The Duodenum-Centered Neurohormonal Hypothesis of Type 2 Diabetes: A Mechanistic Review and Therapeutic Perspective
Abstract
1. Introduction
2. Methods
3. Evidence Supporting the Hypothesis
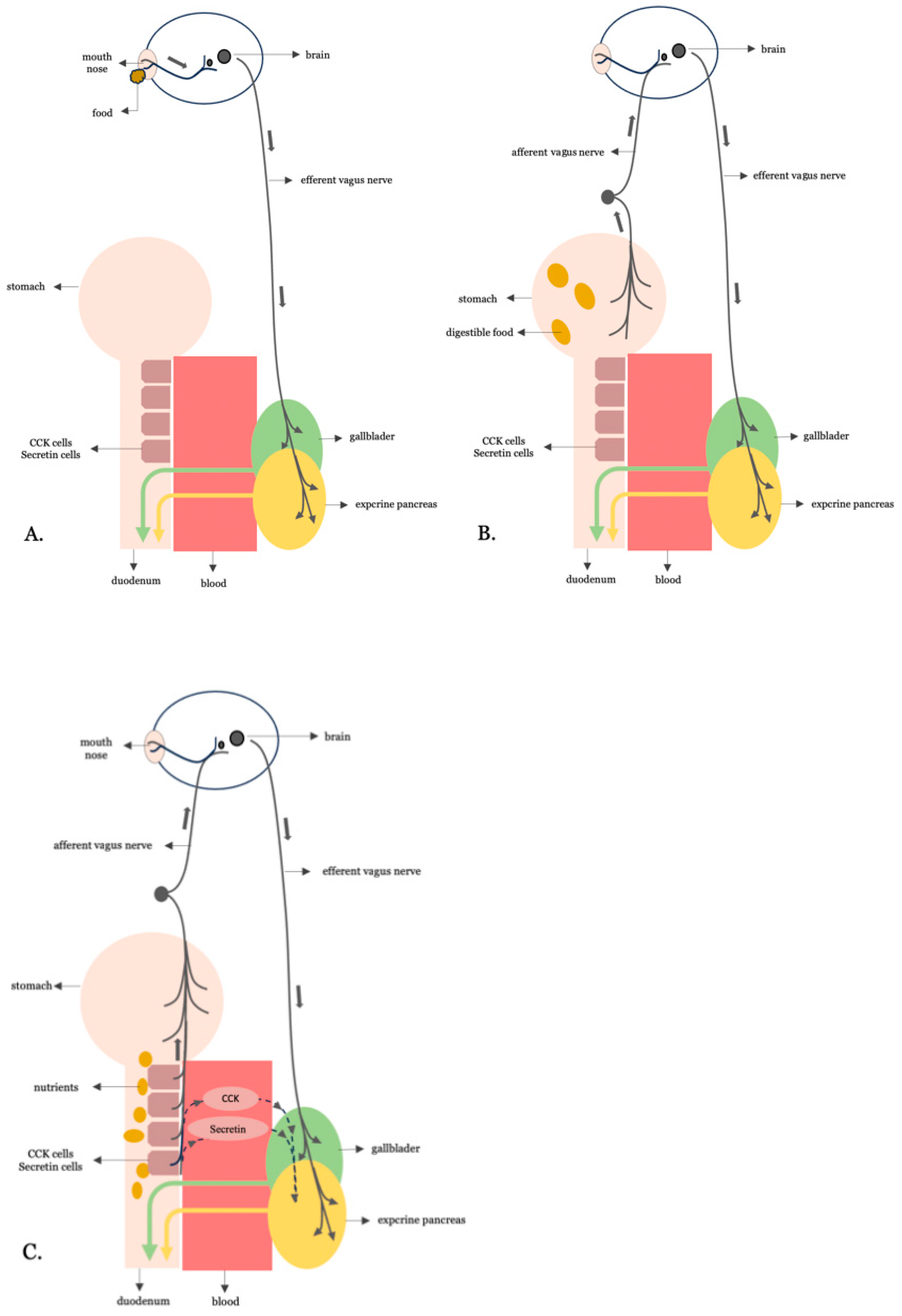
3.1. Digestive Neurohormonal Regulation: From Physiology to Pathogenesis
3.2. Is the Overstimulative Neurohormonal Digestive Axis the Missing Link Between Western Dietary Patterns and Insulin Resistance?
