shift in β-diversity, although the effect was not attributed solely to exercise, suggesting that dietary confounding may influence diversity outcomes.
3.2.4. Gut Microbiota Composition
Three studies evaluated taxonomic shifts after exercise interventions. Verheggen et al. [31] observed increases in Ruminococcus gauvreauii, Lachnospiraceae FCS020 group, and Anaerostipes, with positive correlations between R. gauvreauii and VO2max, indicating a link between aerobic fitness and microbial composition.
Motiani et al. [29] corroborated previous findings with SIT enhancing Lachnospira and MICT favoring Veillonella and Faecalibacterium. Both exercise types also contributed to a favorable reduction in the Bacillota/Bacteroidetes ratio.
4. Discussion
This systematic review identified eleven studies, six observational and five interventional, examining the relationship between physical activity and gut microbiota composition in individuals with T2D or at risk of developing the disease. Despite heterogeneity in study populations, methodologies, and outcome measures, several consistent patterns emerged.
4.1. Observational Evidence on Physical Activity and Gut Microbiota in T2D
Cross-sectional studies consistently reported that lower levels of physical activity and higher sedentary behavior were associated with unfavorable gut microbiota profiles in adults with or at risk of T2D. For example, sedentary individuals showed an increased abundance of potentially pathogenic taxa such as Escherichia/Shigella and reduced levels of beneficial bacteria like Lachnospiraceae and Veillonella, microbial groups linked to anti-inflammatory functions and metabolic health. These findings are in line with evidence that physical inactivity can promote gut dysbiosis, which may exacerbate insulin resistance and systemic inflammation [22,25]. From a physiological perspective, this microbial imbalance may impair glucose uptake, increase gut permeability, and elevate inflammatory cytokines, worsening glycemic control and promoting T2D progression. Recent research has shown that gut-derived inflammation can also contribute to impaired mitochondrial function in skeletal muscle, further diminishing glucose disposal [33].
α-diversity, often considered a proxy for microbial resilience, was consistently lower among less active individuals. Notably, Antush et al. [22] reported a direct correlation between sedentary time and a higher Bacillota/Bacteroidetes ratio, a microbial marker commonly associated with metabolic disorders. Similarly, Chen et al. [24] linked higher microbial diversity with lower insulin resistance, suggesting that even in observational settings, microbial richness may mediate the beneficial effects of physical activity on glucose metabolism. Clinically, this supports the idea that promoting physical activity may not only improve cardiometabolic profiles but also enhance gut microbial balance, potentially delaying the onset or progression of T2D. These associations align with the concept of the gut–muscle axis, where microbial composition may impact insulin signaling and muscle glucose uptake [34].
The metabolite TMAO, a gut-derived metabolite implicated in cardiovascular and metabolic diseases, emerged as a relevant outcome in two studies. Argyridou et al. [23] found that moderate-to-vigorous physical activity was associated with reduced TMAO levels, while Kalagi et al. [26] showed that the association between TMAO and T2D risk lost significance after adjusting for physical activity and diet. These results suggest that TMAO levels may not only reflect dietary intake but also lifestyle-related microbiota changes, offering a potential biomarker for tracking microbiota–host interactions in response to physical activity. Physiologically, lower TMAO levels may reduce endothelial dysfunction, oxidative stress, and chronic inflammation—all of which are implicated in the pathogenesis of T2D and its vascular complications [35]. Clinically, tracking TMAO could help identify individuals at higher cardiometabolic risk and monitor the efficacy of lifestyle interventions, including exercise. In addition, elevated TMAO concentrations have been associated with impaired insulin signaling and pancreatic β-cell dysfunction, which are central features of T2D pathophysiology [36].
4.2. Interventional Studies: Effects of Exercise Type and Intensity on Gut Microbiota
Interventional studies provide more robust causal evidence that exercise influences gut microbial composition. Both aerobic and resistance-based exercise protocols induced taxonomic changes, although they did not consistently alter microbial diversity. Exercise decreases the Bacillota/Bacteroides ratio, providing multiple benefits for individuals with T2D. Motiani et al. [29] showed that both HIIT and SIT reduced the Bacillota/Bacteroides ratio in individuals with prediabetes and T2D. However, Wei et al. [32] reported no significant differences in gut microbiota between a lifestyle intervention and standard care interventions. This discrepancy may be explained by the fact that the standard care group was not monitored for diet or daily physical activity, potentially introducing a confounding variable. Nonetheless, it is worth noting that beneficial changes were observed between the start of the intervention and after 12 months.