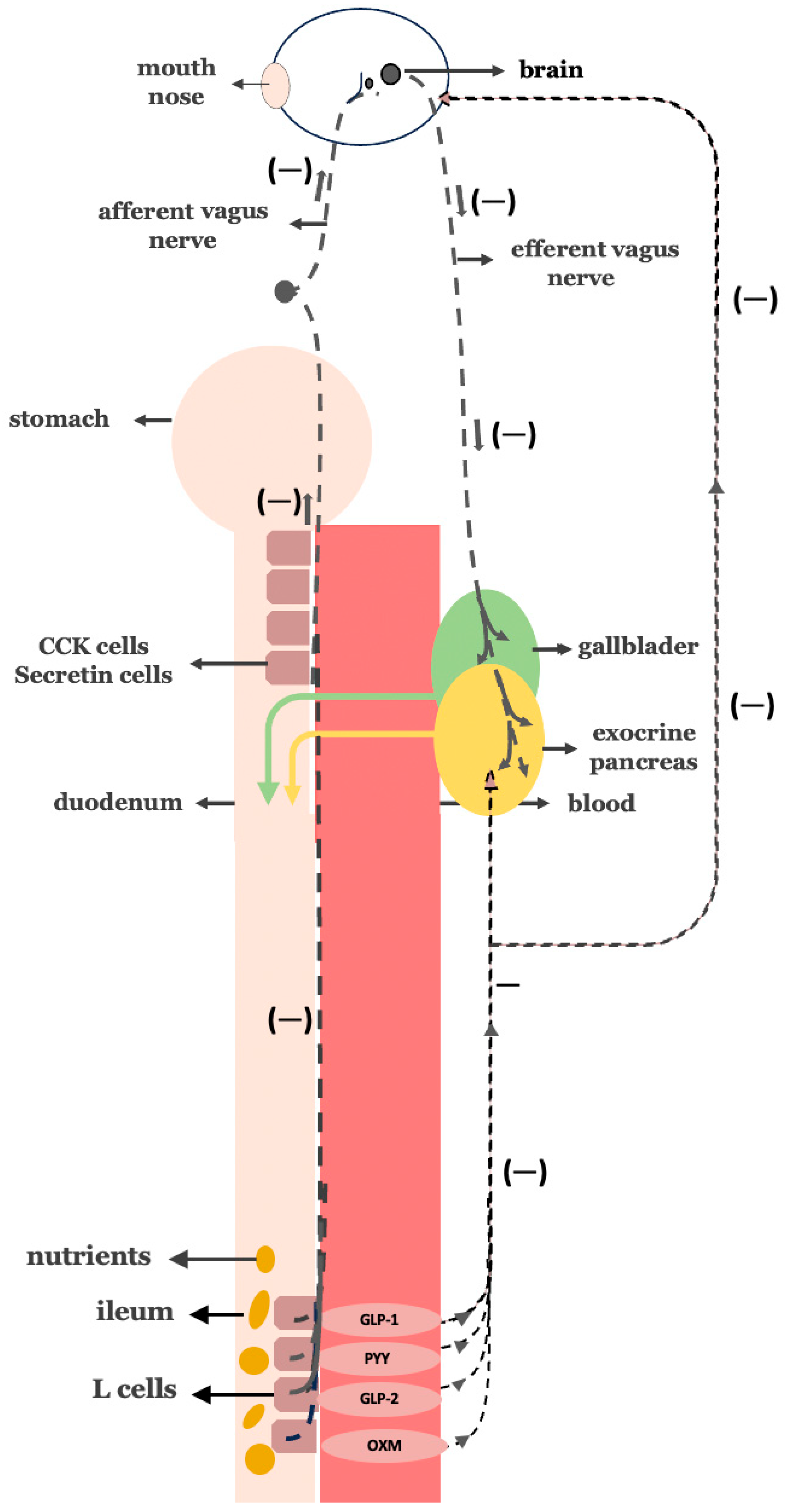
3.3. Validating the Anti-Incretin Hypothesis: Digestive Neurohormonal Hyperactivity
3.4. A Increased Vagal Activity in the Insulin Resistance Stage of T2DM
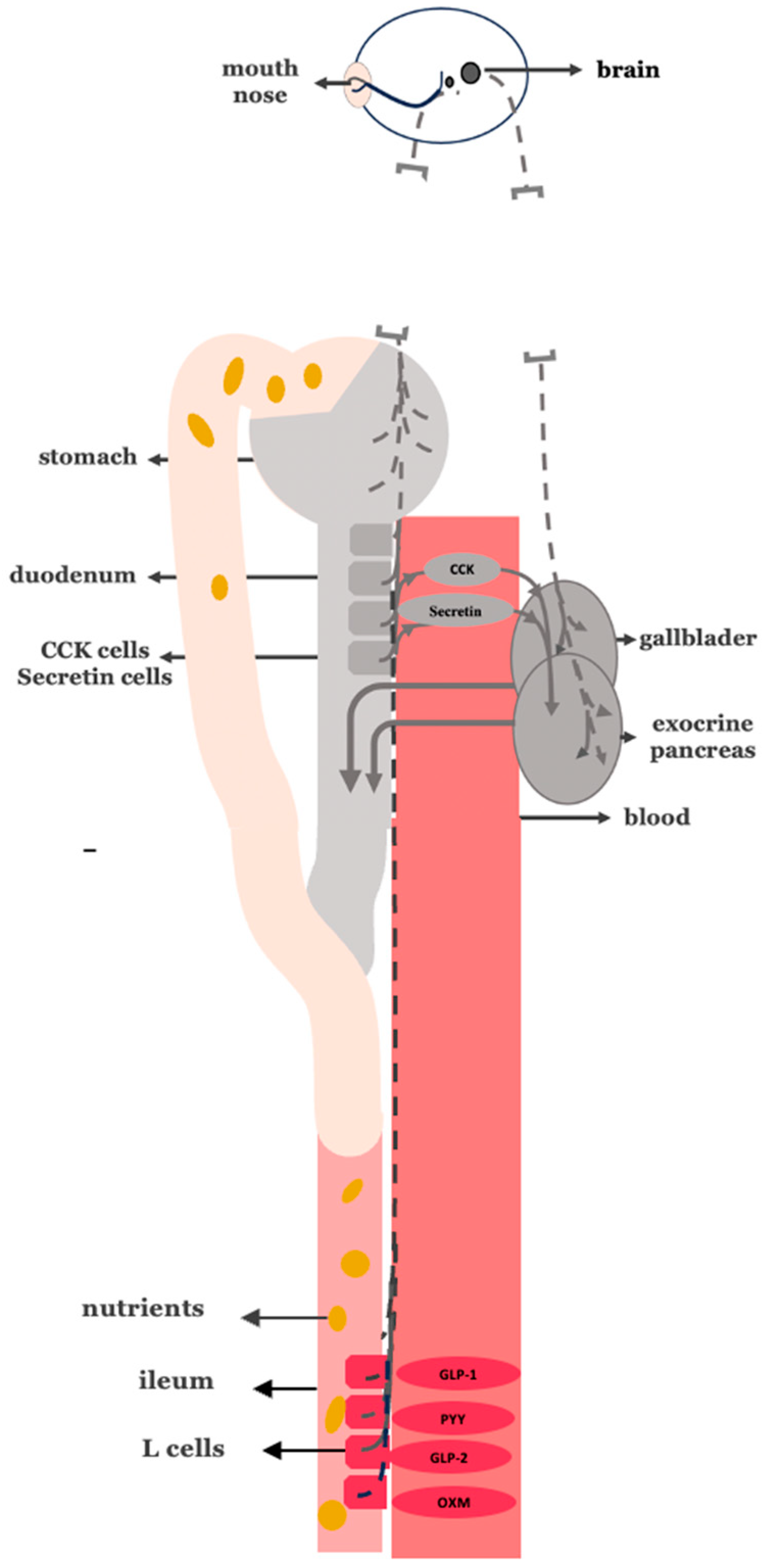
3.5. B Truncal Vagotomy for an Improvement in Insulin Sensitivity in T2DM
3.6. C Excessive Nutrient-Stimulated CCK and Secretin Hormonal Signaling in the Insulin Resistance Stage of T2DM
3.7. D Proximal Intestinal Bypass Procedures Are Most Effective for the Remission of T2DM
3.8. The Hypothetical Model for the Establishment of the Insulin Resistance Stage in T2DM
3.9. Corresponding Corrective Antidiabetic Actions of Bariatric Surgery on the Hypothetical Model of Insulin Resistance
3.10. Disrupting the Overactive Digestive Neurohormonal Axis: Truncal Vagotomy Combined with Gastric Bypass as a Targeted Strategy for Diabetes Remission
3.11. Lessons from Upper GI Surgeries for Cancer and Peptic Ulcer Disease
3.12. Potential Drawbacks and Limitations of Adding Truncal Vagotomy in Gastric Bypass Procedures
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| Ras | receptor agonists |
| TZDs | Thiazolidinediones |
| GLP-1 | glucagon-like peptide-1 |
| SGLT2 | Sodium–glucose cotransporter 2 |
| DPP-4 | Dipeptidyl peptidase-4 |
| LPS | Lipopolysaccharide |
| GAD | Glutamic acid decarboxylase |
References
- Islam, S.; Moinuddin Mir, A.R.; Arfat, M.Y.; Alam, K.; Ali, A. Studies on glycoxidatively modified human IgG: Implications in immuno-pathology of type 2 diabetes mellitus. Int. J. Biol. Macromol. 2017, 104, 19–29. [Google Scholar] [CrossRef]
- Rubino, F.; Amiel, S.A. Is the gut the “sweet spot” for the treatment of diabetes? Diabetes 2014, 63, 2225–2228. [Google Scholar] [CrossRef]
- Rubino, F. Medical research: Time to think differently about diabetes. Nature 2016, 533, 459–461. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Rubino, F.; Gagner, M. Potential of Surgery for Curing Type 2 Diabetes Mellitus. Ann. Surg. 2002, 236, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Regulation of Pancreatic Secretion. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar][Green Version]
- Dave, H.D.; Shumway, K.R.; Al Obaidi, N.M. Physiology, Biliary. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar][Green Version]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef]
- Schulze, M.B.; Liu, S.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am. J. Clin. Nutr. 2004, 80, 348–356. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Glycemic Index. JAMA 2002, 287, 2414. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The High-Fat Diet–Fed Mouse. Diabetes 2004, 53 (Suppl_3), S215–S219. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, W.M.; Nauck, M.A.; Zinman, B.; Daniels, G.H.; Bergenstal, R.M.; Mann, J.F.; Ravn, L.S.; Moses, A.C.; Stockner, M.; Baeres, F.M.; et al. LEADER 3—lipase and amylase activity in subjects with type 2 diabetes: Baseline data from over 9000 subjects in the LEADER Trial. Pancreas 2014, 43, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Gurney, K.; Malloy, J.; Shan, K.; Yan, P.; Chen, S. Increased variability and abnormalities in pancreatic enzyme concentrations in otherwise asymptomatic subjects with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2012, 5, 419–424. [Google Scholar] [CrossRef]