Additionally, Motiani et al. [29] and Torquati et al. [30] demonstrated that both SIT and MICT increased the relative abundance of beneficial taxa such as Veillonella, Faecalibacterium, and butyrate producers like Lachnospira and Anaerostipes. These microbes are known to produce SCFAs, which enhance gut barrier function and improve insulin sensitivity [29,30]. From a physiological standpoint, SCFAs contribute to energy metabolism, promote GLP-1 secretion, regulate immune responses, and inhibit histone deacetylases, all critical in preventing or managing insulin resistance [37]. The increased abundance of SCFA producers may therefore underline some of the glycemic improvements observed in exercise trials. Clinically, identifying individuals with a low baseline abundance of these taxa may help personalize exercise prescriptions to enhance microbiota responsiveness and optimize metabolic outcomes. Further, some taxa enriched through exercise, such as Faecalibacterium prausnitzii, have been inversely associated with HbA1c levels and pro-inflammatory markers in patients with T2D [38].
Only one study [30] assessed short-chain fatty acids directly, reporting no significant changes in SCFA concentrations post-intervention. However, the increase in butyrate-producing taxa in both HIIT and MICT groups suggests the potential for longer-term benefits not captured within the study duration [30]. Since SCFAs play critical roles in glucose homeostasis, lipid metabolism, and inflammation regulation, future studies should combine taxonomic profiling with metagenomics or metabolomics to evaluate functional microbial outputs in response to exercise. Physiologically, SCFAs act as signaling molecules that influence insulin sensitivity, adipose tissue metabolism, hepatic glucose production, and gut–brain axis signaling. Clinically, enhancing SCFA production through exercise may reduce the need for pharmacologic glycemic control and lower systemic inflammation, important goals in T2D management. Importantly, butyrate has been shown to enhance mitochondrial function and improve glucose uptake in skeletal muscle [39].
Interestingly, the magnitude and direction of microbial shifts varied by exercise type. SIT appeared to favor the growth of Lachnospira and Clostridium species, whereas MICT enriched Veillonella and Bifidobacterium, suggesting that microbial responses to exercise may be intensity- and modality-dependent. This distinction could have practical implications for tailoring exercise prescriptions to optimize gut health in people with or at risk of T2D.
Contrary to expectations, most intervention studies failed to show significant changes in α- or β-diversity following exercise, even in protocols lasting several weeks. Beals et al. [28] reported some shifts in β-diversity, but effects could not be disentangled from dietary influences. These inconsistencies may reflect the inherent stability of microbial diversity in adults, requiring longer interventions or more intense stimuli to yield measurable change. Alternatively, taxonomic shifts may be more functionally relevant than diversity indices alone, highlighting the need for integrative analyses that include microbial metabolites and host metabolic endpoints. From a physiological and clinical standpoint, the lack of diversity change does not necessarily imply a lack of benefit. It suggests instead that compositional and functional changes may precede or substitute for shifts in diversity, especially in the short term. Clinically, functional redundancy in microbial ecosystems may allow for resilience in metabolic pathways even when diversity metrics remain stable.
4.3. Limitations of This Systematic Review
This review has several limitations. First, the number of eligible studies was small, reflecting the novelty of the field. Second, considerable methodological heterogeneity existed in microbiota sequencing methods, physical activity assessments, and population characteristics, limiting cross-study comparability. Third, only a few studies controlled dietary intake, a critical confounder in microbiota research.
Nevertheless, these findings suggest promising directions for future research. Standardized protocols incorporating diet-controlled interventions, metagenomic sequencing, and longitudinal designs are needed to clarify the causal links between exercise, gut microbiota, and metabolic health. Additionally, stratifying analyses by sex, medication use, and metabolic status may reveal subpopulations most responsive to microbiota-modulating effects of physical activity. From a clinical standpoint, this line of research may help integrate microbiome profiling into personalized lifestyle interventions for T2D prevention and treatment. Trials could also explore synergistic interventions combining diet, prebiotics, or fecal microbiota transplantation with exercise to enhance metabolic outcomes.
Over the years, approximately 2000 articles have been published exploring the relationship between gut microbiota and type 2 diabetes. However, when narrowing the focus to studies in which physical exercise is the primary variable, only five clinical trials were identified and included in this review. This highlights a clear gap in the literature and underscores the need for further research to elucidate the role of physical exercise, considering its key components such as volume, type, intensity, and frequency, in modulating gut microbiota and its impact on the regulation of T2D.