- Srihardyastutie, A.; Soeatmadji, D.W.; Fatchiyah, F. Relation of Elevated Serum Lipase to Indonesian Type 2 Diabetes Mellitus Progression the Role of Astaxanthin Compared with Metformin in Preventing Glycated Human Serum Albumin from Possible Unfolding: A Molecular Dynamic Study View Project Sweet Potatoes Anthocyanin as Anti-Depressant to Regulate Mechanism in Brain View Project [Internet]. Available online: www.biomedres.info (accessed on 1 February 2015).
- Rahman, M.A.; Ripon, M.A.R.; Amin, M.T.; Bhowmik, D.R.; Bhuiyan, M.S.; Hossain, M.S. Alpha-amylase Activity in Serum is Positively Associated with C-reactive Protein in Obesity and Diabetes. Dhaka Univ. J. Pharm. Sci. 2024, 23, 7–12. [Google Scholar] [CrossRef]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of glucose absorption in the small intestine in health and metabolic diseases and their role in appetite regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef]
- Fujita, Y.; Kojima, H.; Hidaka, H.; Fujimiya, M.; Kashiwagi, A.; Kikkawa, R. Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long-Evans Tokushima Fatty Rats. Diabetologia 1998, 41, 1459–1466. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Li, J.; Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef]
- Bashary, R.; Vyas, M.; Nayak, S.K.; Suttee, A.; Verma, S.; Narang, R.; Khatik, G.L. An Insight of Alpha-amylase Inhibitors as a Valuable Tool in the Management of Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 16, 117–136. [Google Scholar]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Handelsman, Y. Role of bile acid sequestrants in the treatment of type 2 diabetes. Diabetes Care 2011, 34, S244–S250. [Google Scholar] [CrossRef] [PubMed]
- Raghava, A.; Islam, S.; Mishra, B.K.; Gupta, R.K. Optical Screening of Glycation Induced Structural Alterations in Serum Proteins of Diabetes Patients Using Spectroscopic Techniques [Internet]. Available online: www.ijntr.org (accessed on 5 May 2016).
- Rohner-Jeanrenaud, F.; Hochstrasser, A.C.; Jeanrenaud, B. Hyperinsulinemia of preobese and obese fa/fa rats is partly vagus nerve mediated. Am. J. Physiol.-Endocrinol. Metab. 1983, 244, E317–E322. [Google Scholar] [CrossRef] [PubMed]
- Rohner-Jeanrenaud, F.; Jeanrenaud, B. A role for the vagus nerve in the etiology and maintenance of the hyperinsulinemia of genetically obese fa/fa rats. Int. J. Obes. 1985, 9 (Suppl 1), 71–75. [Google Scholar] [PubMed]
- Rohner-Jeanrenaud, F.; Jeanrenaud, B. Involvement of the Cholinergic System in Insulin and Glucagon Oversecretion of Genetic Preobesity. Endocrinology 1985, 116, 830–834. [Google Scholar] [CrossRef]
- Rohner-Jeanrenaud, F.; Walker, C.D.; Greco-Perotto, R.; Jeanrenaud, B. Central Corticotropin-Releasing Factor Administration Prevents the Excessive Body Weight Gain of Genetically Obese (fa/fa) Rats. Endocrinology 1989, 124, 733–739. [Google Scholar] [CrossRef]
- Scomparin, D.X.; Gomes, R.M.; Grassiolli, S.; Rinaldi, W.; Martins, A.G.; de Oliveira, J.C.; Gravena, C.; Mathias, P.C.d.F. Autonomic activity and glycemic homeostasis are maintained by precocious and low intensity training exercises in MSG-programmed obese mice. Endocrine 2009, 36, 510–517. [Google Scholar] [CrossRef]
- Balbo, S.L.; Ribeiro, R.A.; Mendes, M.C.; Lubaczeuski, C.; Maller, A.C.P.A.; Carneiro, E.M.; Bonfleur, M.L. Vagotomy diminishes obesity in cafeteria rats by decreasing cholinergic potentiation of insulin release. J. Physiol. Biochem. 2016, 72, 625–633. [Google Scholar] [CrossRef]
- Kral, J.G. Effects of truncal vagotomy on body weight and hyperinsulinemia in morbid obesity. Am. J. Clin. Nutr. 1980, 33 (Suppl. 2), 416–419. [Google Scholar] [CrossRef]
- Smith, D.; Sarfeh, J.; Howard, L. Truncal vagotomy in hypothalamic obesity. Lancet 1983, 321, 1330–1331. [Google Scholar] [CrossRef]
- Gortz, L.; Bjorkman, A.C.; Andersson, H.; Kral, J.G. Truncal Vagotomy Reduces Food and Liquid Intake in Man. Physiol. Behav. 1990, 48, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Kral, J. Vagotomy for Treatment of Severe Obesity. Lancet 1978, 311, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Regulation of cholecystokinin secretion by intraluminal releasing factors. Am. J. Physiol.-Gastrointest. Liver Physiol. 1995, 269, G319–G327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Prpic, V.; Green, G.M.; Reeve, J.R.; Liddle, R.A. Luminal CCK-releasing factor stimulates CCK release from human intestinal endocrine and STC-1 cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 282, G16–G22. [Google Scholar] [CrossRef]
- Tarasova, N.; Spannagel, A.W.; Green, G.M.; Gomez, G.; Reed, J.T.; Thompson, J.C.; Hellmich, M.R.; Reeve, J.R.; Liddle, R.A.; Greeley, G.H. Distribution and Localization of a Novel Cholecystokinin-Releasing Factor in the Rat Gastrointestinal Tract*. Endocrinology 1997, 138, 5550–5554. [Google Scholar] [CrossRef]
- Tanday, N.; English, A.; Lafferty, R.A.; Flatt, P.R.; Irwin, N. Benefits of Sustained Upregulated Unimolecular GLP-1 and CCK Receptor Signalling in Obesity-Diabetes. Front. Endocrinol. 2021, 12, 674704. [Google Scholar] [CrossRef]
- Graf, R.; Bimmler, D. Biochemistry and Biology of SPINK-PSTI and Monitor Peptide. Endocrinol. Metab. Clin. N. Am. 2006, 35, 333–343. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Sathyapalan, T.; Banach, M.; Sahebkar, A. Incretins and microRNAs: Interactions and physiological relevance. Pharmacol. Res. 2020, 153, 104662. [Google Scholar] [CrossRef]
- Gilliam-Vigh, H.; Jorsal, T.; Rehfeld, J.F.; Pedersen, J.; Poulsen, S.S.; Vilsbøll, T.; Knop, F.K. Expression of Cholecystokinin and its Receptors in the Intestinal Tract of Type 2 Diabetes Patients and Healthy Controls. J. Clin. Endocrinol. Metab. 2021, 106, 2164–2170. [Google Scholar] [CrossRef]