5. Conclusions
In summary, this systematic review indicates that physical activity, particularly when structured and sustained, can beneficially modulate gut microbiota composition in individuals with or at risk of T2D. While changes in microbial diversity were inconsistent, several taxa associated with improved metabolic outcomes were enriched through exercise, suggesting a role for the gut microbiome as a mediator of exercise benefits. These insights support the integration of exercise as a non-pharmacological strategy to optimize gut health and metabolic control in the context of diabetes prevention and management. Clinically, these findings underscore the potential of exercise as an adjunctive therapy to current pharmacologic regimens for T2D, particularly when combined with strategies that target gut microbial function and composition.
Author Contributions
Conceptualization, J.C.-P., J.G.P.-G. and C.C.; methodology, J.C.-P., J.G.P.-G. and C.C.; formal analysis, L.M.-R. and A.M.; investigation, L.M.-R. and A.M.; resources, L.M.-R. and A.M.; writing—original draft preparation, L.M.-R. and A.M.; writing—review and editing, J.C.-P., J.G.P.-G. and C.C.; supervision, J.C.-P., J.G.P.-G. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body Mass Index |
| DNA | Deoxyribonucleic Acid |
| HbA1c | Hemoglobin A1c |
| HIIT | High-Intensity Interval Training |
| MICT | Moderate-Intensity Continuous Training |
| N | Total participants |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| rRNA | Ribosomal Ribonucleic Acid |
| SCFAs | Short-Chain Fatty Acids |
| SIT | Sprint Interval Training |
| TMAO | Trimethylamine N-oxide |
| TS | Topic Search |
| VO2max | Maximal oxygen uptake |
References
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diabetes Rep. 2018, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, I.; Fulci, V.; Palone, F.; Stronati, L.; Cucchiara, S.; Carissimi, C. Quantitative Assessment of Shotgun Metagenomics and 16S rDNA Amplicon Sequencing in the Study of Human Gut Microbiome. OMICS A J. Integr. Biol. 2018, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V. Human Microbiome Acquisition and Bioinformatic Challenges in Metagenomic Studies. Int. J. Mol. Sci. 2018, 19, 383. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 121, pp. 91–119. ISBN 978-0-12-800100-4. [Google Scholar]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between Microbiota-Dependent Metabolite Trimethylamine-N-Oxide and Type 2 Diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Zhang, N.; Der Pluijm, W.V. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018–2028). Diabetes 2018, 67, 202-LB. [Google Scholar] [CrossRef]
- Egshatyan, L.; Kashtanova, D.; Popenko, A.; Tkacheva, O.; Tyakht, A.; Alexeev, D.; Karamnova, N.; Kostryukova, E.; Babenko, V.; Vakhitova, M.; et al. Gut Microbiota and Diet in Patients with Different Glucose Tolerance. Endocr. Connect. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Hao, W.; Zhang, J.; Li, H.; Yang, C.; Yang, J.; Chen, X.; Wang, H. Gut Microbiota and Type 2 Diabetes Mellitus: Association, Mechanism, and Translational Applications. Mediat. Inflamm. 2021, 2021, 5110276. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise Influence on the Microbiome–Gut–Brain Axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Pourabdi, R.; Shahidi, F.; Tabandeh, M.R.; Salehpour, M. Aerobic Exercise Timing Affects Mitochondrial Dynamics and Insulin Resistance by Regulating the Circadian Clock Protein Expression and NAD+-SIRT1-PPARα-MFN2 Pathway in the Skeletal Muscle of High-Fat-Diet-Induced Diabetes Mice. J. Physiol. Biochem. 2025, 81, 199–214. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. Impact of Different Training Modalities on Glycaemic Control and Blood Lipids in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Cutuli, D.; Decandia, D.; Giacovazzo, G.; Coccurello, R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut–Muscle–Brain Partnership. Int. J. Mol. Sci. 2023, 24, 14686. [Google Scholar] [CrossRef]
- Silva, J.S.C.; Seguro, C.S.; Naves, M.M.V. Gut Microbiota and Physical Exercise in Obesity and Diabetes–A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 863–877. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Díaz, J.; Jorquera-Aguilera, C.; Rodríguez-Rodríguez, A.; Rodríguez-Rodríguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9518. [Google Scholar] [CrossRef]