- Rogers, R.C.; Hermann, G.E. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides 2008, 29, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, A.K.; Davis, D.B. Glucagon-like peptide-1 and cholecystokinin production and signaling in the pancreatic islet as an adaptive response to obesity. J. Diabetes Investig. 2016, 7 (Suppl. 1), 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rushakoff, R.A.; Goldfine, I.D.; Beccaria, L.J.; Mathur, A.; Brand, R.J.; Liddle, R.A. Reduced postprandial cholecystokinin (CCK) secretion in patients with noninsulin-dependent diabetes mellitus: Evidence for a role for CCK in regulating postprandial hyperglycemia. J. Clin. Endocrinol. Metab. 1993, 76, 489–493. [Google Scholar] [PubMed]
- Ahrén, B.; Holst, J.J.; Efendic, S. Antidiabetogenic Action of Cholecystokinin-8 in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2000, 85, 1043–1048. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, J.; Liu, D.; Lv, Y.; Zhang, C.; Su, X.; Li, L.; Merlotti, D. The Prevalence and Characteristics of Exocrine Pancreatic Insufficiency in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 7764963. [Google Scholar] [CrossRef]
- Rubino, F.; Marescaux, J. Effect of Duodenal-Jejunal Exclusion in a Non-obese Animal Model of Type 2 Diabetes: A New Perspective for an Old Disease. Ann. Surg. 2004, 239, 1–11. [Google Scholar] [CrossRef]
- Rubino, F.; Forgione, A.; Cummings, D.E.; Vix, M.; Gnuli, D.; Mingrone, G.; Castagneto, M.; Marescaux, J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann. Surg. 2006, 244, 741–749. [Google Scholar] [CrossRef]
- Inabnet, W.B.; Winegar, D.A.; Sherif, B.; Sarr, M.G. Early outcomes of bariatric surgery in patients with metabolic syndrome: An analysis of the bariatric outcomes longitudinal database. J. Am. Coll. Surg. 2012, 214, 550–556. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Aminian, A.; Romero-Talamás, H.; Batayyah, E.; Mackey, J.; Kennedy, L.; Kashyap, S.R.; Kirwan, J.P.; Rogula, T.; Kroh, M.; et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann. Surg. 2013, 258, 628–637. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; Belle, S.H.; Neiberg, R.H.; Pierson, S.K.; Eagleton, J.K.; Kalarchian, M.A.; DeLany, J.P.; Lang, W.; Jakicic, J.M. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment. JAMA Surg. 2015, 150, 931–940. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Vidal, J.; Salminen, P.; Still, C.D.; Nor Hanipah, Z.; Sharma, G.; Tu, C.; Wood, G.C.; Ibarzabal, A.; Jimenez, A.; et al. Late Relapse of Diabetes After Bariatric Surgery: Not Rare, but Not a Failure. Diabetes Care 2020, 43, 534–540. [Google Scholar] [CrossRef] [PubMed]
- McTigue, K.M.; Wellman, R.; Nauman, E.; Anau, J.; Coley, R.Y.; Odor, A.; Tice, J.; Coleman, K.J.; Courcoulas, A.; Pardee, R.E.; et al. Comparing the 5-Year Diabetes Outcomes of Sleeve Gastrectomy and Gastric Bypass. JAMA Surg. 2020, 155, e200087. [Google Scholar] [CrossRef] [PubMed]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef]
- Arneth, B. Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines 2024, 5, 153–165. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Rodin, J.; Wack, J.; Ferrannini, E.; DeFronzo, R.A. Effect of Insulin and Glucose on Feeding Behavior. Metabolism 1985, 34, 826–831. [Google Scholar] [CrossRef]
- Rodin, J. Insulin Levels, Hunger, and Food Intake: An Example of Feedback Loops in Body Weight Regulation. Health Psychol. 1985, 4, 1. [Google Scholar] [CrossRef]
- Kapralou, A.N.; Chrousos, G.P. Metabolic effects of truncal vagotomy when combined with bariatric-metabolic surgery. Metabolism 2022, 135, 155263. [Google Scholar] [CrossRef]
- Campbell, J.; Berry, J.; Liang, Y. Anatomy and Physiology of the Small Intestine. In Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set [Internet]; Elsevier: Amsterdam, The Netherlands, 2019; pp. 817–841. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323402323000716 (accessed on 5 May 2016).
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.F.; Jacques, P.F. Carbohydrate Nutrition, Insulin Resistance, and the Prevalence of the Metabolic Syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef]
- Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Ochner, C.N.; Gibson, C.; Shanik, M.; Goel, V.; Geliebter, A. Changes in neurohormonal gut peptides following bariatric surgery. Int. J. Obes. 2011, 35, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Meek, C.L.; Lewis, H.B.; Reimann, F.; Gribble, F.M.; Park, A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016, 77, 28–37. [Google Scholar] [CrossRef]
- Cazzo, E.; Pareja, J.C.; Chaim, E.A.; Geloneze, B.; Barreto, M.R.L.; Magro, D.O. GLP-1 and GLP-2 Levels are Correlated with Satiety Regulation After Roux-en-Y Gastric Bypass: Results of an Exploratory Prospective Study. Obes. Surg. 2017, 27, 703–708. [Google Scholar] [CrossRef]
- Manning, S.; Pucci, A.; Batterham, R.L. GLP-1: A Mediator of the Beneficial Metabolic Effects of Bariatric Surgery? Physiology 2015, 30, 50–62. [Google Scholar] [CrossRef]
- Romero, F.; Nicolau, J.; Flores, L.; Casamitjana, R.; Ibarzabal, A.; Lacy, A.; Vidal, J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc. 2012, 26, 2231–2239. [Google Scholar] [CrossRef]
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexandrides, T.K. Weight Loss, Appetite Suppression, and Changes in Fasting and Postprandial Ghrelin and Peptide-YY Levels After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy. Ann. Surg. 2008, 247, 401–407. [Google Scholar] [CrossRef]
- Albrechtsen, N.J.W.; Hornburg, D.; Albrechtsen, R.; Svendsen, B.; Toräng, S.; Jepsen, S.L.; Kuhre, R.E.; Hansen, M.; Janus, C.; Floyd, A.; et al. Oxyntomodulin Identified as a Marker of Type 2 Diabetes and Gastric Bypass Surgery by Mass-spectrometry Based Profiling of Human Plasma. eBioMedicine 2016, 7, 112–120. [Google Scholar] [CrossRef]
- Wahlström, A.; Aydin, Ö.; Olsson, L.M.; Sjöland, W.; Henricsson, M.; Lundqvist, A.; Marschall, H.-U.; Franken, R.; van de Laar, A.; Gerdes, V.; et al. Alterations in bile acid kinetics after bariatric surgery in patients with obesity with or without type 2 diabetes. eBioMedicine 2024, 106, 105265. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Shan, X.; Cheng, Y.; Xu, J.; Fu, H.; Wang, W.; Yan, R.; Cai, Q. Clinical Course of Diabetes After Gastrectomy According to Type of Reconstruction in Patients with Concurrent Gastric Cancer and Type 2 Diabetes. Obes. Surg. 2015, 25, 673–679. [Google Scholar] [CrossRef]
- Wang, K.; Huang, K.; Lan, Y.; Fang, W.; Lo, S.; Li, A.F.; Wu, C. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World J. Surg. 2014, 38, 431–438. [Google Scholar] [CrossRef]
- Kim, J.W.; Cheong, J.H.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J. Gastroenterol. 2012, 18, 49–54. [Google Scholar] [CrossRef]
- Lee, W.; Ahn, S.H.; Lee, J.H.; Park, D.J.; Lee, H.-J.; Kim, H.-H.; Yang, H.-K. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes. Surg. 2012, 22, 1238–1243. [Google Scholar] [CrossRef]
- Peng, D.; Cheng, Y.X.; Zhang, W. Does Roux-en-Y Construction Really Bring Benefit of Type 2 Diabetes Mellitus Remission After Gastrectomy in Patients with Gastric Cancer? A Systematic Review and Meta-Analysis. Diabetes Ther. 2020, 11, 2863–2872. [Google Scholar] [CrossRef]
- An, J.Y.; Kim, Y.M.; Yun, M.A.; Jeon, B.H.; Noh, S.H. Improvement of type 2 diabetes mellitus after gastric cancer surgery: Short-term outcome analysis after gastrectomy. World J. Gastroenterol. 2013, 19, 9410–9417. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, D.H.; Park, B.J.; Kim, S.; Park, S.; Rosenthal, R.J. Impact of preoperative visceral fat proportion on type 2 diabetes in patients with low body mass index after gastrectomy. Surg. Obes. Relat. Dis. 2017, 13, 1361–1368. [Google Scholar] [CrossRef]
- Kral, J.G.; Paez, W.; Wolfe, B.M. Vagal nerve function in obesity: Therapeutic implications. World J. Surg. 2009, 33, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Inaba, C.S.; Koh, C.Y.; Sujatha-Bhaskar, S.; Gallagher, S.; Chen, Y.; Nguyen, N.T. Operative time as a marker of quality in bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y. Postgastrectomy syndrome. Foregut Surg. 2022, 2, 17. [Google Scholar] [CrossRef]