- Antush, M.T.; Balemba, O.B.; Hendricks, S.A.; Flynn, M.; Geidl, R.; Vella, C.A. Associations of Sedentary Behavior and Screen Time with Human Gut Microbiome Composition and Diversity. Life 2024, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Argyridou, S.; Bernieh, D.; Henson, J.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Suzuki, T.; Yates, T. Associations between Physical Activity and Trimethylamine N -Oxide in Those at Risk of Type 2 Diabetes. BMJ Open Diab. Res. Care 2020, 8, e001359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity: A Microbiome-Wide Analysis from Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef] [PubMed]
- Houttu, V.; Boulund, U.; Nicolaou, M.; Holleboom, A.G.; Grefhorst, A.; Galenkamp, H.; Van Den Born, B.-J.; Zwinderman, K.; Nieuwdorp, M. Physical Activity and Dietary Composition Relate to Differences in Gut Microbial Patterns in a Multi-Ethnic Cohort—The HELIUS Study. Metabolites 2021, 11, 858. [Google Scholar] [CrossRef]
- Kalagi, N.A.; Thota, R.N.; Stojanovski, E.; Alburikan, K.A.; Garg, M.L. Association between Plasma Trimethylamine N-Oxide Levels and Type 2 Diabetes: A Case Control Study. Nutrients 2022, 14, 2093. [Google Scholar] [CrossRef]
- Somnuk, S.; Komindr, S.; Monkhai, S.; Poolsawat, T.; Nakphaichit, M.; Wanikorn, B. Metabolic and Inflammatory Profiles, Gut Microbiota and Lifestyle Factors in Overweight and Normal Weight Young Thai Adults. PLoS ONE 2023, 18, e0288286. [Google Scholar] [CrossRef]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary Weight Loss-Induced Improvements in Metabolic Function Are Enhanced by Exercise in People with Obesity and Prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.-J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Torquati, L.; Gajanand, T.; Cox, E.R.; Willis, C.R.G.; Zaugg, J.; Keating, S.E.; Coombes, J.S. Effects of Exercise Intensity on Gut Microbiome Composition and Function in People with Type 2 Diabetes. Eur. J. Sport Sci. 2023, 23, 530–541. [Google Scholar] [CrossRef]
- Verheggen, R.J.H.M.; Konstanti, P.; Smidt, H.; Hermus, A.R.M.M.; Thijssen, D.H.J.; Hopman, M.T.E. Eight-week Exercise Training in Humans with Obesity: Marked Improvements in Insulin Sensitivity and Modest Changes in Gut Microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef]
- Wei, S.; Brejnrod, A.D.; Trivedi, U.; Mortensen, M.S.; Johansen, M.Y.; Karstoft, K.; Vaag, A.A.; Ried-Larsen, M.; Sørensen, S.J. Impact of Intensive Lifestyle Intervention on Gut Microbiota Composition in Type 2 Diabetes: A Post-Hoc Analysis of a Randomized Clinical Trial. Gut Microbes 2022, 14, 2005407. [Google Scholar] [CrossRef] [PubMed]
- Da, C.; Pinaffi-Langley, A.C.; Melia, E.; Hays, F.A. Exploring the Gut–Mitochondrial Axis: p66Shc Adapter Protein and Its Implications for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 3656. [Google Scholar] [CrossRef]
- Thome, T.; Salyers, Z.R.; Kumar, R.A.; Hahn, D.; Berru, F.N.; Ferreira, L.F.; Scali, S.T.; Ryan, T.E. Uremic Metabolites Impair Skeletal Muscle Mitochondrial Energetics through Disruption of the Electron Transport System and Matrix Dehydrogenase Activity. Am. J. Physiol. Cell Physiol. 2019, 317, C701–C713. [Google Scholar] [CrossRef]
- Jiang, J.-Y.; Liu, W.-M.; Zhang, Q.-P.; Ren, H.; Yao, Q.-Y.; Liu, G.-Q.; Lu, P.-R. Trimethylamine N-Oxide Aggravates Vascular Permeability and Endothelial Cell Dysfunction under Diabetic Condition: In Vitro and in Vivo Study. Int. J. Ophthalmol. 2024, 17, 25–33. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; Von Scholten, B.J. Trimethylamine N-Oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Łagowska, K.; Drzymała-Czyż, S. A Low Glycemic Index, Energy-Restricted Diet but Not Lactobacillus Rhamnosus Supplementation Changes Fecal Short-Chain Fatty Acid and Serum Lipid Concentrations in Women with Overweight or Obesity and Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 917–926. [Google Scholar] [CrossRef]
- Hecker, M.; Sommer, N.; Mayer, K. Assessment of Short- and Medium-Chain Fatty Acids on Mitochondrial Function in Severe Inflammation. In Mitochondrial Medicine; Weissig, V., Edeas, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2277, pp. 125–132. ISBN 978-1-0716-1269-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).