- Ganipisetti, V.M.; Naha, S. Bariatric Surgery Malnutrition Complications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Scarpellini, E.; Arts, J.; Karamanolis, G.; Laurenius, A.; Siquini, W.; Suzuki, H.; Ukleja, A.; Van Beek, A.; Vanuytsel, T.; Bor, S.; et al. International consensus on the diagnosis and management of dumping syndrome. Nat. Rev. Endocrinol. 2020, 16, 448–466. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl_2), S157–S163. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans With Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Tuomi, T.; Santoro, N.; Caprio, S.; Cai, M.; Weng, J.; Groop, L. The many faces of diabetes: A disease with increasing heterogeneity. Lancet 2014, 383, 1084–1094. [Google Scholar] [CrossRef]



| Target Organ(s) | Origin | Mechanism | Pathophysiological Features | Potential Interventions |
|---|---|---|---|---|
| Adipose tissue, [91] | Excess visceral adiposity | Inflammatory adipokine signaling from visceral fat | ↑ TNF-α, IL-6, resistin; ↓ adiponectin → systemic inflammation → insulin resistance | Weight loss, TZDs, anti-inflammatory agents, lifestyle modification |
| Liver, [92] | High-fat diets, metabolic syndrome | Hepatic insulin resistance and ectopic lipid accumulation | ↑ Gluconeogenesis; ↑ hepatic glucose output; lipotoxicity and hepatic steatosis | Metformin, GLP-1 RAs, low-carb diets, SGLT2 inhibitors, TZDs |
| Intestine, [93] | Western diet, antibiotics | Gut microbiota alterations and endotoxemia | Dysbiosis → ↑ LPS → metabolic endotoxemia → chronic low-grade inflammation | Prebiotics, probiotics, fiber-rich diet, GLP-1 Ras, metformin |
| Skeletal muscle, [94] | Physical inactivity, chronic nutrient overload | Impaired glucose uptake in skeletal muscle | ↓ GLUT4 translocation; mitochondrial dysfunction; insulin signaling defects | Exercise, metformin, TZDs, weight loss |
| Pancreatic β-cells, [95] | Chronic glucolipotoxicity, genetic susceptibility, inflammation | Pancreatic β-cell dysfunction and failure | Progressive β-cell mass loss and impaired insulin secretion; oxidative stress, ER stress, islet inflammation | Early insulin, GLP-1 RAs, DPP-4 inhibitors, β-cell protective therapies |
| Pancreatic β-cells, [96] | Pancreatic β-cells with autoimmune destruction | Autoimmune-like features | Presence of GAD or other islet autoantibodies; β-cell destruction resembling T1DM | Early insulin therapy, immunomodulators (investigational) |
| Hypothetical: Duodenum | Hypothetical: Chronic high-fat, high glycemic index diets | Hypothetical: Amplified digestion–driven neurohormonal dysregulation | Hypothetical: Excessive vagal and hormonal (CCK, secretin) stimulation → increased biliopancreatic secretion → accelerated nutrient absorption → insulin resistance | Hypothetical: Truncal vagotomy+proximal intestinal bypass; vagal or hormonal pathway modulators (investigational) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapralou, A.N.; Yapijakis, C.; Chrousos, G.P. The Duodenum-Centered Neurohormonal Hypothesis of Type 2 Diabetes: A Mechanistic Review and Therapeutic Perspective. Curr. Issues Mol. Biol. 2025, 47, 657. https://doi.org/10.3390/cimb47080657
Kapralou AN, Yapijakis C, Chrousos GP. The Duodenum-Centered Neurohormonal Hypothesis of Type 2 Diabetes: A Mechanistic Review and Therapeutic Perspective. Current Issues in Molecular Biology. 2025; 47(8):657. https://doi.org/10.3390/cimb47080657
Chicago/Turabian StyleKapralou, Athena N., Christos Yapijakis, and George P. Chrousos. 2025. "The Duodenum-Centered Neurohormonal Hypothesis of Type 2 Diabetes: A Mechanistic Review and Therapeutic Perspective" Current Issues in Molecular Biology 47, no. 8: 657. https://doi.org/10.3390/cimb47080657
APA StyleKapralou, A. N., Yapijakis, C., & Chrousos, G. P. (2025). The Duodenum-Centered Neurohormonal Hypothesis of Type 2 Diabetes: A Mechanistic Review and Therapeutic Perspective. Current Issues in Molecular Biology, 47(8), 657. https://doi.org/10.3390/cimb47080657







